Abstract
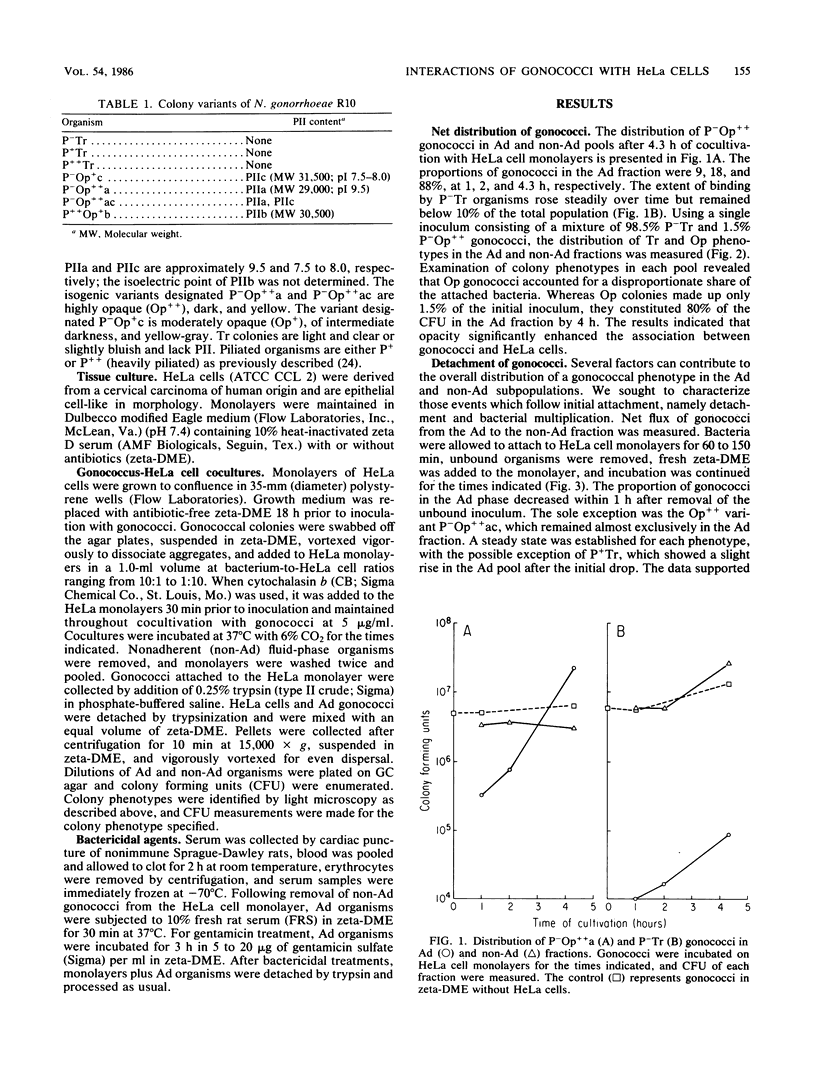
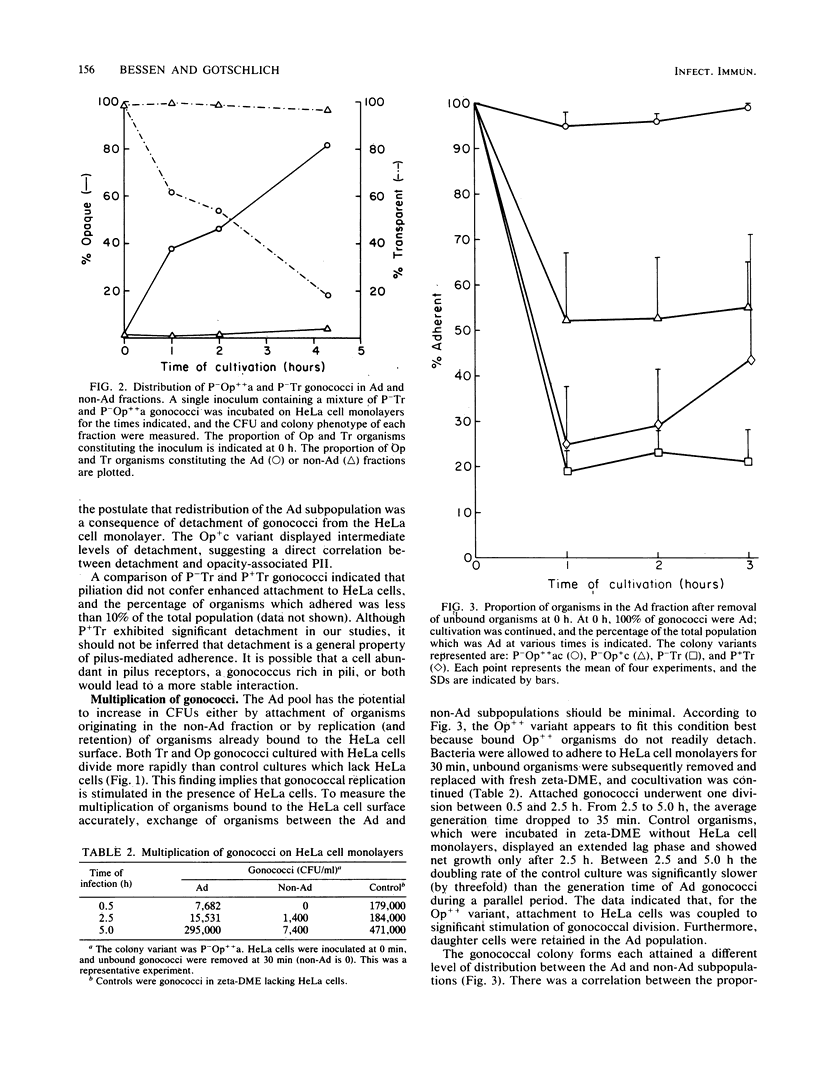
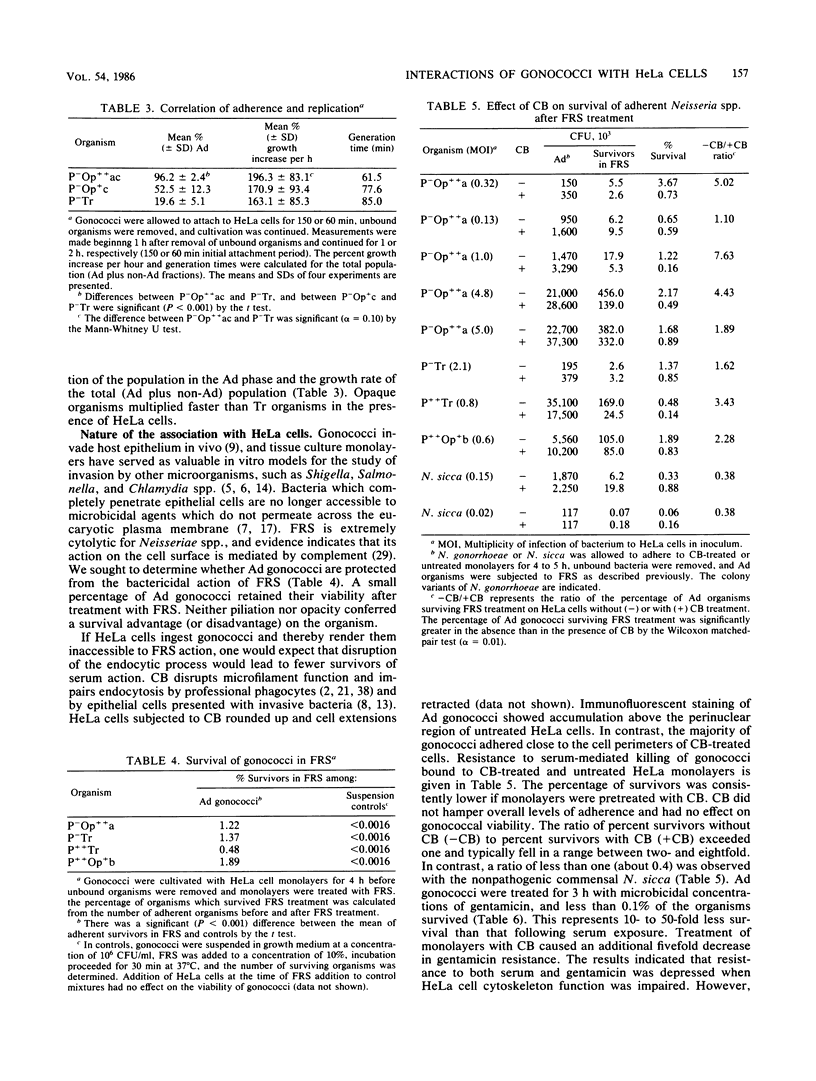
Colony variants of Neisseria gonorrhoeae differ in their interactions with eucaryotic cells. When gonococci were cultivated with HeLa cell monolayers, the opacity phenotype (Op) became increasingly dominant in the subpopulation of organisms which adhered to the HeLa cells. Once bound, Op organisms displayed very low levels of detachment. Adherent Op gonococci exhibited generation times up to threefold greater than cultures containing gonococci in the absence of HeLa cells. In addition, the progeny of adherent Op organisms remained bound to the HeLa cell monolayer. Both piliated (P+) and transparent (Tr) colony types attached to HeLa cells, but their progeny were retained less efficiently. Gonococci bound to HeLa cells were subjected to the bactericidal action of fresh rat serum and approximately 0.5 to 2.5% survived, irrespective of their opacity or piliation phenotype. Incubation with gentamicin resulted in a 10- to 50-fold further reduction in viability. Pretreatment of HeLa cell monolayers with the microfilament-disrupting agent cytochalasin b diminished gonococcal survival in either serum or gentamicin by up to eightfold. In contrast, cytochalasin b treatment did not decrease survival of the commensal organism N. sicca. The data suggest that very few gonococci are completely interiorized and a small proportion of adherent gonococci are partially protected from the soluble-phase environment by HeLa cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodeur B. R., Johnson W. M., Johnson K. G., Diena B. B. In vitro interaction of Neisseria gonorrhoeae type 1 and type 4 with tissue culture cells. Infect Immun. 1977 Feb;15(2):560–567. doi: 10.1128/iai.15.2.560-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Fox R. I., Polyzonis M., Allison A. C., Haswell A. D. The inhibition of phagocytosis and facilitation of exocytosis in rabbit polymorphonuclear leukocytes by cytochalasin B. Lab Invest. 1973 Jan;28(1):16–22. [PubMed] [Google Scholar]
- Draper D. L., James J. F., Brooks G. F., Sweet R. L. Comparison of virulence markers of peritoneal and fallopian tube isolates with endocervical Neisseria gonorrhoeae isolates from women with acute salpingitis. Infect Immun. 1980 Mar;27(3):882–888. doi: 10.1128/iai.27.3.882-888.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans B. A. Ultrastructural study of cervical gonorrhea. J Infect Dis. 1977 Aug;136(2):248–255. doi: 10.1093/infdis/136.2.248. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Washington O., Gemski P., Formal S. B. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J Infect Dis. 1973 Jul;128(1):69–75. doi: 10.1093/infdis/128.1.69. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E., Nilsson L. Endocytosis of Salmonella typhimurium 395 MS and MR10 by HeLa cells. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):322–328. doi: 10.1111/j.1699-0463.1977.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Interaction of intraleukocytic bacteria and antibiotics. J Clin Invest. 1973 Jul;52(7):1673–1679. doi: 10.1172/JCI107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Stephens D. S., Hoffman L. H., Schlech W. F., 3rd, Horn R. G. Mechanisms of mucosal invasion by pathogenic Neisseria. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S708–S714. doi: 10.1093/clinids/5.supplement_4.s708. [DOI] [PubMed] [Google Scholar]
- Ota F., Pontefract R., Ashton F. E., Diena B. B. Studies on gonococcal infection. II. Attachment and fate of gonococci in tissue-culture cells. Can J Microbiol. 1975 Nov;21(11):1698–1704. doi: 10.1139/m75-249. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Lin S. Cytochalasin B, its interaction with actin and actomyosin from muscle (cell movement-microfilaments-rabbit striated muscle). Proc Natl Acad Sci U S A. 1972 Feb;69(2):442–446. doi: 10.1073/pnas.69.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Nickel P., Meyer T. F., So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984 Jun;37(2):447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Sugasawara R. J., Cannon J. G., Black W. J., Nachamkin I., Sweet R. L., Brooks G. F. Inhibition of Neisseria gonorrhoeae attachment to HeLa cells with monoclonal antibody directed against a protein II. Infect Immun. 1983 Dec;42(3):980–985. doi: 10.1128/iai.42.3.980-985.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Bergström S., Barrera O., Robbins K., Corwin D. Pilus- gonococcal variants. Evidence for multiple forms of piliation control. J Exp Med. 1985 Aug 1;162(2):729–744. doi: 10.1084/jem.162.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Goldschneider I. The serum bactericidal system: ultrastructural changes in Neisseria meningitidis exposed to normal rat serum. J Exp Med. 1969 Jan 1;129(1):51–79. doi: 10.1084/jem.129.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Gonococcal adherence: selected topics. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S678–S684. doi: 10.1093/clinids/5.supplement_4.s678. [DOI] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Quan A. L., Rene A. A., Weiss E. Phase transition of gonococci in mammalian cell cultures. Infect Immun. 1974 Dec;10(6):1401–1411. doi: 10.1128/iai.10.6.1401-1411.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitkins S. A., Flynn J. Intracellular growth and type variation of Neisseria gonorrhoeae in tissue cell-cultures. J Med Microbiol. 1973 Aug;6(3):399–403. doi: 10.1099/00222615-6-3-399. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J. Adherence of Neisseria gonorrhoeae to urethral mucosal cells: an electron-microscopic study of human gonorrhea. J Infect Dis. 1972 Dec;126(6):601–605. doi: 10.1093/infdis/126.6.601. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]


