Abstract
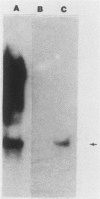
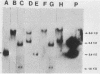
The Streptococcus mutans LM7 gene gtfA was cloned in Escherichia coli along with flanking regions of the chromosome as a fragment representing 10.3 kilobases (kb) of streptococcal DNA. Restriction endonuclease mapping revealed that the cloned DNA consisted of four EcoRI fragments with gtfA sucrase activity localized to one fragment, EcoRI-B (2.4 kb). Subsequent analysis with E. coli minicells indicated that three polypeptides were encoded on the 10.3-kb insert (55 [GtfA], 45, and 35 kilodaltons). Neither the 45- nor 35-kilodalton polypeptide exhibited any detectable sucrase activity. The approximate positions and directions of transcription of the two larger proteins were determined from minicell protein profiles displaying truncated versions of these polypeptides. The restriction endonuclease data for the cloned gtfA gene were used to develop a strategy for insertional inactivation of this locus in vivo. An internal HincII fragment of the gtfA gene was removed and replaced with a DNA fragment containing a tetracycline resistance determinant. This new recombinant plasmid was linearized and then transformed into S. mutans GS5 and S. mutans V403 where it was incapable of replication. It was predicted that Tcr colonies would result from double-crossover recombinational events involving homologous regions flanking the gtfA gene. This was verified by Southern DNA hybridization analyses. The inactivation of the gtfA gene in both S. mutans GS5 and S. mutans V403 resulted in a decrease of water-soluble exopolysaccharide but no detectable changes in the amounts of water-insoluble polymers.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Beall J. R., Bielawski R. M., Porter E. V., Donkersloot J. A. Occurrence and distribution of sucrose-metabolizing enzymes in oral streptococci. Infect Immun. 1976 Aug;14(2):408–415. doi: 10.1128/iai.14.2.408-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic analysis of Streptococcus mutans virulence. Curr Top Microbiol Immunol. 1985;118:253–277. doi: 10.1007/978-3-642-70586-1_14. [DOI] [PubMed] [Google Scholar]
- Freedman M., Birked D., Granath K. Analyses of glucans from cariogenic and mutant Streptococcus mutans. Infect Immun. 1978 Jul;21(1):17–27. doi: 10.1128/iai.21.1.17-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSSON B. E., QUENSEL C. E., LANKE L. S., LUNDQVIST C., GRAHNEN H., BONOW B. E., KRASSE B. The Vipeholm dental caries study; the effect of different levels of carbohydrate intake on caries activity in 436 individuals observed for five years. Acta Odontol Scand. 1954 Sep;11(3-4):232–264. doi: 10.3109/00016355308993925. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Harlander S. K., Leung W. L., Schachtele C. F. Streptococcus mutans dextransucrase: functioning of primer dextran and endogenous dextranase in water-soluble and water-insoluble glucan synthesis. Infect Immun. 1977 May;16(2):637–648. doi: 10.1128/iai.16.2.637-648.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. Genetic analysis of bacterial virulence determinants in Bordetella pertussis and the pathogenetic Yersinia. Curr Top Microbiol Immunol. 1985;118:1–11. doi: 10.1007/978-3-642-70586-1_1. [DOI] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Koomey J. M., Gill R. E., Falkow S. Genetic and biochemical analysis of gonococcal IgA1 protease: cloning in Escherichia coli and construction of mutants of gonococci that fail to produce the activity. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7881–7885. doi: 10.1073/pnas.79.24.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral T. A., Daneo-Moore L. Glycerol incorporation in certain oral streptococci. Infect Immun. 1980 Dec;30(3):759–765. doi: 10.1128/iai.30.3.759-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., Jones K. R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983 Nov;25(1):145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Tobian J. A., Jones K. R., Evans R. P., Clewell D. B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982 Oct;19(3):345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Perry D., Kuramitsu H. K. Genetic transformation of Streptococcus mutans. Infect Immun. 1981 Jun;32(3):1295–1297. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Wondrack L. M., Kuramitsu H. K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983 Aug;41(2):722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Macrina F. L. Cloned gtfA gene of Streptococcus mutans LM7 alters glucan synthesis in Streptococcus sanguis. Infect Immun. 1985 Jun;48(3):704–712. doi: 10.1128/iai.48.3.704-712.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robeson J. P., Barletta R. G., Curtiss R., 3rd Expression of a Streptococcus mutans glucosyltransferase gene in Escherichia coli. J Bacteriol. 1983 Jan;153(1):211–221. doi: 10.1128/jb.153.1.211-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rölla G., Iversen O. J., Bonesvoll P. Lipoteichoic acid - the key to the adhesiveness of sucrose grown Streptococcus mutans. Adv Exp Med Biol. 1978;107:607–617. doi: 10.1007/978-1-4684-3369-2_69. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M. Essential dependence of smooth surface caries on, and augmentation of fissure caries by, sucrose and Streptococcus mutans infection. Infect Immun. 1979 Aug;25(2):526–531. doi: 10.1128/iai.25.2.526-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobian J. A., Cline M. L., Macrina F. L. Characterization and expression of a cloned tetracycline resistance determinant from the chromosome of Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):556–563. doi: 10.1128/jb.160.2.556-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter R. B., Gold L. Overproduction of bacteriophage Q beta maturation (A2) protein leads to cell lysis. Cell. 1983 Jul;33(3):877–885. doi: 10.1016/0092-8674(83)90030-2. [DOI] [PubMed] [Google Scholar]