Abstract
It has often been proposed that regions of the human parietal and/or frontal lobe may modulate activity in visual cortex, for example during selective attention or saccade preparation. However, direct evidence for such causal claims is largely missing in human studies, and it remains unclear to what degree the putative roles of parietal and frontal regions in modulating visual cortex may differ. Here we used concurrent TMS-fMRI to show that stimulating right human intra-parietal sulcus (IPS, at a site previously implicated in attention) elicits a pattern of activity-changes in visual cortex that strongly depends on current visual context. Increased intensity of IPS TMS affected the BOLD signal in V5/MT+ only when moving stimuli were present to drive this visual region, whereas TMS-elicited BOLD signal changes were observed in areas V1-V4 only during the absence of visual input. These influences of IPS-TMS upon remote visual cortex differed significantly from corresponding effects of frontal (eye field) TMS, in terms of how they related to current visual input and their spatial topography for retinotopic areas V1-V4. Our results show directly that parietal and frontal regions can indeed have distinct patterns of causal influence upon functional activity in human visual cortex.
Keywords: parietal cortex, frontal cortex, functional Magnetic Resonance Imaging, attention, top-down, Transcranial Magnetic Stimulation
INTRODUCTION
Activity in visual cortex can be modulated by top-down factors. For instance, human neuroimaging studies have shown that the BOLD signal in visual cortex can change even in the absence of any visual stimulus. This can arise when the retinotopically corresponding part of visual space is covertly attended (Hopfinger, Buonocore, and Mangun 2000; Kastner and others 1999; Ress, Backus, and Heeger 2000; Ruff and others 2006), or during eye movements (Super and others 2004), even in darkness (Paus and others 1995; Sylvester, Haynes, and Rees 2005). It is generally thought that such activity modulations in visual areas may reflect ‘top-down’ influences from a fronto-parietal network involved in selective attention and eye-movement control (Corbetta and Shulman 2002; Desimone and Duncan 1995; Driver and Frackowiak 2001; Duncan, Humphreys, and Ward 1997; Kastner and Ungerleider 2000; Miller 2000; Serences and Yantis 2006). More anatomically-specific suggestions have argued in particular (Kastner and Ungerleider, 2000; Macaluso and Driver 2005; Moore and Armstrong 2003; Tehovnik and others 2000) for top-down influences from the intraparietal sulcus (IPS) and/or from the frontal eye fields (FEF). In apparent general accord with such proposals, many neuroimaging studies have found that such areas in frontal and parietal cortex often show activity-increases in situations where visual activity is modulated in a top-down manner (Corbetta and Shulman 2002; Hagler, Jr. and Sereno 2006; Schluppeck and others 2006; Silver, Ress, and Heeger 2005). However, such findings typically fall short of demonstrating a truly causal influence from frontal or parietal cortex upon visual cortex, due to the non-interventional nature of typical neuroimaging studies.
One intervention increasingly used in human studies involves non-invasive transcranial magnetic stimulation (TMS). Several purely behavioural TMS studies have now shown that TMS to frontal or parietal areas can affect some types of visual judgments (Chambers and Mattingley 2005; Ellison, Lane, and Schenk 2007; Grosbras and Paus 2003; Grosbras and Paus 2002; Koch and others 2005; Muggleton and others 2003; Muggleton and others 2006; O'Shea and others 2004; Pourtois and others 2001; Silvanto, Lavie, and Walsh 2006). Such effects might in principle reflect remote influences upon activity in retinotopic visual cortex, but this has rarely been directly tested hitherto. However, in a recent study (Ruff and others 2006) we applied TMS to human FEF during fMRI scanning (see also Paus and others 1997; Taylor, Nobre, and Rushworth 2007). As described in more detail below, we found that FEF TMS could modulate BOLD signal in retinotopic visual areas V1-V4 systematically (see also Armstrong, Fitzgerald, and Moore 2006; Moore and Armstrong 2003 for potentially related microstimulation work in non-human primates; and Kayser and Logothetis 2006 for discussion). It remains unclear whether parietal TMS might exert similar or qualitatively different influences upon human visual cortex. Assessing this with concurrent TMS-fMRI may provide a new approach to determining whether specific parietal and frontal regions can make distinct contributions to ‘top-down’ modulation of visual cortex.
Accordingly, we used concurrent TMS-fMRI in the present study to examine any activity modulations in visual cortex elicited by stimulation of human intraparietal sulcus (IPS). We used an analogous method to that employed in our recent study of FEF TMS during fMRI (Ruff and others 2006). Comparing the outcomes of the present with the previous experiment should reveal whether frontal and parietal TMS can have distinct (or common) effects on activity in visual cortex - any differences would imply some regional specificity in the causal influences observed. In analogy to the FEF, the region in the anterior IPS we targeted with TMS here has already been potentially implicated in covert spatial attention and eye-movements, via activation in fMRI studies (e.g., Brown and others 2004; Connolly and others 2000; Connolly and others 2002; Corbetta and others 1998; Curtis, Rao, and D'Esposito 2004; Gagnon and others 2002; Grosbras, Laird, and Paus 2005; Koyama and others 2004; Perry and Zeki 2000; Petit and Haxby 1999). The new question here was whether stimulating the IPS with TMS would lead to a similar outcome as FEF TMS, or to qualitative differences, in terms of any induced changes in activity within remote visual cortex. There are emerging proposals that frontal versus parietal regions might fulfil somewhat different, but potentially complementary, functions in the control of visual attention (e.g., Buschman and Miller, 2007; Culham, Cavanagh, and Kanwisher 2001; Kastner and others 1999; Shulman and others 2003; Wardak and others 2006), eye movements (e.g., Connolly and others, 2002), or working memory (e.g., Curtis, 2006; Postle 2003). For instance, it has recently been argued that frontal areas (in particular, FEF and LPFC) may be more involved in top-down or endogenous aspects of visual attention, whereas parietal areas may be involved in more bottom-up or exogenous aspects (see Buschman and Miller, 2007). Any differences we might find here between possible effects of IPS TMS upon activity in visual cortex, versus those of FEF TMS as we recently reported (Ruff and others, 2006), would extend such proposals, by demonstrating directly that frontal and parietal cortex may exert qualitatively different influences on visual cortex.
The experimental procedure and participants for the present IPS TMS experiment were as for our prior TMS study of right FEF, to allow direct comparison. Inside an MR scanner, we now applied TMS over the scalp site corresponding to right IPS (Figure 1A), at one of four different intensities on every trial (see Materials and Methods). This strategy allowed us to identify any areas in visual cortex that showed activity changes (as revealed by fMRI) related to the intensity of IPS TMS, rather than merely to TMS presence versus absence. Note that while our approach is somewhat analogous to physiological studies in animals that intervene in a targeted region (e.g., via lesion, cooling, or chemical inactivation; see e.g., Fuster, Bauer, and Jervey 1985; Wardak and others 2006), and then measure the physiological consequences for remote interconnected regions, TMS itself is likely to have a different mechanism of action than, say, local cooling. TMS of the type used here (see Methods) can be considered as a form of stimulation of the targeted local neural population, as when TMS to motor cortex induces a twitch (e.g., Di Lazzaro and others 2004), or TMS to visual cortex induces an illusory flash or phosphene (e.g., Bestmann and others 2007). Rather than using TMS to disrupt behaviour, here our intention was to use TMS to stimulate IPS (or FEF), in order to characterise how this manipulation may causally influence BOLD signals in remote but interconnected structures of visual cortex, as measured with concurrent fMRI (see also Paus and others 1997; Massimini and others, 2005; Taylor, Nobre, and Rushworth 2007, for related uses of TMS in combinations with other neuroimaging methods). For this reason, participants were asked simply to fixate centrally, as confirmed by on-line eye tracking throughout scanning, with no other task. This ensured that any physiological influences of parietal TMS upon functional activity in visual cortex could not be contaminated by any TMS-induced changes in behaviour. As in our prior FEF study, we applied TMS (now to IPS) during two different visual contexts, either showing a blank screen or else bilateral moving and changing visual stimuli that should activate many visual regions (see Figure 1B-C). We could thereby assess whether any influences of IPS TMS upon visual cortex might depend on the current level of bottom-up activation via visual input. Our previous FEF TMS study had found TMS influences that were unaffected by this visual manipulation. As shown below, the pattern we now found for IPS TMS was very different, with the critical effects upon BOLD activity in visual cortex depending strongly on the current visual context.
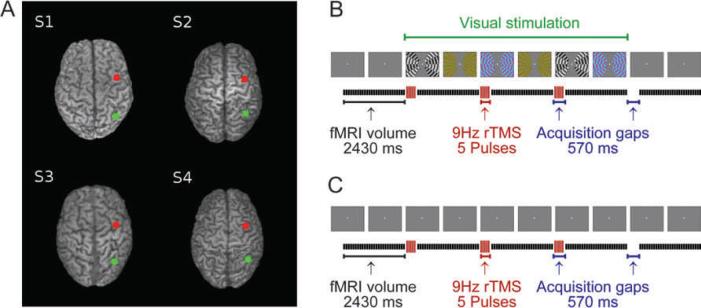
Figure 1. TMS sites and experimental protocol.
Panel (A) shows the parietal (green dot, over IPS) and frontal (red dot, over FEF as in Ruff and others 2006) TMS sites projected on images of the individual structural scans of our participants (S1=Subject 1, etc). The corresponding scalp positions were determined in each individual with Brainsight frameless stereotaxy (see Methods). Panels (B) and (C) show a schematic timecourse of a single block of interleaved TMS-fMRI: (B) with visual stimuli on the screen during TMS or (C) without visual stimuli other than the constant central fixation point (illustrated here by successive blank grey screens). For each block, three TMS trains were delivered in the 570 ms gaps between the acquisition of subsequent image volumes, at one of the four intensities used (see Materials and Methods). Seven rest scans were included between successive blocks. Visual stimuli (when present, as in the illustration-panels for B) remained visible during all three TMS trains and during the acquisition of the three image volumes following the TMS trains.
MATERIALS AND METHODS
Participants
The same four male, right-handed participants (aged 26 to 35 years) took part in the present experiment as in our previous study (Ruff and others 2006). All had good health, normal vision, and no history of neurological or psychiatric illness. Written informed consent was obtained in accord with local ethics.
TMS Stimulation Location
The scalp coordinates for placing the TMS probe over IPS (green dots in Figure 1A) were determined with the Brainsight frameless stereotaxy system (Rogue Research, Montreal, Canada), using individual T1-weighted anatomical MR images of each participant. As for our previous FEF study, we chose here to apply TMS to the IPS in the right hemisphere, for two reasons. In humans there may be some right predominance in networks for top-down modulation of visual processing (e.g., Driver and Mattingley 1998; Karnath, Milner, and Vallar 2002; Mesulam 1999). More importantly, using right sites kept the TMS-stimulated hemisphere constant when comparing the new IPS-TMS data to the existing FEF-TMS data. We used a normalised MNI coordinate (xyz = 36, −52, 48) in the anterior IPS as the TMS site, based on the mean coordinates of published activation-peaks in right IPS during covert shifts of attention or eye-movement planning and execution (Brown and others 2004; Connolly and others 2000; Connolly and others 2002; Corbetta and others 1998; Curtis, Rao, and D'Esposito 2004; Gagnon and others 2002; Perry and Zeki 2000; Petit and Haxby 1999; Sereno, Pitzalis, and Martinez 2001; see also Grosbras, Laird, and Paus 2005; Koyama and others 2004)
Setup and Data Acquisition
This experiment used a comparable setup as for our previous FEF-TMS experiment (Ruff and others 2006). T1-weighted anatomical images were acquired on a 3T head scanner (Magnetom Allegra, Siemens Medical, Erlangen, Germany), using a 3D MDEFT sequence with previously described parameters (1 mm3 isotropic resolution; Deichmann, Schwarzbauer, and Turner 2004). The same scanner was used to acquire data for retinotopic mapping of visual areas (see below), employing a multi-slice gradient echo EPI (echo-planar imaging) sequence (30 oblique axial slices, TR=1950 ms, trapezoidally switched readout gradients, 64 × 64 matrix, in-plane resolution: 3 × 3 mm2, 2 mm slice thickness, 1 mm spatial gap between adjacent slices, TE=30ms, 3551 Hz/pixel receiver bandwidth, echo spacing 330μs). Functional data for the experimental TMS sessions were acquired on a 1.5T whole-body scanner (Magnetom Sonata, Siemens Medical, Erlangen, Germany). We used the standard Siemens CP head coil for the saccade localisers, but a custom-built visual surface-coil (Nova Medical Inc., Boston, Massachusetts, USA) with maximum sensitivity over occipital cortices, extending into temporal cortex for the TMS experiment. This occipital surface-coil maximised power for early visual cortex and was thus ideal for testing our hypotheses that parietal TMS might influence visual cortex functionally. It was also the same MR surface-coil as used in our prior FEF-TMS study, and facilitated combination with concurrent TMS because it has remote electronics (see Ruff and others, 2006). The standard RF body coil was used for transmission.
An identical multi-slice gradient echo EPI sequence was used for all experimental datasets (27 oblique axial slices, 64 × 64 matrix, in-plane resolution: 3 × 3 mm2, 2.5 mm slice thickness, 1.25 mm spatial gap between adjacent slices, TE=50ms, 2298 Hz/pixel receiver bandwidth, echo spacing 500μs). The acquisition time per slice was 90 ms. For the TMS session, a 570 ms gap (see Figure 1B-C) was included between the acquisitions of subsequent volumes (resulting in a TR of 3 seconds) to allow for enough time to apply TMS pulses within the scanner during this gap without corrupting image acquisition (see below). In addition, for the TMS sessions, 50% oversampling was implemented in the phase encoding direction, keeping the spatial resolution at 3 mm, but increasing the FOV in this direction. Thus, any residual Nyquist ghost in the direct vicinity of the TMS probe was shifted outside the brain image.
TMS was employed inside the MR scanner using a Magstim Super Rapid stimulator and a custom-built, figure-of-eight, MRI-compatible non-ferrous coil (53mm inner diameter, 10 turns each winding, 20μH inductance, 5kVA predicted maximal current at 100%; from the MAGSTIM Company, Dyfed, UK). The stimulator box was remotely controlled by a MATLAB script running on a standard PC, which was also used to deliver the visual stimuli (see below). The TMS coil was positioned over the scalp coordinate of the participant's IPS site (see above and Figure 1A) in a tangential orientation, with the initial flow of the induced current in anterior-posterior direction (biphasic pulses were applied). The coil was fixed with a non-ferromagnetic custom holder, and the participant's head was held in place by a standard vacuum-suction cushion (Siemens Medical, Erlangen, Germany). To eliminate interference of RF noise generated by the TMS device with image acquisition, the stimulator box was housed in an RF-shielded metal cabinet; moreover, the custom stimulator cable connecting the box to the TMS coil was channelled through a custom filter box (The MAGSTIM Company, Dyfed, UK) and further ferrite sleeves (Wuerth Elektronik, Waldenburg, Germany). As an additional precaution any slices (less than 1%) containing TMS-capacitor-induced artefacts were replaced by the mean of the spatially equivalent slices from the previous and the subsequent image volume (as also in Ruff and others 2006). Artefacts were easily identified as changes of the slice signal by more than 3 SD (of the mean slice difference in the time series) between two consecutive volumes.
In each TMS-stimulation block, three equal-intensity trains of five TMS-pulses (9 Hz, with intensity either at 85%, 70%, 55%, or 40% of total output) were applied in the 570 ms temporal gap between acquisitions of three subsequent image volumes, thus avoiding image artefacts due to TMS pulses. This TMS protocol did not induce any muscle twitches, as confirmed by piloting and by reports of our participants, and as expected given the distance of the stimulation site from motor cortex. In each run (606 volumes, 30 minutes 18 seconds), 48 TMS-stimulation blocks were delivered, each interleaved with seven image volumes without any stimulation, thus complying with published safety limits for repetitive TMS (Wassermann 1998). An equal number of stimulation blocks (six) were delivered at each of the four TMS intensity levels, crossed with presence or absence of peripheral visual stimulation. The run also contained twelve control blocks without any TMS, during which visual stimuli could be present or absent also.
The visual stimuli - when present - were randomly moving (whole pattern movement, maximum translation in both horizontal and vertical direction 0.3 degrees per 16 ms frame) patterns that spared the fovea and the vertical meridian and randomly changed their form and colour every 500 ms (16 different combinations were possible). These stimuli were projected onto a screen (30 × 22 degrees visual angle, grey background, 0.5 × 0.5 degree central fixation cross always present) mounted at the rear end of the bore, which participants viewed via a mirror system attached to the MR surface coil.
The order of conditions was randomly determined by the program used to deliver all experimental stimulation, which was implemented in the MATLAB (The Mathworks, Natick, MA) stimulus-presentation toolbox COGENT (www.fil.ion.ac.uk/Cogent2000). Eye position, pupil diameter, and any blinks were monitored at 60Hz during scanning with an ASL 504 Remote Optics Eye tracker (Applied Science Laboratories, Bedford USA) via the same mirror used for visual stimulus viewing. Raw eye position data were filtered for blinks (identified as continuous losses of pupil signal for more than 5 frames / 80 ms), and transformed to degree visual angle. Pupil diameter was also recorded by the eye tracker.
Image Processing and Analyses
Data from the IPS experiment underwent the same analyses as the FEF TMS data in (Ruff and others 2006). All image pre-processing and general linear model (GLM) analysis steps were performed with SPM2 (www.fil.ion.ucl.ac.uk). Functional images were reconstructed offline, and the first six images of each run discarded to account for T1 equilibration effects. Images were realigned to the first of the series, corrected for movement-induced image distortions (Andersson and others 2001), normalised to the MNI (Montreal Neurological Institute) anatomical standard space, and spatially smoothed with a three-dimensional 6mm FWHM Gaussian kernel, in accord with the SPM approach (Frackowiak and others, 2003). All reported peak voxel coordinates correspond to the MNI space employed in SPM2.
For initial group stereotactic analyses, the voxel-wise effects of experimental conditions were estimated by multiple regression of the voxel time-series onto a composite model containing ten covariates of interest per session (four TMS stimulation intensities plus no TMS, each with and without visual stimulation). All conditions were modelled as continuous series of delta functions sustained over three image volumes (9 seconds), convolved with the canonical hemodynamic response function employed in SPM2. In addition to the experimental conditions (effects of interest), the model also contained one regressor representing eye blinks (modelled as delta functions convolved with the canonical HRF) and another regressor for mean pupil diameter per scan, taking into account hemodynamic delay. The regression approach in SPM entails that any variance in brain activity that was shared by two regressors (e.g., correlated with both TMS intensity and pupil width) was not considered a unique effect of one regressor, and thus could not be included in our fMRI results (Friston and others 1995). A high-pass filter (128 seconds cut-off) and an AR(1) process accounted for low-frequency drifts and short-term temporal autocorrelation of scans, respectively (Friston and others 2002). Linear compounds (contrasts) were used after model estimation to assess and compare regression parameters for the different conditions. Correlations of BOLD with TMS intensity were modelled as the corresponding weighted linear combination of the four covariates representing different TMS intensities (linear parametric modulation contrast in SPM2). Any effects of mere TMS presence on BOLD signal were estimated as the weighted contrast of trials with TMS present versus the trials with TMS absent. The statistical threshold for all analyses was set to T > 3 and a cluster threshold of p < 0.05, corrected for multiple comparisons across the whole image volume.
In addition to standard SPM group analyses in stereotactic space, we also conducted analyses of TMS-induced activity changes in individually defined visual areas. The FEF-TMS data reported in Ruff and others (2006) had examined retinotopic visual areas V1-V4 in detail; these same retinotopically mapped regions were also inspected for the present analyses. However, we now also provide data for visual area V5MT+, as identified with a separate localiser (see below), examining this region for any TMS effects in both the new IPS-TMS data and the previous FEF-TMS dataset. For all these analyses, mean BOLD signal estimates during the different conditions were extracted from the individually-defined regions in the same fashion for both experiments, and directly compared by means of ANOVAs and subsequent t-tests for planned comparisons.
Retinotopic areas V1-V4 were determined for each subject individually by standard retinotopic meridian mapping procedures, with data acquired in a 5 minute fMRI session of subjects viewing flickering checkerboards, presented either along the horizontal or vertical meridian in alternating manner. To identify cortical regions driven by these stimuli, the unsmoothed data were modelled voxel-wise, using a general linear model that included two meridian conditions. The borders of visual areas V1-V4 (Sereno and others 1995) were then plotted onto cortical flatmaps derived by segmentation and cortical flattening in MrGray (Teo, Sapiro, and Wandell 1997; Wandell, Chial, and Backus 2000). The same flatmaps were then used to display flattened representations of the SPM(T)s quantifying the correlation of TMS intensity and BOLD signal from the main experiments. For analysis of TMS effects upon representations of different visual eccentricity - following up on Ruff and others 2006, who found systematically different effects of FEF TMS for representations of the central versus peripheral visual field - each area was divided into four different eccentricity ‘sectors’. For this procedure, the meeting point of the extended exterior borders of V4 and V3d in the foveal confluence was treated as origin for all visual areas and borders, and each area was divided into four sectors of equivalent length along its centre-periphery axis (Schwartz and others 2005). Each voxel within these boundaries was then assigned to one area and eccentricity sector. Note that different parts of the foveal confluence were thus assigned to different visual regions, but in all our experiments the TMS-induced effects in these different central sectors were equivalent, so this did not affect our results. The correlation of BOLD-signal with TMS-intensity (quantified as T-values in relation to voxelwise noise) was averaged across the voxels contained in each sector. This statistic-based approach ensured that comparison of TMS-induced effects in different eccentricity sectors, and across experiments, was not confounded by unspecific effects or noise. Moreover, our conservative strategy of averaging the TMS effects across all voxels in particular eccentricity sectors (rather than just picking the voxels displaying the maximum effects) allowed us to compare effects between regions and experiments in an unbiased manner.
Visual area V5/MT+ was determined for each participant by means of a separate 5 minute fMRI session with alternating presentations of moving or static starfields, which spared the fovea by two degrees to each side. A voxel-wise general linear model (two conditions) of the unsmoothed data was used to determine the cortical region maximally driven by the moving relative to the static starfield stimuli, in lateral occipital cortex corresponding to the putative anatomical location of V5/MT+ (see e.g., Rees, Friston, and Koch 2000; Watson and others 1993). We assessed TMS-intensity-dependent effects in this region by means of Region-of-Interest (ROI) analyses. Mean signals per condition (SPM parameter estimates, scaled for each voxel as percent of the session mean) were extracted from spherical ROIs with 6 mm radius, centred at the individual peak from the motion localiser. Analogously to our previous study (Ruff and others 2006), for the ROI analyses we compared the average of the two highest TMS intensities (85% and 70% total output) versus the two lowest (55% and 40% total output), separately for trials with and without visual stimuli present on the screen.
RESULTS
We used two complementary analysis approaches to the present IPS-TMS data, exactly as for the previous FEF-TMS dataset (Ruff and others 2006). Stereotactic group analyses of activity across the image volume (acquired by the visual surface coil centred over occipital cortex) identified any regions in normalised space that reliably displayed activity changes as a function of IPS-TMS intensity or of its mere presence. To further characterise the pattern of IPS-TMS effects on specific regions of visual cortex, we also used standard retinotopic mapping procedures in conjunction with cortical flattening for V1-V4, as well as a functional localiser for V5/MT+, in each individual participant (see above). Importantly, these analyses allowed us to directly compare any effects upon visual cortex elicited by stimulation of the IPS site with those we had previously obtained for FEF TMS, since we applied the same experimental protocol in the same participants, but now to a different cortical site.
Group stereotactic analyses
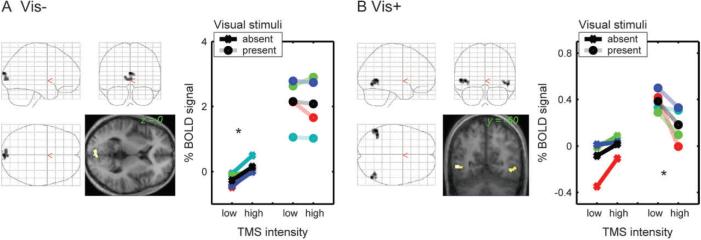
These analyses revealed occipital activity changes due to increased TMS intensity over IPS, which differed qualitatively from those we had previously observed for TMS over FEF. During IPS TMS here, we found two sets of regions that displayed significant interactions of TMS intensity with the presence/absence of visual stimuli on the screen (that is, regions where the impact of TMS depended on the current visual context). A region in the bilateral cuneus (encompassing the calcarine sulci, peak at xyz = 0, −92, 18) showed significant activity increases with IPS TMS intensity, but only in the absence of visual stimuli (Figure 2A). In contrast, for bilateral regions in lateral occipital cortex beyond retinotopic visual areas (corresponding to V5/MT+, as confirmed further via the motion-localiser below), stronger IPS TMS led to significant decreases in activity, but only during the presence of the moving visual stimuli (Figure 2B). The location of these latter effects overlapped with the activations from an independent motion localiser scan (see below for analyses of individually-defined regions), and their peak coordinates (xyz = 50, −66, −3; and xyz = −51, −56, 3) were in close agreement with the location of visual area V5/MT+ as reported in other studies (e.g., Rees, Friston, and Koch 2000; Watson and others 1993).
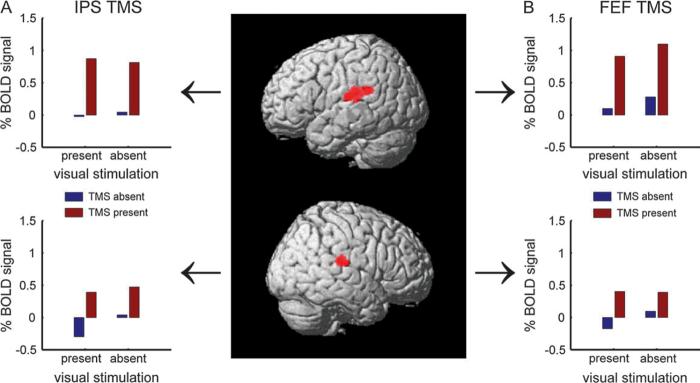
Figure 2. Group stereotactic analyses: Effects of IPS TMS intensity upon BOLD signal in occipital cortex depend on current visual context.
The images in both panels show the SPM(T)s quantifying (A) positive correlations of BOLD with TMS intensity during the absence of visual stimuli (Vis−) or (B) negative correlations of BOLD with TMS intensity during the presence of visual stimuli (Vis+). The SPM(T)s are plotted as 2-D projections onto a transparent schematic of the MNI template, and as renderings onto a transverse slice of the mean structural scan. All thresholds are set to T > 3 and p < 0.05 (cluster-level corrected for multiple comparisons across the image volume). The line plots displayed in each panel show the mean signal intensity during the different experimental conditions, extracted from a spherical region-of-interest (6mm radius) centred in the peak voxel of the corresponding SPM(T). The data for each subject is shown in a different colour, whereas the inter-subject mean is shown in black. For ease of visualisation (and for comparison with the same procedure in Ruff and others 2006), the signal is plotted averaged across the two lowest versus the two highest TMS intensities. Panel (A) shows a region in the calcarine sulcus that displayed activity increases with greater intensity of TMS over IPS, but only during the absence of visual stimulation, not when visual stimuli were present (significant positive correlation of BOLD with TMS intensity during blank-screen trials only, and significant interaction with absence/presence of visual stimuli). Note that the TMS effect is only apparent with a blank screen (asterisked leftmost pair of points in the corresponding plot). Panel (B) displays a bilateral region in occipito-temporal cortex, corresponding to V5/MT+, that showed negative correlations of BOLD signal with IPS-TMS intensity (i.e, reduced activity with higher intensity of TMS), but only when moving visual stimuli were concurrently presented (see asterisked rightmost pair of points in the plot). Note that applying the same TMS protocol over a different site (FEF) elicited occipital activity modulations that, by contrast, did not depend on visual context and had no effect on V5/MT+ (see main text and Figures 3 and 4).
In contrast, the occipital BOLD changes we observed (Ruff and others 2006) during application of the comparable TMS protocol to FEF instead did not depend on visual context, and were localised either more anteriorly in the cuneus or at the occipital poles. Moreover, no effect of FEF-TMS intensity had been found in or around V5/MT+ for the group analyses of the FEF-TMS data, unlike the effect found here during visual stimulation for IPS TMS. These qualitative differences between IPS- and FEF-TMS effects upon activity in visual cortex were confirmed and further specified in analyses of BOLD changes for individually mapped visual areas (V1-V4 and V5/MT+), as described below.
Analyses of individually mapped visual areas
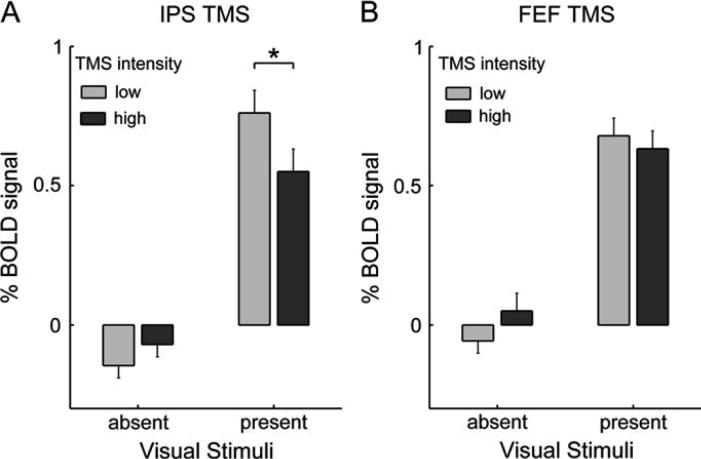
The group analyses in stereotactic space above indicate that increased IPS-TMS intensity led to reduced BOLD signal bilaterally in lateral occipital cortex near putative V5/MT+, but only during visual stimulation. No such effects in that region were found during FEF TMS, and hence none were reported for V5/MT+ in (Ruff and others, 2006). We formally confirmed this difference between the effects of the two TMS stimulation sites on V5/MT+ by Region-of-Interest (ROI) analyses of the mean BOLD signal extracted from V5/MT+ (see Figure 3), as determined for each participant by the individual motion localiser (Materials and Methods). In accord with the normalised stereotactic group results, only the IPS-TMS effects in V5/MT+ depended on visual context (2 × 2 ANOVA on IPS data from both hemispheres; significant interaction of TMS intensity and presence/absence of visual stimuli; F(1,28) = 6.16, p < 0.05), whereas the effects of FEF-TMS did not (F(1,28) = 2.24, n.s.). In direct planned comparison between TMS sites/experiments, the BOLD decrease elicited by increased intensity of IPS TMS, during the presence of visual stimuli, was significantly larger than during FEF TMS (t(7) = 1.99, p < 0.05).
Figure 3. Increased intensity of IPS TMS (but not FEF TMS) elicits BOLD decreases in V5/MT+ specifically during the presence of moving visual stimuli.
The bar graphs show the mean BOLD signal intensity in V5/MT+ (determined for each subject with a motion localiser, see Materials and Methods) during (A) IPS TMS or (B) FEF TMS. The BOLD signal estimates were derived and plotted analogously to the estimates for Figure 2, but now extracted from individually-localised V5/MT+ ROIs, and collapsed across hemispheres (since equivalent results were found for each). Error bars represent the s.e. of the mean difference between high- and low-TMS-intensity trials, under one or the other condition of visual stimulation (i.e, for each pair of adjacent bars). Stars indicate p < 0.05 in paired t-tests (see main text for ANOVA results). The bar graphs show that (A) increasing the intensity of IPS TMS led to activity decreases in V5/MT+ only when the moving visual stimuli were present to activate this visual area, not in the absence of visual stimulation, whereas (B) no such effect was found for increased intensity of TMS over FEF.
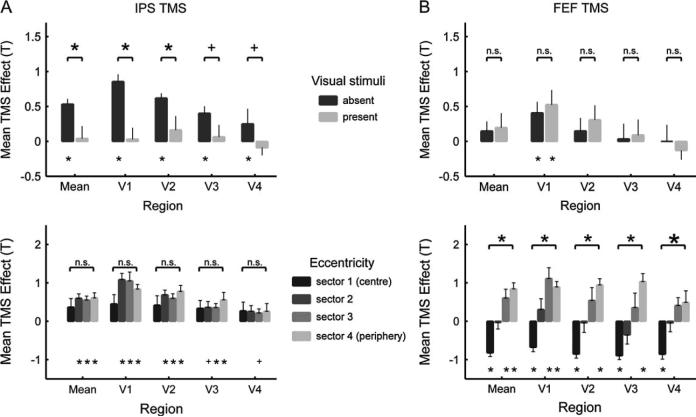
We also further characterised the BOLD-signal changes elicited by IPS TMS in retinotopic visual areas V1-V4, and compared those to the effects of FEF TMS. Areas V1-V4 were defined by means of standard retinotopic-mapping procedures in conjunction with cortical flattening (see Materials and Methods). Four eccentricity sectors (Schwartz and others 2005) were defined in each area, corresponding to more central or more peripheral visual field representations (see also Ruff and others 2006). Figure 4A shows the IPS-TMS intensity effects upon retinotopic visual areas as a function of visual stimulus presence/absence (top) and eccentricity sector (bottom). The IPS-TMS effects on individually mapped retinotopic visual areas were in good accord with the results of the initial group analyses in stereotactic space. Increased IPS-TMS intensity only elicited clear activity increases in retinotopic visual areas during the absence of visual input (Figure 4A, top graph; compare dark bars to light). In contrast, effects of FEF-TMS upon these same individually-mapped regions did not differ as a function of current visual input (Figure 4B, top graph; note no reliable differences between dark and light bars; data from Ruff and others 2006, but presented here in more detail).
Figure 4. Retinotopic analyses of individual areas V1-V4: Effects of IPS (but not FEF) TMS depend on current visual context, whereas FEF (but not IPS) TMS has opposing effects on the peripheral vs central visual field.
The plots show the mean T-values (+/− s.e.m) reflecting the correlation of (A) IPS-TMS or (B) FEF-TMS intensity with BOLD, for V1-V4 averaged across dorsal and ventral. The top plot in each panel shows the effects of TMS over (A) IPS or (B) FEF separately for trials with visual stimuli absent or present. The bottom plot in each panel shows the (A) IPS- or (B) FEF-TMS effects for each of four eccentricity sectors within each retinotopic area, but now only for the absence of visual stimuli (as significant effects of IPS TMS were found in V1-V4 only for these conditions, see the top plots). See main text for how the eccentricity sectors were derived, but note that eccentricity sector number 1 (the first along the x-axis for each visual area) corresponds to the representation of the central visual field, with increasing sector numbers (further to the right along the x-axis, for each visual area) correspond to increasingly eccentric visual field representations. Statistical significance of paired t-tests (top of every plot) or simple t-tests (i.e, against zero, with significance for this indicated at bottom of every plot) is marked according to the following scheme: * p<0.05, + p<0.1, n.s. not significant; see main text for results of ANOVAs. Comparison of the top plots in both panels illustrates that only the effects of IPS TMS depended on visual context (i.e, were significantly stronger when visual stimuli were absent rather than present, compare dark and light bars in top plots). The two plots at the bottom of the figure shows that only FEF TMS had opposite effects on the central vs peripheral visual field (that is, significantly negative effects on the central sector and significantly different effects for the central versus the most peripheral sector). This contrasts with the IPS TMS effects that did not vary reliably with eccentricity sector.
As a second major difference between the impacts of IPS TMS versus FEF TMS on visual cortex, the effects observed for the two TMS sites also differed in their spatial topography across retinotopic visual areas. Activity increases elicited by IPS TMS, during the absence of visual stimuli only, were similarly present across all the different eccentricity sectors within V1-V4 (Figure 4A, bottom graph). In contrast, increased intensity of FEF TMS had opposite effects on the sectors representing the central visual field (eliciting a BOLD decrease there) vs the peripheral visual field (where a BOLD increase was observed instead; see Figure 4B, bottom graph; data from Ruff and others 2006, but presented here in more detail).
Statistical analyses formally confirmed these two qualitative differences between the effects of the two TMS sites. Pooling across areas V1-V4, only the IPS-TMS effects depended on visual context, in a similar manner for all eccentricity sectors: A 2 (TMS site) × 2 (visual context) × 4 (eccentricity sector) ANOVA on the TMS effect revealed a significant interaction of TMS site (i.e., FEF or IPS experiment) with presence/absence of visual stimuli (F(1,21) = 8.98, p < 0.05). Pairwise comparisons confirmed for all early visual areas that IPS TMS elicited activity increases that were significantly stronger during the absence than presence of visual stimuli (see Figure 4A, top graph); this effect was most marked in visual area V1 and V2. In contrast, the effects of FEF stimulation did not depend on visual context, in any retinotopic visual area (Figure 4B, top graph). The 2×2×4 ANOVA also confirmed that FEF versus IPS TMS differentially affected central vs peripheral sectors of the visual field in retinotopic visual cortex (interaction of TMS site and eccentricity sector, F(1, 21) = 6.47, p < 0.05). In direct planned comparisons, the BOLD increases observed with increased IPS-TMS intensity during the absence of visual stimuli were comparable for peripheral and central sectors (Figure 4A, bottom graph). FEF stimulation, in contrast, induced significant BOLD decreases in the central sector, but increases in the more peripheral sectors. (Figure 4B, bottom graph). Significant pairwise comparisons (or non-significant contrasts, n.s.) are all marked in Figure 4.
Control for and analysis of non-specific TMS effects
The effects on BOLD activity in visual regions found here during IPS TMS were specifically related to the intensity of TMS rather than to its mere presence. Moreover, they were clearly distinct from the effects we had observed in the FEF-TMS experiment using the same protocol (i.e., the effects depended on visual context only for IPS TMS, while differentiating the central and peripheral visual field only for FEF TMS). This intensity-dependence and site-specificity make it highly unlikely that any non-specific effects of TMS administration per se explain these results, but we were nevertheless careful to analyse our data for such possible non-specific influences of TMS (see also Ruff and others 2006).
We assessed and directly compared the data from both experiments for any effects of the mere presence or absence of TMS (as opposed to effects of TMS intensity). This revealed activations in bilateral regions in auditory cortex that were similarly found for TMS to either site (see Figure 5), presumably arising due to the ‘click’ sound associated with TMS presence versus absence. This effect could be detected with our occipital MR surface coil, as that extended over temporal cortex and thereby auditory cortex also. Note that visual regions, unlike auditory cortex, were specifically affected by the intensity of TMS rather than by its mere presence, thus showing a very different pattern to auditory cortex in both experiments.
Figure 5. Activity increases in auditory cortex due to TMS presence versus absence are comparable for IPS and FEF TMS.
This figure shows regions that were more active during trials with TMS present than absent, for both IPS and FEF TMS. The central images show the SPM(T) of the conjunction contrast (inclusive masking) of TMS present (all intensities pooled) minus TMS absent, for both IPS and FEF TMS, rendered onto a 3-D version of the normalised template brain employed in SPM2. The same statistical threshold as in Figure 2 was used, with different shades of gray indicating different distances from the cortical surface. Note that TMS to either region elicited similar activation in auditory cortex due to the presence of the sound associated with TMS application. The side panels show the mean signal extracted from the peak voxel in the respective hemisphere (as indicated by the arrows), plotted separately for (A) IPS and (B) FEF TMS. Direct statistical comparisons (paired t-tests) revealed that the effects of mere TMS presence on auditory cortex (TMS present minus absent) were equivalent for both stimulation sites, and did not show lateralisation (consistent with the click sound reaching both ears).
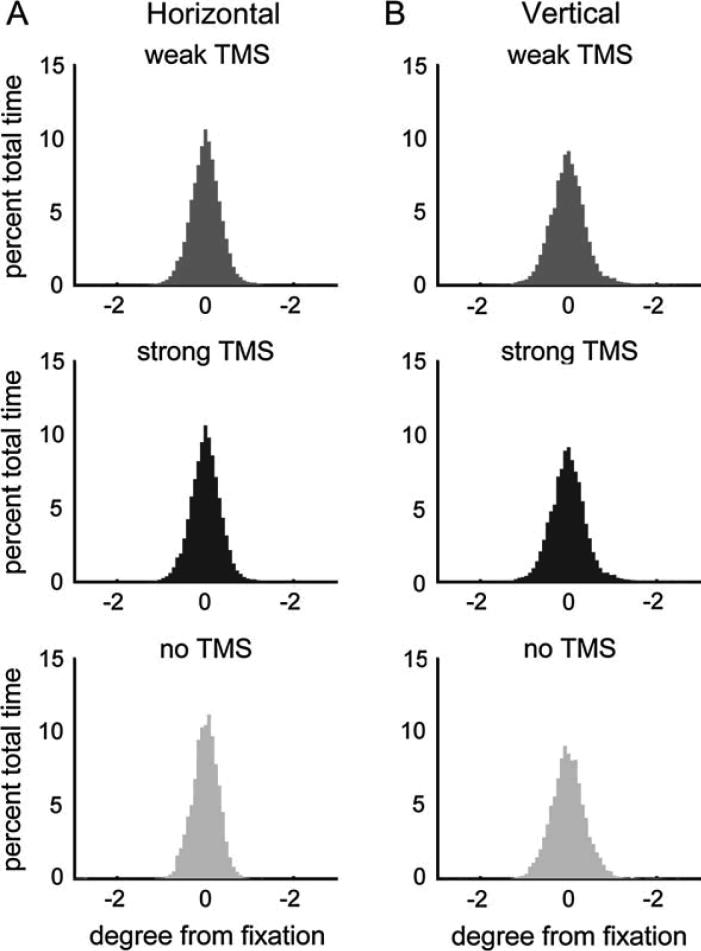
On-line eye tracking throughout scanning (see Materials and Methods) provided detailed measurements of eye-position, blinks, and pupil diameter, and these factors had also been included in our multiple regression procedure as nuisance variables to partial out their effects (see Material and Methods). Similar to what we had observed for FEF TMS (see Ruff, 2006), mean horizontal and vertical eye position, and their variability, did not differ between trials with strong, weak, or no IPS TMS (see Figure 6; 3-way ANOVAs, all F(2,237) < 2.53, all p > 0.05), confirming that the TMS protocol employed here did not induce eye-movements. Moreover, trials with strong, weak, or no IPS TMS also did not differ with respect to mean pupil diameter (3-way ANOVA, F(2,237) = 1.38, p = 0.25), and blinks occurred equally often during the three different trial types (chi-square test, χ2(2) = 1.74, p = 0.84).
Figure 6. IPS-TMS effects upon functional activity in visual cortex cannot be explained by changes in eye position.
Histograms of (A) horizontal and (B) vertical eye position during trials with weak TMS (lowest two TMS intensities pooled, medium gray), strong TMS (highest two intensities, dark gray), and no TMS (light gray) to IPS. The histograms plot for each condition the eye position as percent time at different degrees of visual angle of deviation from fixation. No statistical differences were found in the mean or variance of these eye position distributions across the displayed conditions (see main text), confirming that differential eye movements cannot account for the observed TMS effects. Ruff and others (2006) had likewise observed that FEF TMS did not affect eye position.
DISCUSSION
We applied TMS over human parietal cortex (right IPS) during fMRI with an occipital surface coil, to characterise how stimulating the IPS can causally influence remote but interconnected structures in visual cortex. This revealed that parietal TMS could produce reliable and distinctive effects upon BOLD signals in remote human visual cortex, including area V5/MT+ and retinotopic visual areas V1-V4. These effects differed qualitatively and statistically from those found for a frontal TMS site over right FEF (cf. Ruff and others 2006). The present results provide a clear ‘proof-of-principle’ that circuits involving parietal regions such as the IPS are capable of causally modulating activity in early human visual cortex, in a manner that is qualitatively different to frontal (FEF) influences induced by comparable TMS stimulation there instead.
The clear differences between the effects of the two stimulation sites, discussed in further detail below, rule out any account of our results in terms of general, non-specific effects potentially associated with TMS application, such as the characteristic ‘click’ sound. This sound did have effects in the two experiments, but affected auditory rather than visual cortex in an equivalent fashion for the two TMS sites, as a function of mere TMS presence rather than intensity. By contrast, the TMS effects upon visual cortex depended specifically upon TMS intensity rather than mere presence, and differed strikingly for the IPS- versus FEF-TMS site in terms of spatial topography and dependence on visual context. Note that these qualitative differences between the effects found for the two stimulation sites cannot plausibly be accounted for by potential intrinsic differences associated with TMS application over frontal versus parietal cortex (such as different skull thickness over these regions, see e.g., Stokes and others 2005). Any such general factors could not trivially explain, for instance, why only IPS-TMS (but not FEF-TMS) effects depended on current visual input, or why there were differences in the eccentricity-sectors affected in retinotopic visual cortex.
Frontal and parietal brain areas are often jointly activated in the human brain, for example during covert spatial attention (Corbetta and Shulman 2002; Kastner and Ungerleider 2000; Macaluso and Driver 2005) and eye movements (Grosbras, Laird, and Paus 2005; Sylvester, Haynes, and Rees 2005), leading to questions about whether different components within this ‘fronto-parietal control network’ might subserve different functions. The present results provide rather direct, causal evidence that human IPS and FEF can have clearly distinct influences on activity in retinotopic visual cortex. Specifically, the effects of IPS stimulation strongly depended on the current visual context: TMS over right IPS led to increased activity in retinotopic visual areas (V1-V4) only in the absence of changing retinal input to these visual regions, while affecting V5/MT+ only in the presence of moving visual stimuli. By contrast, TMS over right FEF led to changes in activity in early visual cortex that applied in a strictly ‘top-down’ manner, irrespective of current bottom-up visual input and thus regardless of the overall level of activity in visual cortex.
This difference in outcome for IPS- vs FEF-TMS effects upon V1-V4 may indicate that the presence of strong visual inputs can dominate functional connections between early visual cortex and IPS in a ‘bottom-up’ manner, rendering those neural pathways less responsive to any ‘feedback’ influences from IPS (such as those induced by TMS here) when visual inputs drive the system. From a functional perspective, this may fit the emerging view that neural signals in parietal regions, and their feedback influences upon visual cortex, relate to on-line coding and integration of sensory information about the current environment, as often assumed in the literature on visual (e.g., Culham, Cavanagh, and Kanwisher 2001; Kastner and others 1999; Wardak and others 2006) and intermodal (e.g., Macaluso and Driver 2005) attention. By contrast, neural signals arising from frontal cortex (e.g., FEF) may operate in a more purely ‘top-down’ fashion that could enable such signals to be independent of any activity elicited by current sensory input. Such proposals about differential (in)dependence of frontal vs parietal influences on current visual stimulation are emerging in the literature on attention (e.g., Culham, Cavanagh, and Kanwisher 2001; Kastner and others 1999; Miller 2000; Shulman and others 2003; Wardak and others 2006), eye movement control (e.g., Connolly and others, 2002), and working memory (e.g., Curtis, 2006; Postle 2003). It has even been proposed that some signals in human IPS might represent an intermediate stage of visual processing, more similar to extra-striate visual areas than to frontal areas such as the FEF (Kastner and others 1999). Moreover, a recent extensive study using invasive recording in macaque parietal cortex (LIP) and frontal cortex (FEF and LPFC) concurrently, during a visual attention task, argued that frontal contributions to the task might be more concerned with top-down, endogenous aspects, whereas parietal contributions might reflect bottom-up, exogenous aspects of attentional control (Buschman and Miller, 2007). Our present results clearly indicate that frontal (FEF) and parietal (IPS) regions can have distinct functional signatures in the human brain, in terms of how TMS stimulation there may modulate functional activity in visual cortex, and how these remote influences may depend on (for IPS) or be independent of (for FEF) current task-irrelevant visual input.
A further difference between the fMRI effects of IPS and FEF TMS found here concerned functional activity in V5/MT+. This was unaffected by FEF TMS. By contrast, effects of IPS TMS were found on V5/MT+, but now only in the presence of moving visual stimuli. This dependence of the IPS-TMS effect upon current visual input provides a particularly clear example of context-dependent changes in interplay between brain areas, or “effective connectivity”, as previously proposed in some theoretical work (see e.g., Friston 2002; McIntosh 2000). Note that the concurrent use of TMS and fMRI here allowed us to test for such context-dependent influences of a particular brain region upon others (e.g., IPS on V5/MT+) with conventional fMRI analyses that do not have to rely on more complex mathematical approaches to the effective-connectivity issue. Our finding that the influence of IPS upon visual cortex can vary with contextual state - here as a function of current visual input - generally implies that such remote TMS effects can reflect functional coupling between areas rather than just fixed anatomical connections, as the functional role of connections may change with state (Friston 2002; Massimini and others 2005).
This functional-coupling aspect may explain why IPS TMS affected V5/MT+ activity here while FEF TMS did not, even though both FEF and IPS have some anatomical connections with V5/MT+ in the macaque brain (e.g., Blatt, Andersen, and Stoner 1990; Schall and others 1995; Stanton, Bruce, and Goldberg 1995). Presumably, IPS and interconnected V5/MT+ may show the strongest functional interactions when processing moving stimuli in particular (see also Friston and Buchel 2000; Huk and Shadlen 2005), perhaps related to the constant updating of spatial representations in a dynamic visual environment (e.g., Colby and Goldberg, 1999). This would be consistent with the presumed role for parietal cortex as well as V5/MT+ in aspects of motion processing (Battelli and others 2001; Bremmer and others 2001; Claeys and others 2003; Orban and others 2006; Williams and others 2003). Such a putative involvement of IPS-V5/MT+ circuits in motion processing may also fit the finding here that IPS-TMS influences on V5/MT+ took the form of activity decreases, which might indicate a disrupting TMS effect on neural activity elicited by the moving visual stimuli. It might be interesting for future studies to test with psychophysics whether right-IPS TMS can result in any changes in motion perception (for related suggestions see also e.g., Cowey and others 2006; Ellison, Lane, and Schenk 2007). Moreover, future studies might also test with extensions of the current TMS-fMRI paradigm whether FEF stimulation might have more influence on activity in V5/MT+ if the motion of the visual display becomes task-relevant for current judgements, rather than just being passively watched as here. In the context of such passive viewing, only parietal regions thought to relate to bottom-up processing of visual input may functionally interact with V5/MT+. By contrast, frontal regions thought to be involved in more top-down, endogenous aspects of attentional control (see Buschman and Miller, 2007) might become functionally coupled with V5/MT+ only when motion becomes task-relevant for judgements in a demanding attentional task.
The present parietal TMS results also differed from the frontal TMS findings in the retinotopic pattern of TMS influences upon early visual cortex. Effects of IPS TMS on visual areas V1-V4, found only during the absence of visual input, did not differentiate the central and peripheral visual field. By contrast, increased TMS intensity to FEF led to increased activity for peripheral-visual-field representations in early visual cortex, but to activity decreases instead for central-visual-field representations. This dissociation might relate to distinct neural circuitry for more peripheral versus more central locations in FEF, and for its connections with visual cortex, as suggested by some tracing studies in non-human primates (e.g., Schall and others, 1995; Stanton and others, 1995). In contrast, no distinction of central and peripheral visual field representations was found here for the effects of TMS over IPS, which may not emphasise the peripheral field as much as FEF does. While some recent fMRI studies in humans show some retinotopic (polar-angle) representations within both FEF (Hagler, Jr. and Sereno 2006) and IPS (Schluppeck and others 2006; Sereno, Pitzalis, and Martinez 2001; Silver, Ress, and Heeger 2005), it is not yet fully known to what degree these representations might differentiate the central and the peripheral visual field (Orban and others 2006). The distinct spatial topography of the activity modulations in retinotopic visual areas, found here during TMS over IPS versus FEF, suggest potential differences in anatomical layout and functional connectivity of these regions with respect to central and peripheral visual field representations.
Pioneering invasive work (Armstrong and Moore 2007; Armstrong, Fitzgerald, and Moore 2006; Moore and Armstrong 2003) has shown using microstimulation, rather than TMS as here, that induced FEF-activity can modulate responses of individual occipital visual neurons (e.g., in area V4) in awake non-human primates. It may be interesting to extend that work in future to compare frontal with parietal sites, as done here for combined TMS-fMRI in humans. While invasive microstimulation can be applied with much higher spatial resolution than the present TMS method, it may never be applicable to healthy humans. Hence the concurrent TMS-fMRI methods used here may become of particular utility for studying causal interplay between different regions of the human brain at a systems level, and could in principle be applied to many different cortical areas (and various cognitive domains; see e.g., Miller and D'Esposito 2005; Sack and others 2007). For instance, it might be interesting in future work to use concurrent TMS-fMRI to directly compare modulatory effects of frontal or parietal TMS over the right versus left hemisphere, as the two hemispheres are often assumed to contribute differently to top-down modulation of visual processing (e.g., Driver and Mattingley 1998; Hilgetag, Theoret, and Pascual-Leone 2001; Karnath, Milner, and Vallar 2002; Mesulam 1999). Here we had kept the stimulated hemisphere constant to allow a direct comparison of the IPS and FEF stimulation effects.
In light of the many striking differences between the effects of FEF versus IPS TMS stimulation upon BOLD signals that we found in remote visual cortex, one noteworthy common aspect was that all effects observed in both experiments arose bilaterally in visual cortex. This may presumably reflect inter-hemispheric callosal or subcortical influences, underlining that the effects of both IPS and FEF TMS upon visual cortex may be poly-synaptic and involve intervening brain regions (Blatt, Andersen, and Stoner 1990; Cavada and Goldman-Rakic 1989; Schall and others 1995; Stanton, Bruce, and Goldberg 1995). Here we had deliberately used an occipital surface MR coil to maximise our sensitivity for retinotopic visual cortex. This inevitably meant less sensitivity for more anterior structures (e.g., in parietal and frontal cortex). Future experiments using the new concurrent TMS-fMRI methodology with whole-brain imaging may shed further light on the full anatomical networks subserving interactions between FEF, IPS, and visual cortex in the human brain. However, it was the specific focus on occipital cortex here that enabled us to characterise and compare the distinct patterns of effects of IPS and FEF TMS upon retinotopic visual areas, while using individual mapping of each visual area.
CONCLUSIONS
Using concurrent TMS-fMRI, we show directly that neural circuits involving the IPS can modulate functional activity in human retinotopic visual cortex, in a qualitatively distinct fashion from circuits involving the FEF. Our data therefore provide a clear ‘proof-of-principle’ that human parietal and frontal regions can exert distinct influences on activity in early visual cortex, including area V1. Only the effects of IPS TMS depended on current visual context, whereas only the effects of FEF TMS significantly differentiated the peripheral vs central visual field. These qualitative distinctions in the effects of IPS vs FEF stimulation accord with nascent proposals about distinct functional contributions from parietal versus frontal sites to cognitive function, and in particular to modulation of visual cortex. Finally, our study illustrates that concurrent TMS-fMRI can now be used to directly compare remote causal influences from different human brain areas.
ACKNOWLEDGEMENTS
This work was supported by the Wellcome Trust and the Medical Research Council (UK). We thank Peter Aston and Eric Featherstone for their help.
Abbreviations
- TMS
Transcranial Magnetic Stimulation
- fMRI
functional Magnetic Resonance Imaging
- BOLD
blood-oxygenation-level-dependent
- IPS
intra-parietal sulcus
- FEF
frontal eye-field
REFERENCES
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modelling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Armstrong KM, Moore T. Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0701104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Henaff MA, Michel F, Barton JJ. Unilateral right parietal damage leads to bilateral deficit for high-level motion. Neuron. 2001;32:985–995. doi: 10.1016/s0896-6273(01)00536-0. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Ruff CC, Blakemore C, Driver J, Thilo KV. Spatial attention changes excitability of human visual cortex to direct stimulation. Curr Biol. 2007;17:134–139. doi: 10.1016/j.cub.2006.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and corticocortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Brown MR, DeSouza JF, Goltz HC, Ford K, Menon RS, Goodale MA, Everling S. Comparison of memory- and visually guided saccades using event-related fMRI. J Neurophysiol. 2004;91:873–889. doi: 10.1152/jn.00382.2003. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Claeys KG, Lindsey DT, De Schutter E, Orban GA. A higher order motion region in human inferior parietal lobule: evidence from fMRI. Neuron. 2003;40:631–642. doi: 10.1016/s0896-6273(03)00590-7. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, DeSouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol. 2000;84:1645–1655. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cowey A, Campana G, Walsh V, Vaina LM. The role of human extra-striate visual areas V5/MT and V2/V3 in the perception of the direction of global motion: a transcranial magnetic stimulation study. Exp Brain Res. 2006;171:558–562. doi: 10.1007/s00221-006-0479-6. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanagh P, Kanwisher NG. Attention response functions: characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron. 2001;32:737–745. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Driver J, Frackowiak RS. Neurobiological measures of human selective attention. Neuropsychologia. 2001;39:1257–1262. doi: 10.1016/s0028-3932(01)00115-4. [DOI] [PubMed] [Google Scholar]
- Driver J, Mattingley JB. Parietal neglect and visual awareness. Nat Neurosci. 1998;1:17–22. doi: 10.1038/217. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Curr Opin Neurobiol. 1997;7:255–261. doi: 10.1016/s0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Ellison A, Lane AR, Schenk T. The Interaction of Brain Regions during Visual Search Processing as Revealed by Transcranial Magnetic Stimulation. Cereb Cortex. 2007 doi: 10.1093/cercor/bhl165. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, Penny WD. Human Brain Function. 2nd ed San Diego, CA: Academic Press; 2003. [Google Scholar]
- Friston K. Functional integration and inference in the brain. Prog Neurobiol. 2002;68:113–143. doi: 10.1016/s0301-0082(02)00076-x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buchel C. Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proc Natl Acad Sci USA. 2000;97:7591–7596. doi: 10.1073/pnas.97.13.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical Parametric Maps in Functional Imaging: A General Linear Approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: theory. Neuroimage. 2002;16:465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bauer RH, Jervey JP. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 1985;330:299–307. doi: 10.1016/0006-8993(85)90689-4. [DOI] [PubMed] [Google Scholar]
- Gagnon D, O'Driscoll GA, Petrides M, Pike GB. The effect of spatial and temporal information on saccades and neural activity in oculomotor structures. Brain. 2002;125:123–139. doi: 10.1093/brain/awf005. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Laird AR, Paus T. Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp. 2005;25:140–154. doi: 10.1002/hbm.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Sereno MI. Spatial maps in frontal and prefrontal cortex. Neuroimage. 2006;29:567–577. doi: 10.1016/j.neuroimage.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced 'virtual lesions' of human parietal cortex. Nat Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Huk AC, Shadlen MN. Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making. J Neurosci. 2005;25:10420–10436. doi: 10.1523/JNEUROSCI.4684-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Milner AD, Vallar G. The cognitive and neural bases of spatial neglect. Oxford: Oxford University Press; 2002. [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Driver J. Multisensory spatial interactions: a window onto functional integration in the human brain. Trends Neurosci. 2005;28:264–271. doi: 10.1016/j.tins.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Netw. 2000;13:861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M. Searching for "the top" in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Miller EK. The neural basis of the top-down control of visual attention in the prefrontal cortex. In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, Mass: MIT Press; 2000. pp. 511–534. [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Haxby, JV. Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J Neurophysiol. 1999;82:463–471. doi: 10.1152/jn.1999.82.1.463. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Zeki S. The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain. 2000;123(Pt 11):2273–2288. doi: 10.1093/brain/123.11.2273. [DOI] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J. Imaging the Brain Activity Changes Underlying Impaired Visuospatial Judgments: Simultaneous fMRI, TMS, and Behavioural Studies. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cereb Cortex. 2005;15:770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d'Avossa G, Corbetta M. Quantitative analysis of attention and detection signals during visual search. J Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- Super H, van der Togt C, Spekreijse H, Lamme VA. Correspondence of presaccadic activity in the monkey primary visual cortex with saccadic eye movements. Proc Natl Acad Sci USA. 2004;101:3230–3235. doi: 10.1073/pnas.0400433101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester R, Haynes JD, Rees G. Saccades differentially modulate human LGN and V1 responses in the presence and absence of visual stimulation. Curr Biol. 2005;15:37–41. doi: 10.1016/j.cub.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev. 2000;32:413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Teo PC, Sapiro G, Wandell BA. Creating connected representations of cortical gray matter for functional MRI visualization. IEEE Trans Med Imaging. 1997;16:852–863. doi: 10.1109/42.650881. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Chial S, Backus BT. Visualization and measurement of the cortical surface. J Cogn Neurosci. 2000;12:739–752. doi: 10.1162/089892900562561. [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Elfar JC, Eskandar EN, Toth LJ, Assad JA. Parietal activity and the perceived direction of ambiguous apparent motion. Nat Neurosci. 2003;6:616–623. doi: 10.1038/nn1055. [DOI] [PubMed] [Google Scholar]