Abstract
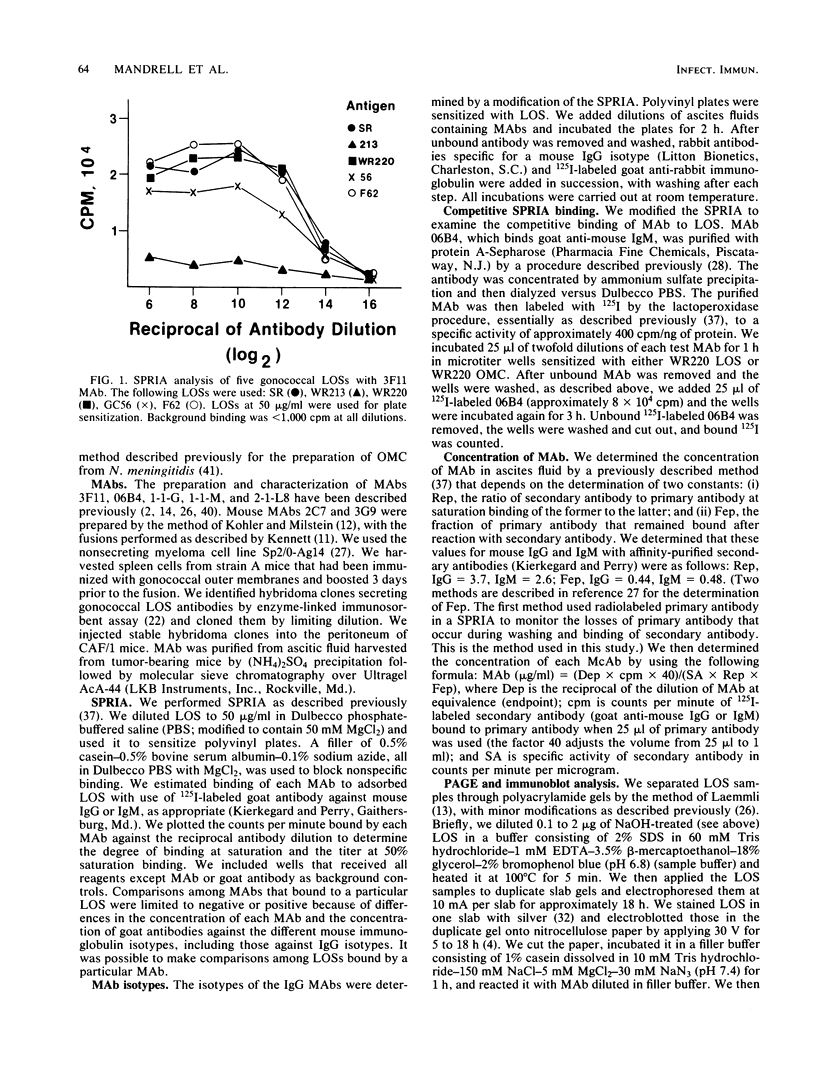
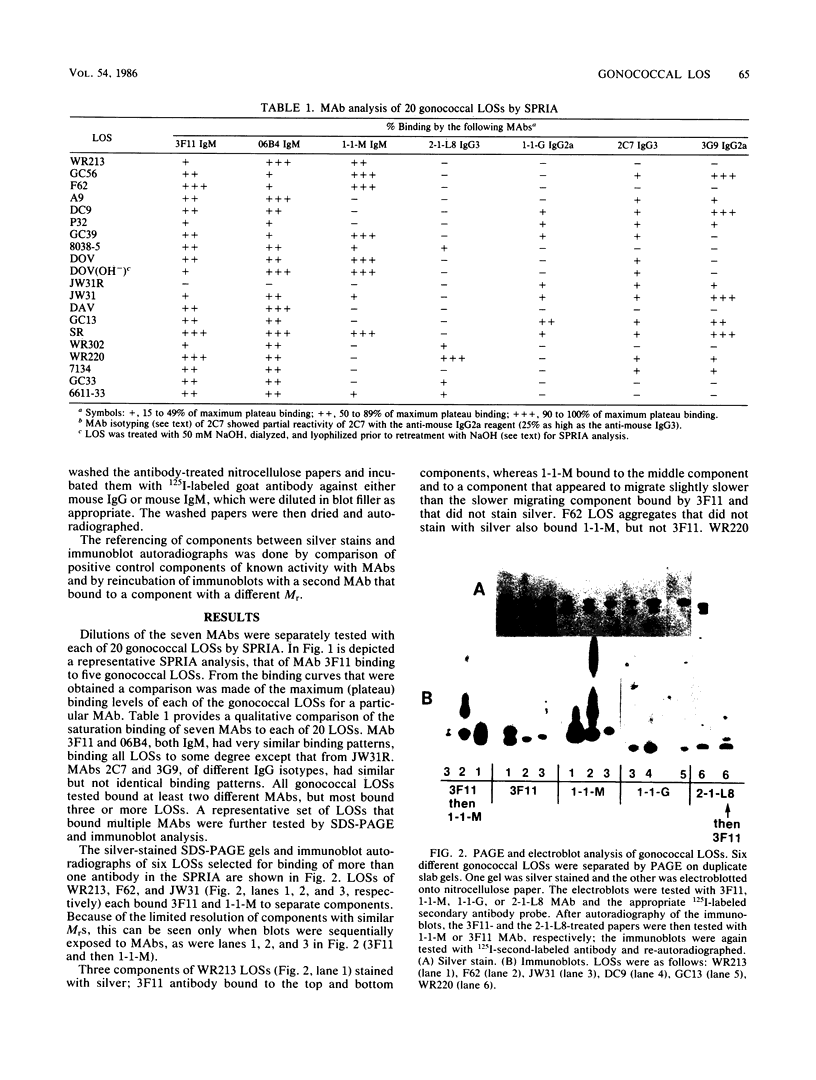
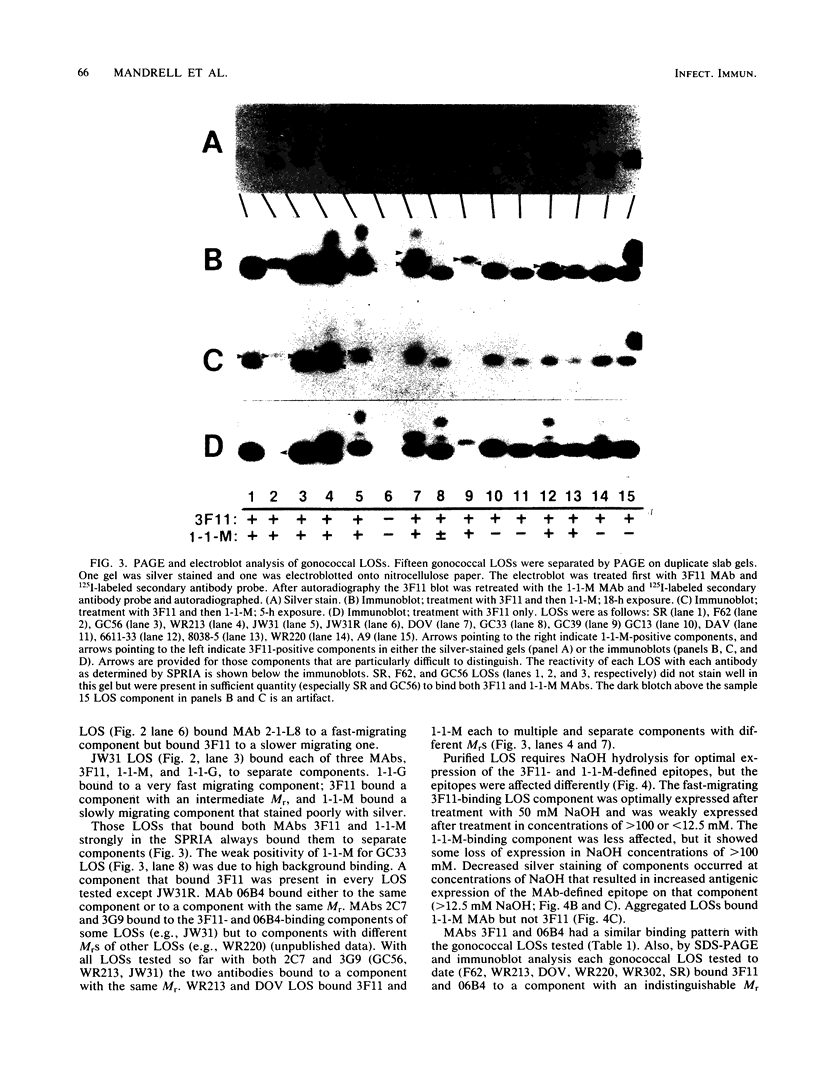
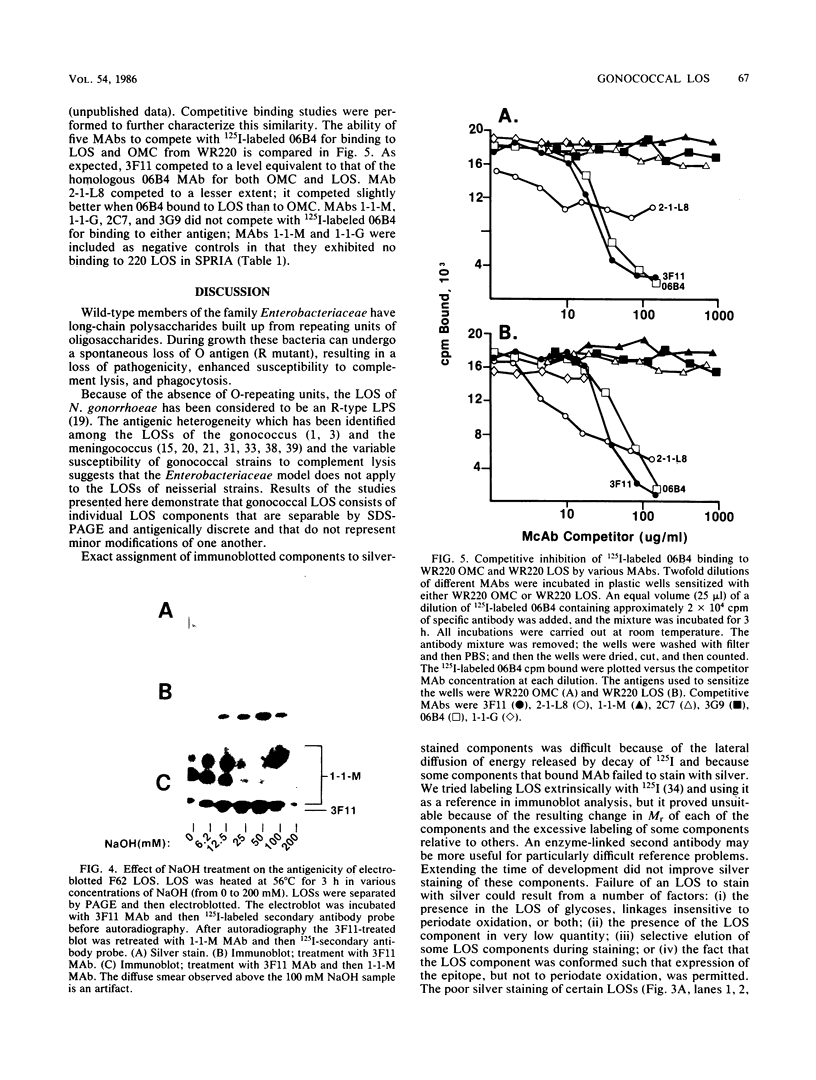
We used mouse monoclonal antibodies (MAbs) to characterize Neisseria gonorrhoeae lipooligosaccharide (LOS). LOSs that bound two or more MAbs in a solid-phase radioimmunoassay usually bound them to different LOS components, as separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE); strains with multiple LOS components on SDS-PAGE usually bound more than one MAb. However, the LOS of some strains bound the same MAb to two LOS components with different relative molecular weights, and some individual LOS components bound more than one MAb. LOSs from different strains bound different amounts of the same MAb at saturation, reflecting differences in the quantitative expression of individual LOS components. Not all components recognized by MAbs were stained by silver after periodate oxidation. Treatment with NaOH variously affected epitopes defined by different MAbs. MAb 3F11 completely inhibited and MAb 2-1-L8 partially inhibited the binding of 125I-labeled 06B4 MAb to WR220 LOS and WR220 outer membranes in competitive binding studies. Other MAbs did not compete with the binding of 125I-labeled 06B4 to either antigen. We conclude that a strain of N. gonorrhoeae elaborates multiple LOSs that can be separated by SDS-PAGE and that are antigenically distinct. Epitope expression within these glycolipids is complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Bennett K. M., Hermerath C. A., Roberts D. E. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1981 Dec;34(3):751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Gagliardi N. C. Antigenic heterogeneity of the non-serogroup antigen structure of Neisseria gonorrhoeae lipopolysaccharides. Infect Immun. 1979 Dec;26(3):870–874. doi: 10.1128/iai.26.3.870-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A. Serogrouping of Neisseria gonorrhoeae: identification of four immunologically distinct acidic polysaccharides. J Infect Dis. 1976 Oct;134(4):377–383. doi: 10.1093/infdis/134.4.377. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Elkins K., Metcalf E. S. Monoclonal antibodies demonstrate multiple epitopes on the O antigens of Salmonella typhimurium LPS. J Immunol. 1984 Oct;133(4):2255–2260. [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Esser M., Shafer W. M. Pyocin-resistant lipopolysaccharide mutans of Neisseria gonorrhoeae: alterations in sensitivity to normal human serum and polymyxin B. Infect Immun. 1982 May;36(2):541–547. doi: 10.1128/iai.36.2.541-547.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect Immun. 1984 Oct;46(1):202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect Immun. 1977 May;16(2):471–475. doi: 10.1128/iai.16.2.471-475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz C. S., Apicella M. A., Morse S. A. Electrophoretic and serological characterization of the lipopolysaccharide produced by Neisseria gonorrhoeae. J Infect Dis. 1984 Apr;149(4):544–552. doi: 10.1093/infdis/149.4.544. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Apicella M. A. Isolation of a lipopolysaccharide mutant of Neisseria gonorrhoeae: an analysis of the antigenic and biologic difference. J Infect Dis. 1982 Feb;145(2):206–216. doi: 10.1093/infdis/145.2.206. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Mintz C. S., Sarafian S. K., Bartenstein L., Bertram M., Apicella M. A. Effect of dilution rate on lipopolysaccharide and serum resistance of Neisseria gonorrhoeae grown in continuous culture. Infect Immun. 1983 Jul;41(1):74–82. doi: 10.1128/iai.41.1.74-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Colony variants of Neisseria meningitidis strain 2996 (B:2b:P1.2): influence of class-5 outer membrane proteins and lipopolysaccharides. J Med Microbiol. 1985 Apr;19(2):203–209. doi: 10.1099/00222615-19-2-203. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate. Roles of blocking antibody and gonococcal outer membrane proteins. J Clin Invest. 1982 Jul;70(1):157–167. doi: 10.1172/JCI110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M. A bactericidal microassay for testing serum sensitivity of Neisseria gonorrhoeae. J Immunol Methods. 1982 Oct 15;54(1):101–105. doi: 10.1016/0022-1759(82)90118-1. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Mandrell R. E., Jarvis G. A. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect Immun. 1985 Dec;50(3):672–677. doi: 10.1128/iai.50.3.672-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä I., Sarvas H., Péterfy F., Mäkelä O. The four subclasses of IgG can be isolated from mouse serum by using Protein A-Sepharose. Scand J Immunol. 1981 Oct;14(4):335–342. doi: 10.1111/j.1365-3083.1981.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Stone M. R., Nowinski R. C. Topological mapping of murine leukemia virus proteins by competition-binding assays with monoclonal antibodies. Virology. 1980 Jan 30;100(2):370–381. doi: 10.1016/0042-6822(80)90528-0. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Wilson C. Variability of the lytic susceptibility of Neisseria gonorrhoeae to human sera. J Immunol. 1977 May;118(5):1843–1851. [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J. The preparation and characterization of a radioiodinated bacterial lipopolysaccharide. Immunochemistry. 1978 Mar;15(3):157–164. doi: 10.1016/0161-5890(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983 Apr;40(1):257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]