Abstract
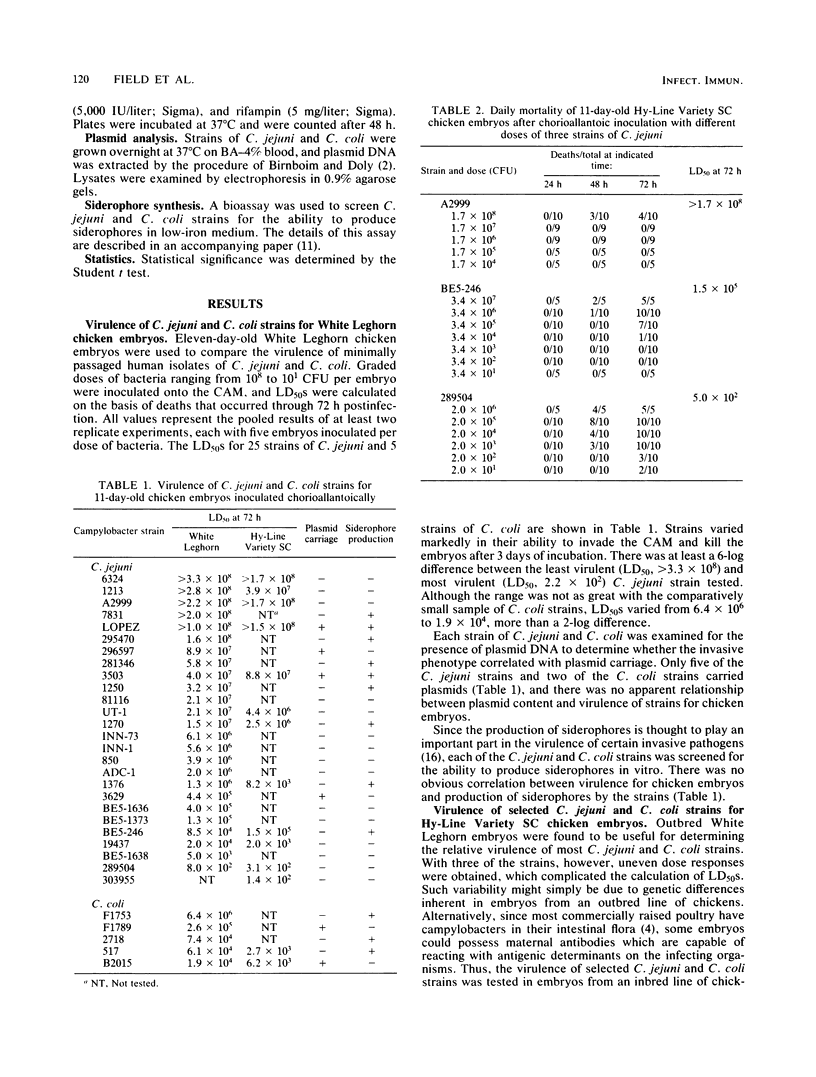
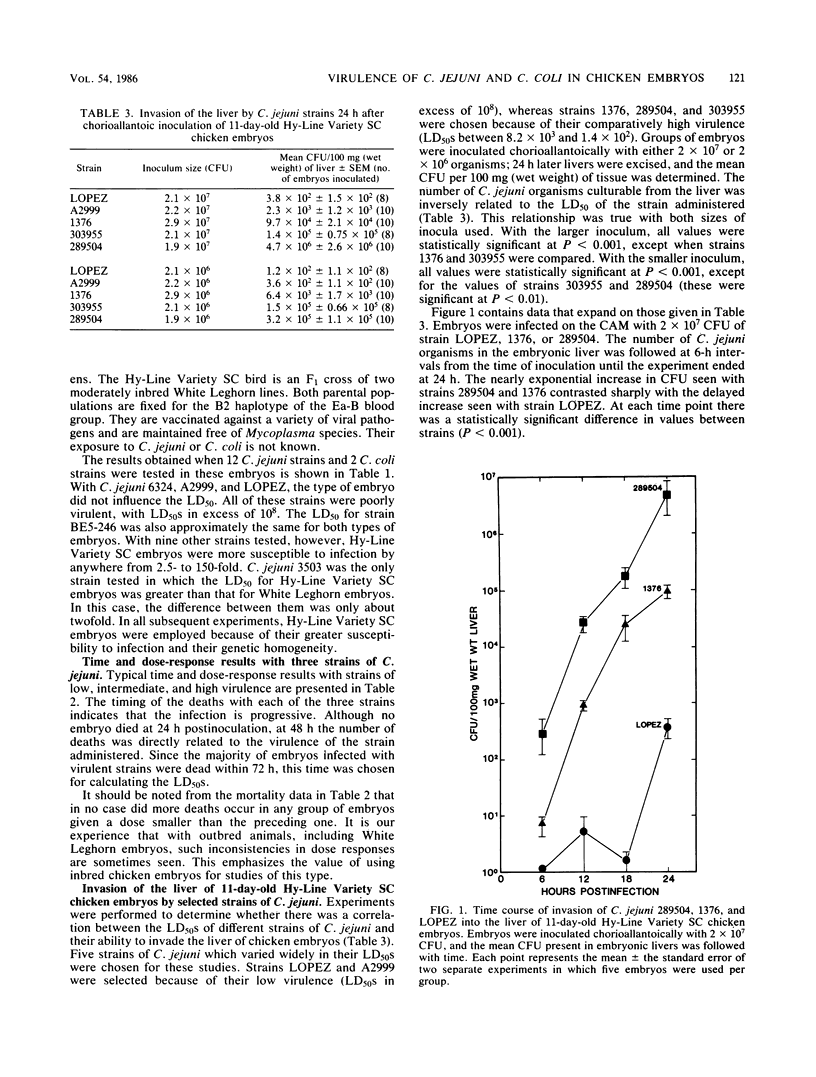
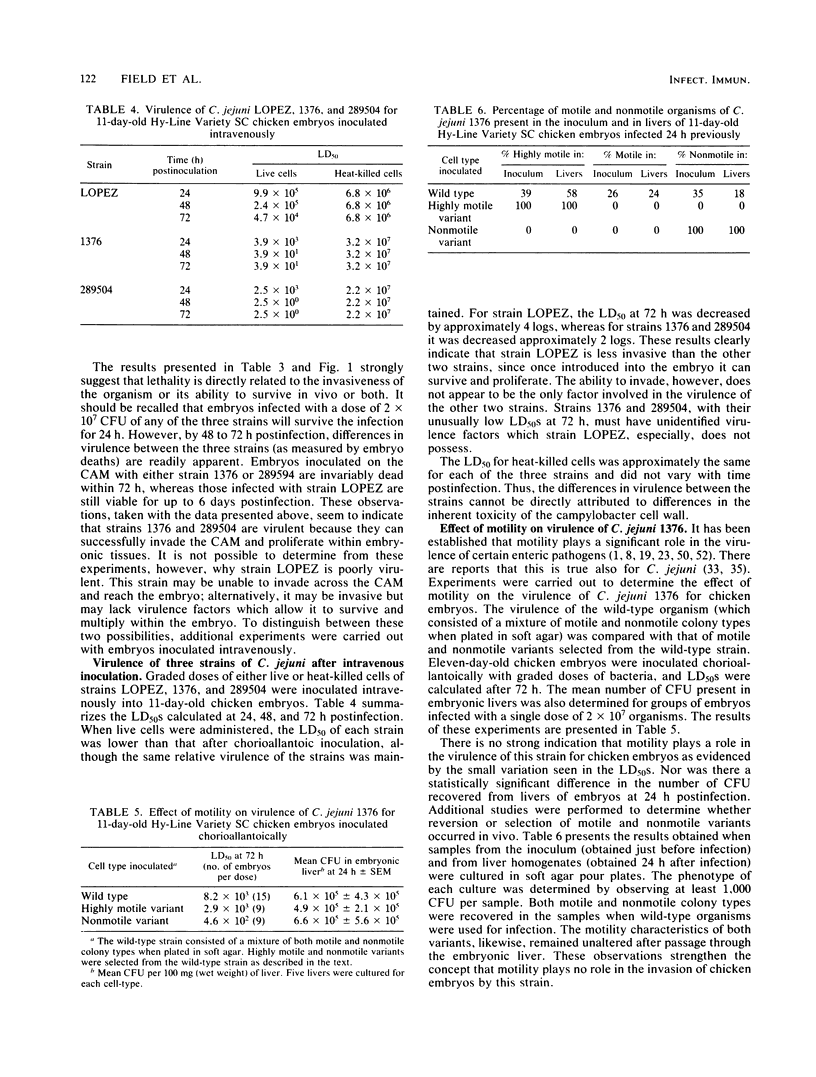
Eleven-day-old chicken embryos were used to compare the relative virulence of minimally passaged human isolates of Campylobacter jejuni and Campylobacter coli. Graded doses of bacteria were inoculated onto the chorioallantoic membrane, and 50% lethal doses were calculated at 72 h postinfection. Strains varied markedly in their ability to invade the chorioallantoic membrane and kill the embryos. The 50% lethal doses varied by about 6 logs for 25 strains of C. jejuni, and by 2 logs for 5 strains of C. coli. Although both outbred and inbred embryos were employed in the study, the latter were found to be more susceptible to infection with most strains. All isolates were screened for plasmid DNA, but there was no apparent relationship between plasmid content and virulence of strains for the embryos. Neither could virulence be associated with the production of siderophores by the strains. The ability of selected strains of C. jejuni to invade the liver of embryos was also studied. The number of campylobacters culturable from the liver was found to be inversely related to the 50% lethal dose of the strain. By inoculating 11-day-old embryos intravenously, it was possible to demonstrate that a strain of C. jejuni which was poorly virulent after chorioallantoic inoculation was relatively noninvasive. Invasiveness alone, however, could not fully account for the lethality of two highly virulent strains of C. jejuni administered by the intravenous route. Finally, there was no correlation between motility and virulence in this model system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983 May;147(5):864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Duncan D. J., Warren G. H., Wang W. L. Experimental Campylobacter jejuni infection of adult mice. Infect Immun. 1983 Feb;39(2):908–916. doi: 10.1128/iai.39.2.908-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- Caldwell M. B., Guerry P., Lee E. C., Burans J. P., Walker R. I. Reversible expression of flagella in Campylobacter jejuni. Infect Immun. 1985 Dec;50(3):941–943. doi: 10.1128/iai.50.3.941-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell M. B., Walker R. I., Stewart S. D., Rogers J. E. Simple adult rabbit model for Campylobacter jejuni enteritis. Infect Immun. 1983 Dec;42(3):1176–1182. doi: 10.1128/iai.42.3.1176-1182.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Weinstein D. L., Karch H., Holder I. A., O'Brien A. D. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect Immun. 1984 Dec;46(3):814–818. doi: 10.1128/iai.46.3.814-818.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN R. A. OBSERVATIONS ON MODE OF ACTION OF ENDOTOXIN IN CHICK EMBRYOS. Proc Soc Exp Biol Med. 1964 Mar;115:702–707. doi: 10.3181/00379727-115-29012. [DOI] [PubMed] [Google Scholar]
- Field L. H., Headley V. L., Payne S. M., Berry L. J. Influence of iron on growth, morphology, outer membrane protein composition, and synthesis of siderophores in Campylobacter jejuni. Infect Immun. 1986 Oct;54(1):126–132. doi: 10.1128/iai.54.1.126-132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. H., Underwood J. L., Berry L. J. The role of gut flora and animal passage in the colonisation of adult mice with Campylobacter jejuni. J Med Microbiol. 1984 Feb;17(1):59–66. doi: 10.1099/00222615-17-1-59. [DOI] [PubMed] [Google Scholar]
- Field L. H., Underwood J. L., Pope L. M., Berry L. J. Intestinal colonization of neonatal animals by Campylobacter fetus subsp. jejuni. Infect Immun. 1981 Sep;33(3):884–892. doi: 10.1128/iai.33.3.884-892.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgeorge R. B., Baskerville A., Lander K. P. Experimental infection of Rhesus monkeys with a human strain of Campylobacter jejuni. J Hyg (Lond) 1981 Jun;86(3):343–351. doi: 10.1017/s0022172400069096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H., De Boeck M., Butzler J. P. A new selective medium for the isolation of Campylobacter jejuni from human faeces. Eur J Clin Microbiol. 1983 Aug;2(4):389–393. doi: 10.1007/BF02019476. [DOI] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey C. D., Montag D. M., Pittman F. E. Experimental infection of hamsters with Campylobacter jejuni. J Infect Dis. 1985 Mar;151(3):485–493. doi: 10.1093/infdis/151.3.485. [DOI] [PubMed] [Google Scholar]
- Hébert G. A., Hollis D. G., Weaver R. E., Steigerwalt A. G., McKinney R. M., Brenner D. J. Serogroups of Campylobacter jejuni, Campylobacter coli, and Campylobacter fetus defined by direct immunofluorescence. J Clin Microbiol. 1983 Mar;17(3):529–538. doi: 10.1128/jcm.17.3.529-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. M., Lior H. Toxins produced by Campylobacter jejuni and Campylobacter coli. Lancet. 1984 Jan 28;1(8370):229–230. doi: 10.1016/s0140-6736(84)92155-x. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982 Nov;38(2):476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Properties of crude Campylobacter jejuni heat-labile enterotoxin. Infect Immun. 1984 Aug;45(2):314–319. doi: 10.1128/iai.45.2.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Short H., Schenk E. A. Pathogenic properties of Campylobacter jejuni: assay and correlation with clinical manifestations. Infect Immun. 1985 Oct;50(1):43–49. doi: 10.1128/iai.50.1.43-49.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastovica A. J., Penner J. L. Serotypes of campylobacter jejuni and campylobacter coli in bacteremic, hospitalized children. J Infect Dis. 1983 Mar;147(3):592–592. doi: 10.1093/infdis/147.3.592. [DOI] [PubMed] [Google Scholar]
- Manninen K. I., Prescott J. F., Dohoo I. R. Pathogenicity of Campylobacter jejuni isolates from animals and humans. Infect Immun. 1982 Oct;38(1):46–52. doi: 10.1128/iai.38.1.46-52.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCardell B. A., Madden J. M., Lee E. C. Production of cholera-like toxin by Campylobacter jejuni/coli. Lancet. 1984 Feb 25;1(8374):448–449. doi: 10.1016/s0140-6736(84)91772-0. [DOI] [PubMed] [Google Scholar]
- Mizejewski G. J., Ramm G. M. Phagocytosis as related to the reticuloendothelial system (RES) in the developing chick. Growth. 1969 Mar;33(1):47–56. [PubMed] [Google Scholar]
- Morooka T., Umeda A., Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol. 1985 Aug;131(8):1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Dolby J. M. Investigations on the role of flagella in the colonization of infant mice with Campylobacter jejuni and attachment of Campylobacter jejuni to human epithelial cell lines. J Hyg (Lond) 1985 Oct;95(2):217–227. doi: 10.1017/s0022172400062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Saunders F., Dehele Y., Pearson A. D. The virulence of clinical and environmental isolates of Campylobacter jejuni. J Hyg (Lond) 1985 Feb;94(1):45–54. doi: 10.1017/s0022172400061118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., Pearson A. The invasion of epithelial cell lines and the intestinal epithelium of infant mice by Campylobacter jejuni/coli. J Diarrhoeal Dis Res. 1984 Mar;2(1):19–26. [PubMed] [Google Scholar]
- Rollins D. M., Coolbaugh J. C., Walker R. I., Weiss E. Biphasic culture system for rapid Campylobacter cultivation. Appl Environ Microbiol. 1983 Jan;45(1):284–289. doi: 10.1128/aem.45.1.284-289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., Escamilla E., Torres N. Experimental Campylobacter diarrhea in chickens. Infect Immun. 1981 Oct;34(1):250–255. doi: 10.1128/iai.34.1.250-255.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., Torres J., Torres N. I., Escamilla E., Ruiz-Palacios B. R., Tamayo J. Cholera-like enterotoxin produced by Campylobacter jejuni. Characterisation and clinical significance. Lancet. 1983 Jul 30;2(8344):250–253. doi: 10.1016/s0140-6736(83)90234-9. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., d'Hauteville H., Formal S. B., Toucas M. Plasmid-mediated invasiveness of "Shigella-like" Escherichia coli. Ann Microbiol (Paris) 1982 May-Jun;133(3):351–355. [PubMed] [Google Scholar]
- Sanyal S. C., Islam K. M., Neogy P. K., Islam M., Speelman P., Huq M. I. Campylobacter jejuni diarrhea model in infant chickens. Infect Immun. 1984 Mar;43(3):931–936. doi: 10.1128/iai.43.3.931-936.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Bryner J. H. Plasmid content and pathogenicity of Campylobacter jejuni and Campylobacter coli strains in the pregnant guinea pig model. Am J Vet Res. 1984 Oct;45(10):2201–2202. [PubMed] [Google Scholar]
- Warner D. P., Bryner J. H. Campylobacter jejuni and Campylobacter coli inoculation of neonatal calves. Am J Vet Res. 1984 Sep;45(9):1822–1824. [PubMed] [Google Scholar]
- Weinstein D. L., Carsiotis M., Lissner C. R., O'Brien A. D. Flagella help Salmonella typhimurium survive within murine macrophages. Infect Immun. 1984 Dec;46(3):819–825. doi: 10.1128/iai.46.3.819-825.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S. L. Experimental gastroenteritis in newly-hatched chicks infected with Campylobacter jejuni. J Med Microbiol. 1984 Oct;18(2):233–248. doi: 10.1099/00222615-18-2-233. [DOI] [PubMed] [Google Scholar]
- Yancey R. J., Willis D. L., Berry L. J. Role of motility in experimental cholera in adult rabbits. Infect Immun. 1978 Nov;22(2):387–392. doi: 10.1128/iai.22.2.387-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeen W. P., Puthucheary S. D., Pang T. Demonstration of a cytotoxin from Campylobacter jejuni. J Clin Pathol. 1983 Nov;36(11):1237–1240. doi: 10.1136/jcp.36.11.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink D. L., Feeley J. C., Wells J. G., Vanderzant C., Vickery J. C., Roof W. D., O'Donovan G. A. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980 Jan 10;283(5743):224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]
- van Spreeuwel J. P., Duursma G. C., Meijer C. J., Bax R., Rosekrans P. C., Lindeman J. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. 1985 Sep;26(9):945–951. doi: 10.1136/gut.26.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]


