Abstract
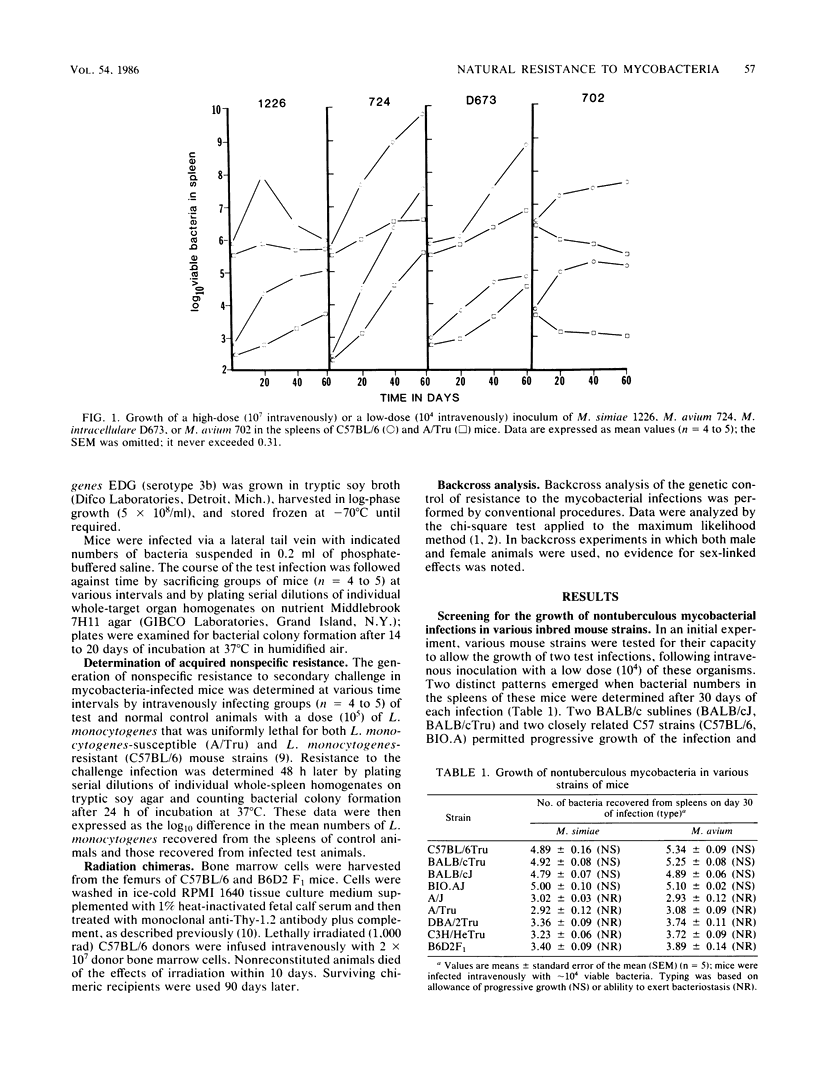
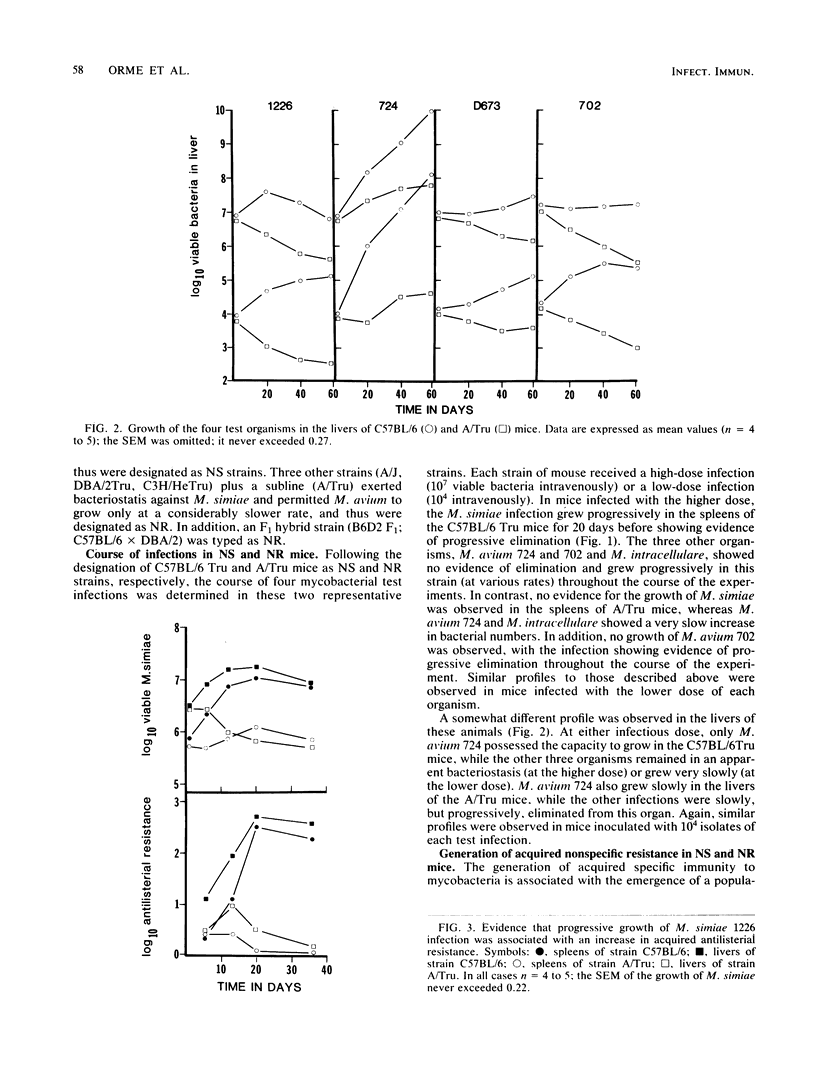
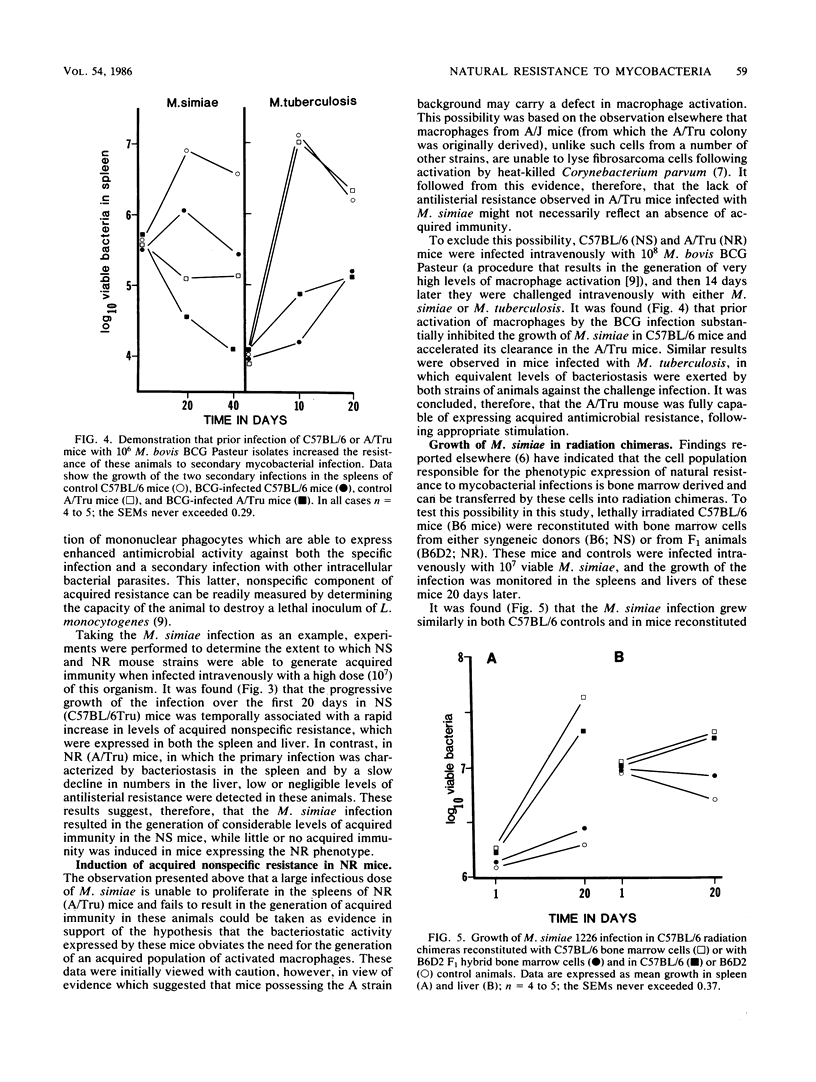
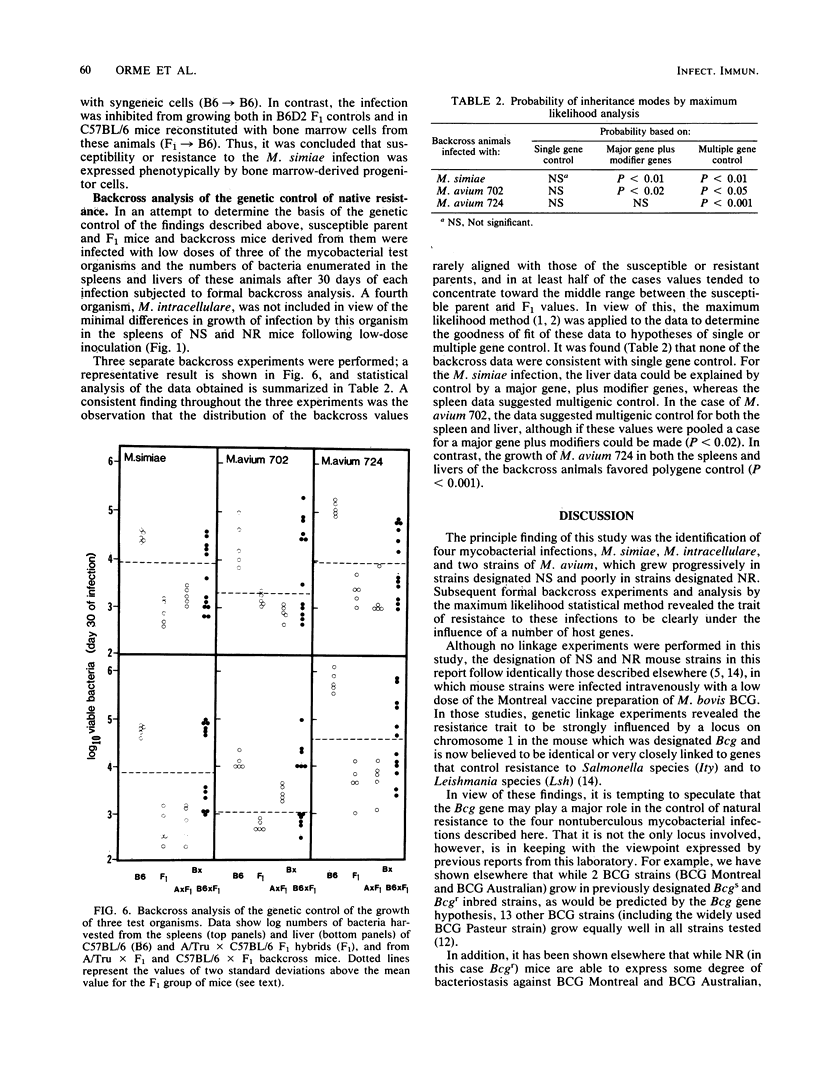
Results show that various inbred strains of mice can be segregated into two distinct groups, based on their capacity to allow a number of nontuberculous mycobacterial infections to grow in target organs following experimental intravenous infection. The first group, which allowed these infections to grow progressively, was thus designated as naturally susceptible to these infections; in contrast, those strains which were able to exert detectable bacteriostasis were designated as naturally resistant. It was then found that segregation of mouse strains based on this distinction also mirrored the capacity of these animals to generate acquired immunity to the mycobacterial infections. For example, Mycobacterium simiae grew progressively in susceptible C57BL/6 mice, subsequently triggering acquired mechanisms of immunity, whereas no evidence for acquired immunity could be found in resistant A/Tru mice infected with this organism. The possibility that acquired immunity could not be expressed in the latter strain as a result of a defect in macrophage activation was excluded. Moreover, it was found that the trait of resistance to these infections could be transferred by bone marrow cells into radiation chimeras, thus indicating that this trait was expressed by the progeny of hemopoietic precursor cells. Subsequent backcross analysis to determine the mode of inheritance of the trait of resistance to these mycobacterial infections revealed data that were consistent with the hypothesis that this resistance is controlled by more than one gene. Statistical analysis of the data by the maximum likelihood method suggested polygenic control, although in some cases the probability values suggested control by a major gene, influenced by modifier genes. These findings suggest that the previous hypothesis that the growth of mycobacterial infections in inbred strains of mice is controlled by a single gene should be reevaluated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elston R. C., Stewart J. The analysis of quantitative traits for simple genetic models from parental, F 1 and backcross data. Genetics. 1973 Apr;73(4):695–711. doi: 10.1093/genetics/73.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nakamura R. M., Takahashi H., Tokunaga T. Genetic control of resistance to Mycobacterium intracellulare infection in mice. Infect Immun. 1984 Oct;46(1):135–140. doi: 10.1128/iai.46.1.135-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Cellular mechanisms of genetically controlled host resistance to Mycobacterium bovis (BCG). J Immunol. 1983 Oct;131(4):1966–1972. [PubMed] [Google Scholar]
- James S. L., Skamene E., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. V. Variation in macrophage schistosomulacidal and tumoricidal activities among mouse strains and correlation with resistance to reinfection. J Immunol. 1983 Aug;131(2):948–953. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Demonstration of acquired resistance in Bcgr inbred mouse strains infected with a low dose of BCG montreal. Clin Exp Immunol. 1984 Apr;56(1):81–88. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Resistance of various strains of mycobacteria to killing by activated macrophages in vivo. J Immunol. 1983 Sep;131(3):1452–1454. [PubMed] [Google Scholar]
- Pelletier M., Forget A., Bourassa D., Gros P., Skamene E. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J Immunol. 1982 Nov;129(5):2179–2185. [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]


