Abstract
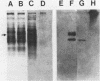
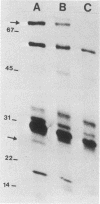
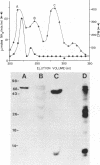
Providencia stuartii was the most prevalent isolate recovered from urine specimens taken weekly over a 1-year period from 51 nursing home patients with urinary catheters in place. Thirty percent of the isolates were urease positive. Urease, which is implicated in renal stone formation, was shown to be transmissible on an 82-kilobase conjugative plasmid in one isolate. Plasmid DNA isolated from this strain was digested with EcoRI, ligated into the EcoRI site of pBR322, and used to transform Escherichia coli HB101. Ampicillin-resistant clones were replica plated onto urea segregation agar, and a urease-positive clone, designated pMID101, was isolated. Recombinant and native urease from cell lysates had identical electrophoretic mobilities on nondenaturing polyacrylamide urease activity gels. The native enzyme was induced fourfold when cells were grown in the presence of 0.1% urea and had a km of 9.4 mM and a Vmax of 3.2 mumol of NH3 per min per mg of protein. Its molecular weight was estimated to be 375,000 +/- 35,000 by Sephacryl S-300 chromatography. The enzyme was cytoplasmic in P. stuartii, was inhibited in vitro by hydroxyurea, acetohydroxamic acid, and EDTA, and appears to have a complex subunit structure and a unique molecular size within genera of the Proteeae tribe.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler L. W., Ichikawa T., Hasan S. M., Tsuchiya T., Rosen B. P. Orientation of the protonmotive force in membrane vesicles of Escherichia coli. J Supramol Struct. 1977;7(1):15–27. doi: 10.1002/jss.400070103. [DOI] [PubMed] [Google Scholar]
- Alagna L., Hasnain S. S., Piggott B., Williams D. J. The nickel ion environment in jack bean urease. Biochem J. 1984 Jun 1;220(2):591–595. doi: 10.1042/bj2200591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUDE A. I., SIEMIENSKI J. Role of bacterial urease in experimental pyelonephritis. J Bacteriol. 1960 Aug;80:171–179. doi: 10.1128/jb.80.2.171-179.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicmanec J. F., Helmers S. L., Evans A. T. Office practice survey of urease positive bacterial pathogens causing urinary tract infections. Urology. 1980 Sep;16(3):274–276. doi: 10.1016/0090-4295(80)90041-2. [DOI] [PubMed] [Google Scholar]
- Dixon N. E., Gazzola C., Asher C. J., Lee D. S., Blakeley R. L., Zerner B. Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the "abnormal" ultraviolet spectrum. The nickel content of jack beans. Can J Biochem. 1980 Jun;58(6):474–480. doi: 10.1139/o80-063. [DOI] [PubMed] [Google Scholar]
- Dixon N. E., Hinds J. A., Fihelly A. K., Gazzola C., Winzor D. J., Blakeley R. L., Zerner B. Jack bean urease (EC 3.5.1.5). IV. The molecular size and the mechanism of inhibition by hydroxamic acids. Spectrophotometric titration of enzymes with reversible inhibitors. Can J Biochem. 1980 Dec;58(12):1323–1334. doi: 10.1139/o80-180. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Hickman F. W., Brenner D. J., Schreiber M., Rickenbach D. G. Unusual Enterobacteriaceae. "Proteus rettgeri" that "change" into Providencia stuartii. J Clin Microbiol. 1977 Oct;6(4):373–378. doi: 10.1128/jcm.6.4.373-378.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Urease of Klebsiella aerogenes: control of its synthesis by glutamine synthetase. J Bacteriol. 1977 Aug;131(2):446–452. doi: 10.1128/jb.131.2.446-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO M. M., LIU P. V. SEROLOGICAL SPECIFICITIES OF UREASES OF PROTEUS SPECIES. J Gen Microbiol. 1965 Mar;38:417–422. doi: 10.1099/00221287-38-3-417. [DOI] [PubMed] [Google Scholar]
- Grant R. B., Penner J. L., Hennessy J. N., Jackowski B. J. Transferable urease activity in Providencia stuartii. J Clin Microbiol. 1981 Mar;13(3):561–565. doi: 10.1128/jcm.13.3.561-565.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Gargan R. A. Rapid screening for urease inhibitors. Invest Urol. 1979 Mar;16(5):327–328. [PubMed] [Google Scholar]
- Hickman-Brenner F. W., Farmer J. J., 3rd, Steigerwalt A. G., Brenner D. J. Providencia rustigianii: a new species in the family Enterobacteriaceae formerly known as Providencia alcalifaciens biogroup 3. J Clin Microbiol. 1983 Jun;17(6):1057–1060. doi: 10.1128/jcm.17.6.1057-1060.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W. J., Summers A. O. Polypeptides encoded by the mer operon. J Bacteriol. 1982 Feb;149(2):479–487. doi: 10.1128/jb.149.2.479-487.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D. B., Habets W. J., Marugg J. T., Van Der Drift C. Nitrogen control in Pseudomonas aeruginosa: mutants affected in the synthesis of glutamine synthetase, urease, and NADP-dependent glutamate dehydrogenase. J Bacteriol. 1982 Jul;151(1):22–28. doi: 10.1128/jb.151.1.22-28.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING J. A routine method for the estimation of lactic dehydrogenase activity. J Med Lab Technol. 1959 Oct;16:265–272. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacLaren D. M. The significance of urease in proteus pyelonephritis: a histological and biochemical study. J Pathol. 1969 Jan;97(1):43–49. doi: 10.1002/path.1710970107. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chen C. M., Silver S., Rosen B. P. Cloning and expression of R-factor mediated arsenate resistance in Escherichia coli. Mol Gen Genet. 1983;191(3):421–426. doi: 10.1007/BF00425757. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Fraiman M. H., Tenney J. H., Warren J. W. Variable phenotypes of Providencia stuartii due to plasmid-encoded traits. J Clin Microbiol. 1985 Nov;22(5):851–853. doi: 10.1128/jcm.22.5.851-853.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasaki H., Matsushima T., Sato S., Kawachi T. Purification and properties of alkaline phosphatase from the mucosa of rat small intestine. J Biochem. 1979 Nov;86(5):1225–1231. doi: 10.1093/oxfordjournals.jbchem.a132637. [DOI] [PubMed] [Google Scholar]
- Nikakhtar B., Vaziri N. D., Khonsari F., Gordon S., Mirahmadi M. D. Urolithiasis in patients with spinal cord injury. Paraplegia. 1981;19(6):363–366. doi: 10.1038/sc.1981.68. [DOI] [PubMed] [Google Scholar]
- Romano N., La Licata R. Cell fractions and enzymatic activities of Ureaplasma urealyticum. J Bacteriol. 1978 Dec;136(3):833–838. doi: 10.1128/jb.136.3.833-838.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Hamilton-Miller J. M., Brumfitt W. Role of urease in the formation of infection stones: comparison of ureases from different sources. Infect Immun. 1981 Apr;32(1):32–37. doi: 10.1128/iai.32.1.32-37.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Hamilton-Miller J. M. Inhibitors of urease as chemotherapeutic agents. Crit Rev Microbiol. 1984;11(1):1–12. doi: 10.3109/10408418409105901. [DOI] [PubMed] [Google Scholar]
- Rosenstein I., Hamilton-Miller J. M., Brumfitt W. The effect of acetohydroxamic acid on the induction of bacterial ureases. Invest Urol. 1980 Sep;18(2):112–114. [PubMed] [Google Scholar]
- Schlammadinger J., Szabó G. The effect of theophylline upon induced -galactosidase synthesis in Escherichia coli. Acta Microbiol Acad Sci Hung. 1971;18(1):55–59. [PubMed] [Google Scholar]
- Senior B. W., Bradford N. C., Simpson D. S. The ureases of Proteus strains in relation to virulence for the urinary tract. J Med Microbiol. 1980 Nov;13(4):507–512. doi: 10.1099/00222615-13-4-507. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Kobashi K., Yoshida O. Prevention of infected urinary stones in rats by urease inhibitor: a new hydroxamic acid derivative. Invest Urol. 1980 Sep;18(2):102–105. [PubMed] [Google Scholar]
- Takeuchi H., Takayama H., Konishi T., Tomoyoshi T. Scanning electron microscopy detects bacteria within infection stones. J Urol. 1984 Jul;132(1):67–69. doi: 10.1016/s0022-5347(17)49466-3. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Williams J. J., Rodman J. S., Peterson C. M. A randomized double-blind study of acetohydroxamic acid in struvite nephrolithiasis. N Engl J Med. 1984 Sep 20;311(12):760–764. doi: 10.1056/NEJM198409203111203. [DOI] [PubMed] [Google Scholar]