Abstract
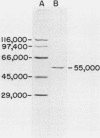
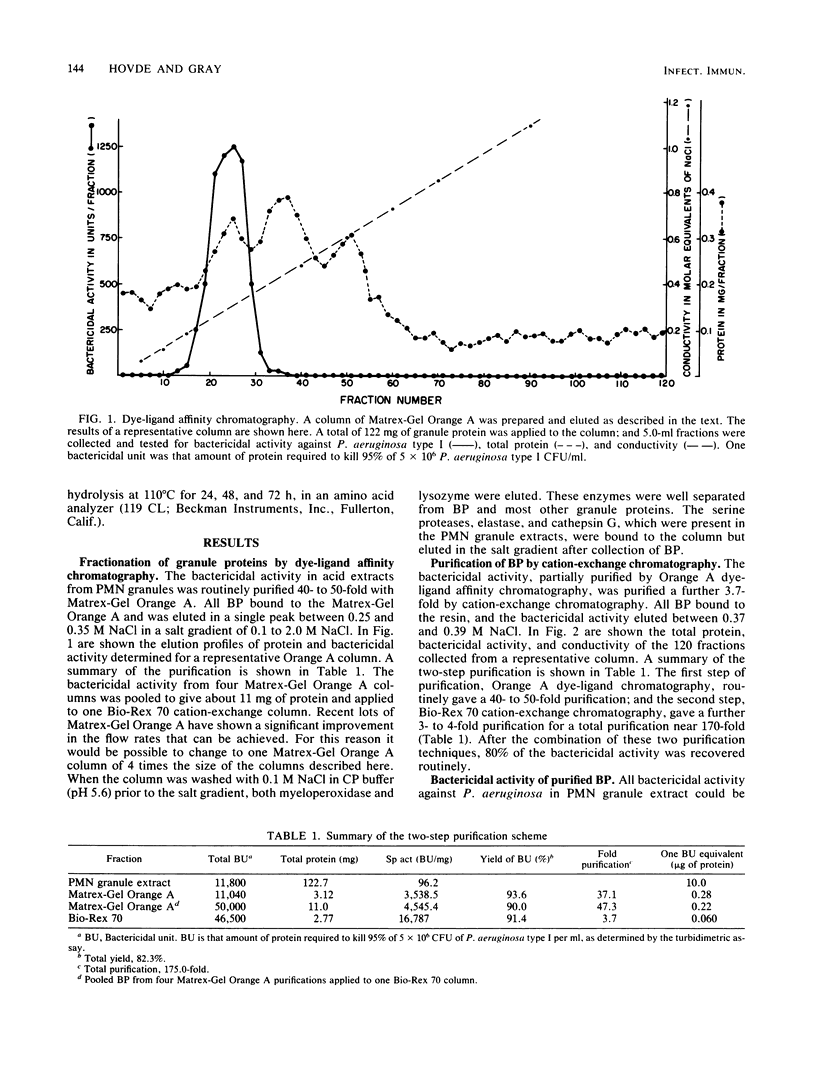
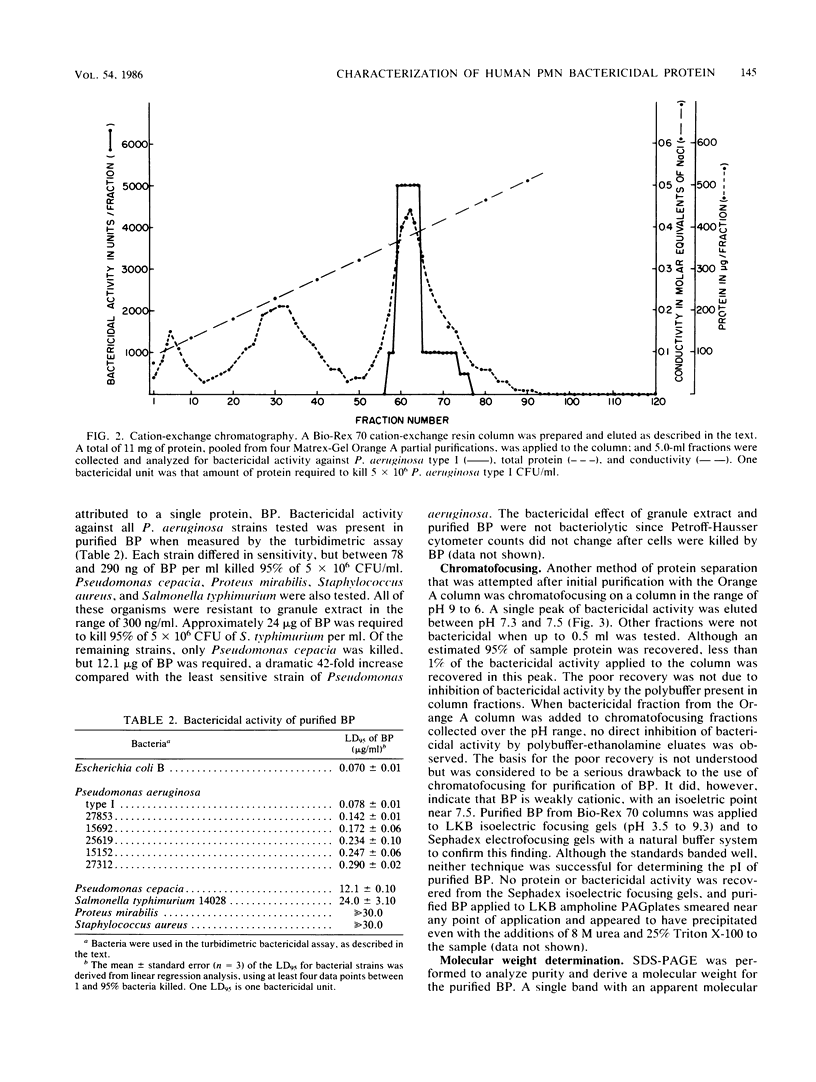
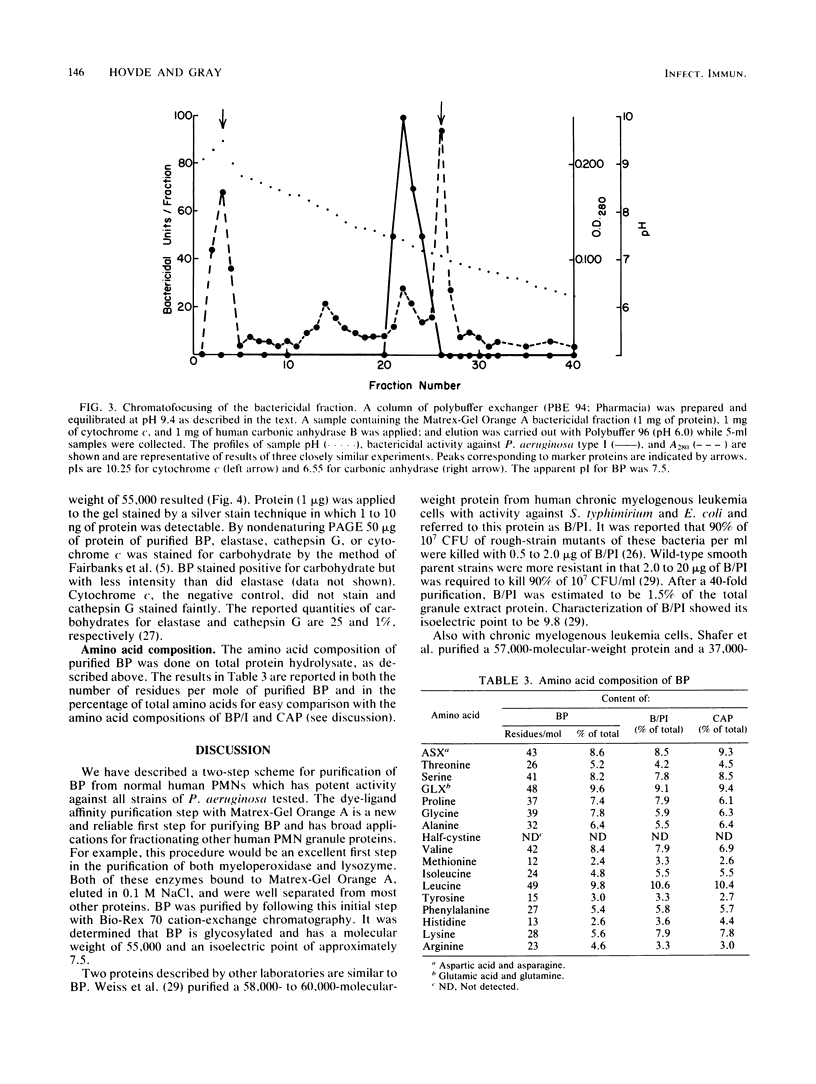
Purification of a bactericidal protein (BP) from the cytoplasmic granules of normal human polymorphonuclear leukocytes (PMN) with activity against Pseudomonas aeruginosa is described. Bactericidal activity from acid extracts of a mixed granule population was purified 175-fold by a two-step chromatographic procedure. The first step, dye-ligand affinity chromatography with Matrex-Gel Orange A, was followed by cation-exchange chromatography with Bio-Rex 70 resin, and this combination routinely gave a yield near 80%. Only one peak of bactericidal activity against P. aeruginosa type I was found after each chromatographic step. Characterization of BP showed a single band with an apparent molecular weight of 55,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Purified BP was most active against the six strains of P. aeruginosa tested and against Escherichia coli B (a deep rough mutant). Purified BP killed 5 X 10(6) CFU of P. aeruginosa type I per ml at a concentration of 60 to 80 ng/ml. Proteus mirabilis and Staphylococcus aureus were both resistant to the bactericidal activity of BP. BP was shown to be glycosylated by periodic acid staining after gel electrophoresis and to have an isoelectric point near 7.5 by chromatofocusing. The amino acid composition of BP is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casey S. G., Shafer W. M., Spitznagel J. K. Anaerobiosis increases resistance of Neisseria gonorrhoeae to O2-independent antimicrobial proteins from human polymorphonuclear granulocytes. Infect Immun. 1985 Feb;47(2):401–407. doi: 10.1128/iai.47.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. A., Lee T. J., Spitznagel J. K., Sparling P. F. Gonococci with mutations to low-level penicillin resistance exhibit increased sensitivity to the oxygen-independent bactericidal activity of human polymorphonuclear leukocyte granule extracts. Infect Immun. 1982 Mar;35(3):826–833. doi: 10.1128/iai.35.3.826-833.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. A reevaluation of the roles of the O2-dependent and O2-independent microbicidal systems of phagocytes. Rev Infect Dis. 1983 Sep-Oct;5(5):843–853. doi: 10.1093/clinids/5.5.843. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Buescher E. S., Seligmann B. E., Nath J., Gaither T., Katz P. NIH conference. Recent advances in chronic granulomatous disease. Ann Intern Med. 1983 Nov;99(5):657–674. doi: 10.7326/0003-4819-99-5-657. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P., Howard J. B. The amino acid sequence of the nitrogenase iron protein from Azotobacter vinelandii. J Biol Chem. 1982 Mar 10;257(5):2483–2490. [PubMed] [Google Scholar]
- Hovde C. J., Gray B. H. Physiological effects of a bactericidal protein from human polymorphonuclear leukocytes on Pseudomonas aeruginosa. Infect Immun. 1986 Apr;52(1):90–95. doi: 10.1128/iai.52.1.90-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MUSCHEL L. H., TREFFERS H. P. Quantitative studies on the bactericidal actions of serum and complement. I. A rapid photometric growth assay for bactericidal activity. J Immunol. 1956 Jan;76(1):1–10. [PubMed] [Google Scholar]
- Mandell G. L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974 Feb;9(2):337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzakowski M. C., Paranavitana C. M. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes: role of bacterial membrane lipid. Infect Immun. 1981 May;32(2):668–674. doi: 10.1128/iai.32.2.668-674.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The role of Pseudomonas aeruginosa in infections. J Antimicrob Chemother. 1983 May;11 (Suppl B):1–13. doi: 10.1093/jac/11.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Prestidge R. L., Hearn M. T. Preparative flatbed electrofocusing in granulated gels with natural pH gradients generated from simple buffers. Anal Biochem. 1979 Aug;97(1):95–102. doi: 10.1016/0003-2697(79)90332-4. [DOI] [PubMed] [Google Scholar]
- Rest R. F. Killing of Neisseria gonorrhoeae by human polymorphonuclear neutrophil granule extracts. Infect Immun. 1979 Aug;25(2):574–579. doi: 10.1128/iai.25.2.574-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. I. Stimulation by phytohaemagglutinin. Biochim Biophys Acta. 1970 Dec 29;222(3):565–582. doi: 10.1016/0304-4165(70)90182-0. [DOI] [PubMed] [Google Scholar]
- SHUGAR D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952 Mar;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K. Nonoxidative antimicrobial reactions of leukocytes. Contemp Top Immunobiol. 1984;14:283–343. doi: 10.1007/978-1-4757-4862-8_10. [DOI] [PubMed] [Google Scholar]
- Thomas J. M., Hodes M. E. A new discontinuous buffer system for the electrophoresis of cationic proteins at near-neutral pH. Anal Biochem. 1981 Nov 15;118(1):194–196. doi: 10.1016/0003-2697(81)90178-0. [DOI] [PubMed] [Google Scholar]
- Travis J., Giles P. J., Porcelli L., Reilly C. F., Baugh R., Powers J. Human leucocyte elastase and cathepsin G: structural and functional characteristics. Ciba Found Symp. 1979;(75):51–68. doi: 10.1002/9780470720585.ch4. [DOI] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Weiss J., Franson R. C., Beckerdite S., Schmeidler K., Elsbach P. Partial characterization and purification of a rabbit granulocyte factor that increases permeability of Escherichia coli. J Clin Invest. 1975 Jan;55(1):33–42. doi: 10.1172/JCI107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherall B. L., Pruul H., McDonald P. J. Oxygen-independent killing of Bacteroides fragilis by granule extracts from human polymorphonuclear leukocytes. Infect Immun. 1984 Mar;43(3):1080–1084. doi: 10.1128/iai.43.3.1080-1084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]