Abstract
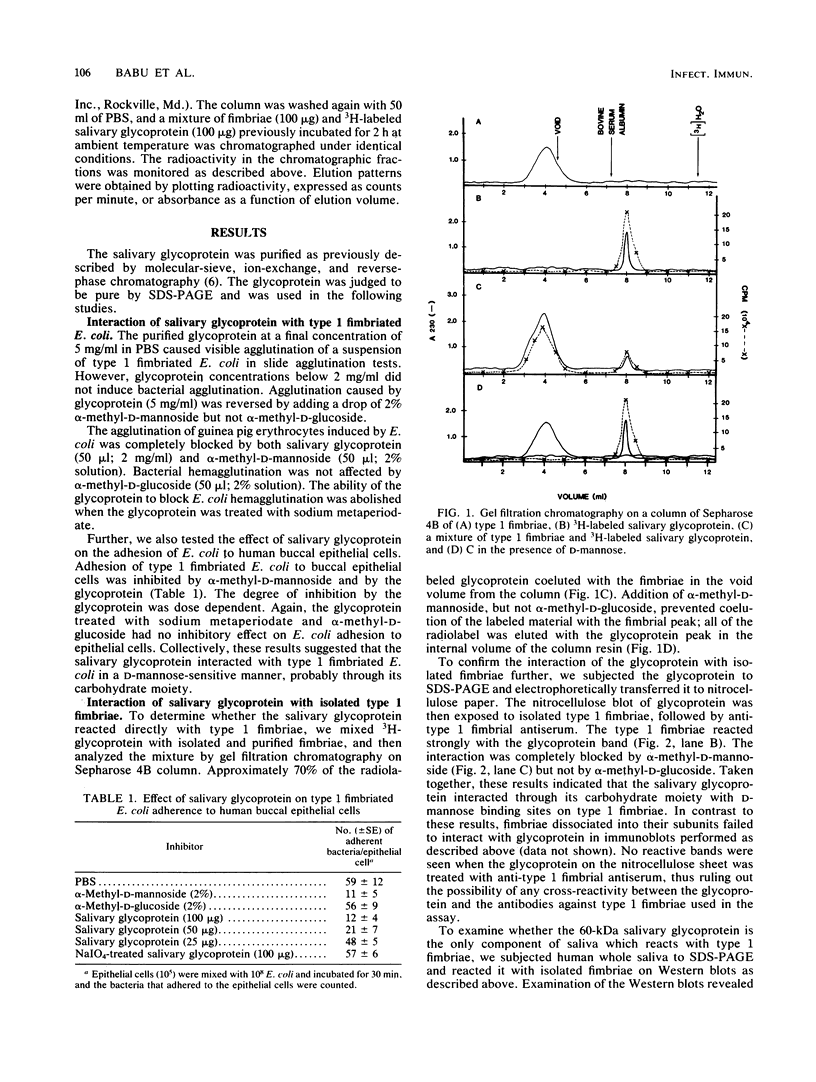
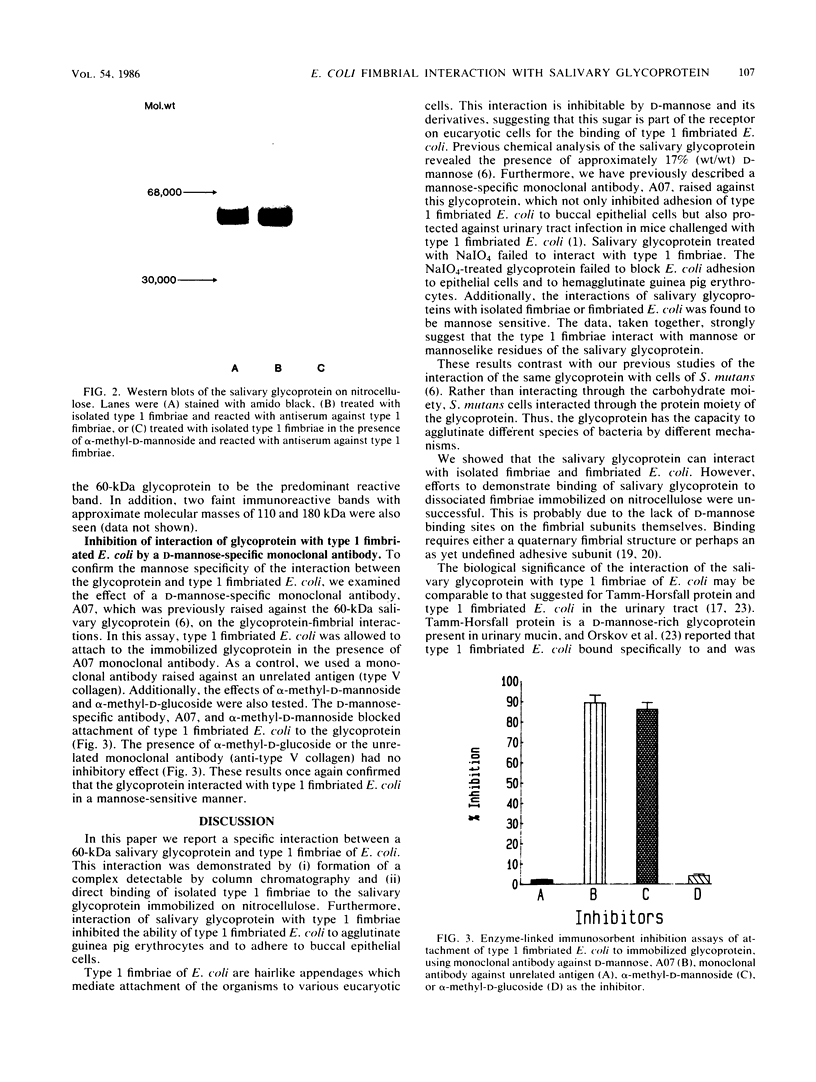
A 60-kilodalton glycoprotein previously isolated and purified from human saliva (J. B. Babu, E. H. Beachey, D. L. Hasty, and W. A. Simpson, Infect. Immun. 51: 405-413, 1986) was found to interact with type 1 fimbriae and prevent adhesion of type 1 fimbriated Escherichia coli to animal cells in a D-mannose-sensitive manner. Purified salivary glycoprotein agglutinated type 1 fimbriated E. coli and, at subagglutinating concentrations, blocked the ability of type 1 fimbriated E. coli to attach to human buccal epithelial cells or agglutinate guinea pig erythrocytes. Both interactions were inhibited by alpha-methyl-D-mannoside but not by alpha-methyl-D-glucoside. Complexing of the glycoprotein to type 1 fimbriae was demonstrated by molecular sieve chromatography and modified Western blots. When mixed with type 1 fimbriae, the radiolabeled salivary glycoprotein coeluted with type 1 fimbriae from a column of Sepharose 4B. When blotted from a sodium dodecyl sulfate gel to nitrocellulose sheets, the glycoprotein interacted directly with type 1 fimbriae applied to the blots. Both of the latter interactions also were blocked by alpha-methyl-D-mannoside but not by alpha-methyl-D-glucoside. Chemical modification of the glycoprotein with sodium metaperiodate abolished its ability to interact with isolated type 1 fimbriae or type 1 fimbriated E. coli. These results suggest that the carbohydrate moiety of the 60-kilodalton glycoprotein serves as a receptor for type 1 fimbriae in the oral cavity, and we postulate that the interaction may cause agglutination and early removal of E. coli, thereby preventing colonization by these organisms of oropharyngeal mucosae and dental tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Babu J. P., Giampapa C. S., Hasty D. L., Simpson W. A., Beachey E. H. Protection against Escherichia coli-induced urinary tract infections with hybridoma antibodies directed against type 1 fimbriae or complementary D-mannose receptors. Infect Immun. 1985 Jun;48(3):625–628. doi: 10.1128/iai.48.3.625-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham S. N., Hasty D. L., Simpson W. A., Beachey E. H. Antiadhesive properties of a quaternary structure-specific hybridoma antibody against type 1 fimbriae of Escherichia coli. J Exp Med. 1983 Oct 1;158(4):1114–1128. doi: 10.1084/jem.158.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneberg P. Partial characterization of five glycoprotein fractions secreted by the human parotid glands. Arch Oral Biol. 1974 Oct;19(10):921–928. doi: 10.1016/0003-9969(74)90055-7. [DOI] [PubMed] [Google Scholar]
- Babu J. P., Beachey E. H., Hasty D. L., Simpson W. A. Isolation and characterization of a 60-kilodalton salivary glycoprotein with agglutinating activity against strains of Streptococcus mutans. Infect Immun. 1986 Feb;51(2):405–413. doi: 10.1128/iai.51.2.405-413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Simpson W. A., Courtney H. S., Beachey E. H. Interaction of human plasma fibronectin with cariogenic and non-cariogenic oral streptococci. Infect Immun. 1983 Jul;41(1):162–168. doi: 10.1128/iai.41.1.162-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bennick A., Connell G. E. Purification and partial characterization of four proteins from human parotid saliva. Biochem J. 1971 Jul;123(3):455–464. doi: 10.1042/bj1230455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D. C., Eisenstein B. I. Antigenic quantitation of type 1 fimbriae on the surface of Escherichia coli cells by an enzyme-linked immunosorbent inhibition assay. Infect Immun. 1982 Nov;38(2):764–773. doi: 10.1128/iai.38.2.764-773.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. W., Russell R. R. The adsorption of human salivary components to strains of the bacterium Streptococcus mutans. Arch Oral Biol. 1984;29(10):751–757. doi: 10.1016/0003-9969(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Ericson T., Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983 Jun 15;133(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast receptor for cell-substratum adhesion: studies on the interaction of baby hamster kidney cells with latex beads coated by cold insoluble globulin (plasma fibronectin). J Cell Biol. 1980 Jul;86(1):104–112. doi: 10.1083/jcb.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hogg S. D., Embery G. The isolation and partial characterization of a sulphated glycoprotein from human whole saliva which aggregates strains of Streptococcus sanguis but not Streptococcus mutans. Arch Oral Biol. 1979;24(10-11):791–797. doi: 10.1016/0003-9969(79)90040-2. [DOI] [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama S. M., Silverblatt F. J. Effect of Tamm-Horsfall urinary glycoprotein on phagocytosis and killing of type I-fimbriated Escherichia coli. Infect Immun. 1986 Jan;51(1):193–198. doi: 10.1128/iai.51.1.193-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Minion F. C., Abraham S. N., Beachey E. H., Goguen J. D. The genetic determinant of adhesive function in type 1 fimbriae of Escherichia coli is distinct from the gene encoding the fimbrial subunit. J Bacteriol. 1986 Mar;165(3):1033–1036. doi: 10.1128/jb.165.3.1033-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oemrawsingh I., Roukema P. A. Isolation, purification and chemical characterization of mucins from human submandibular glands. Arch Oral Biol. 1974 Aug;19(8):615–626. doi: 10.1016/0003-9969(74)90129-0. [DOI] [PubMed] [Google Scholar]
- Oppenheim F. G., Hay D. I., Franzblau C. Proline-rich proteins from human parotid saliva. I. Isolation and partial characterization. Biochemistry. 1971 Nov;10(23):4233–4238. doi: 10.1021/bi00799a013. [DOI] [PubMed] [Google Scholar]
- Orskov I., Ferencz A., Orskov F. Tamm-Horsfall protein or uromucoid is the normal urinary slime that traps type 1 fimbriated Escherichia coli. Lancet. 1980 Apr 19;1(8173):887–887. doi: 10.1016/s0140-6736(80)91396-3. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Mandel I. D., Ellison S. A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982 Feb;11(1):1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]