Abstract
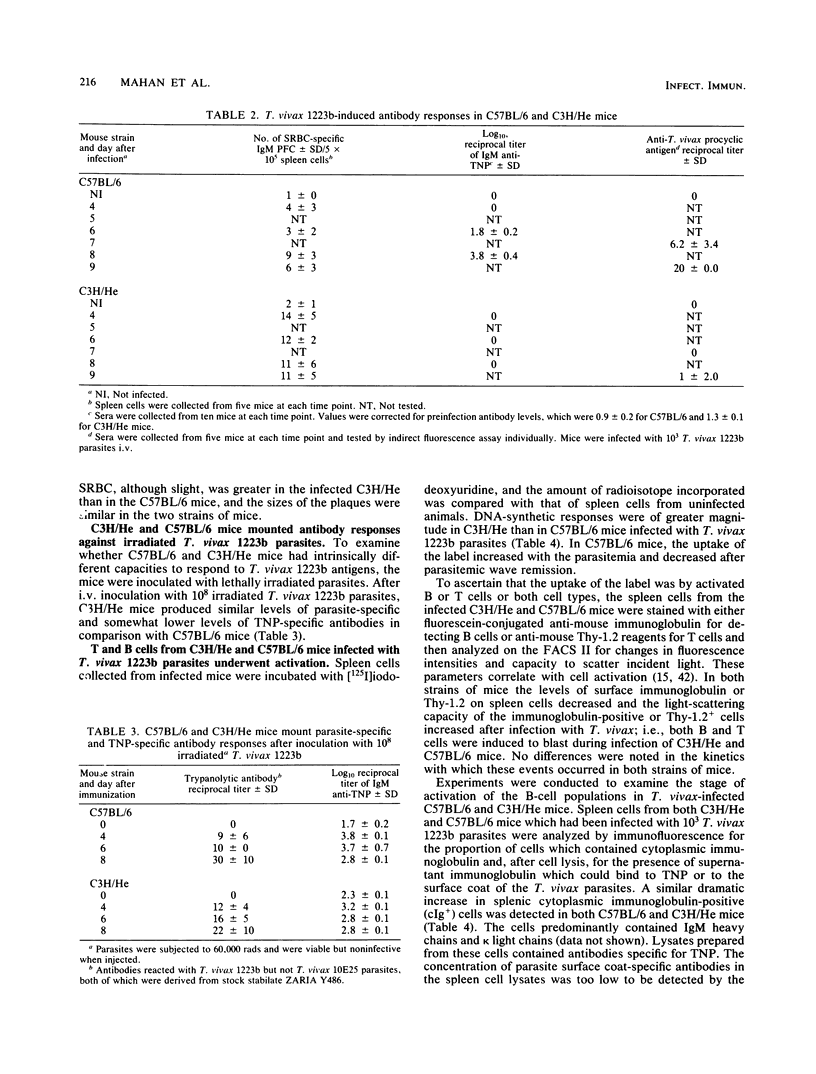
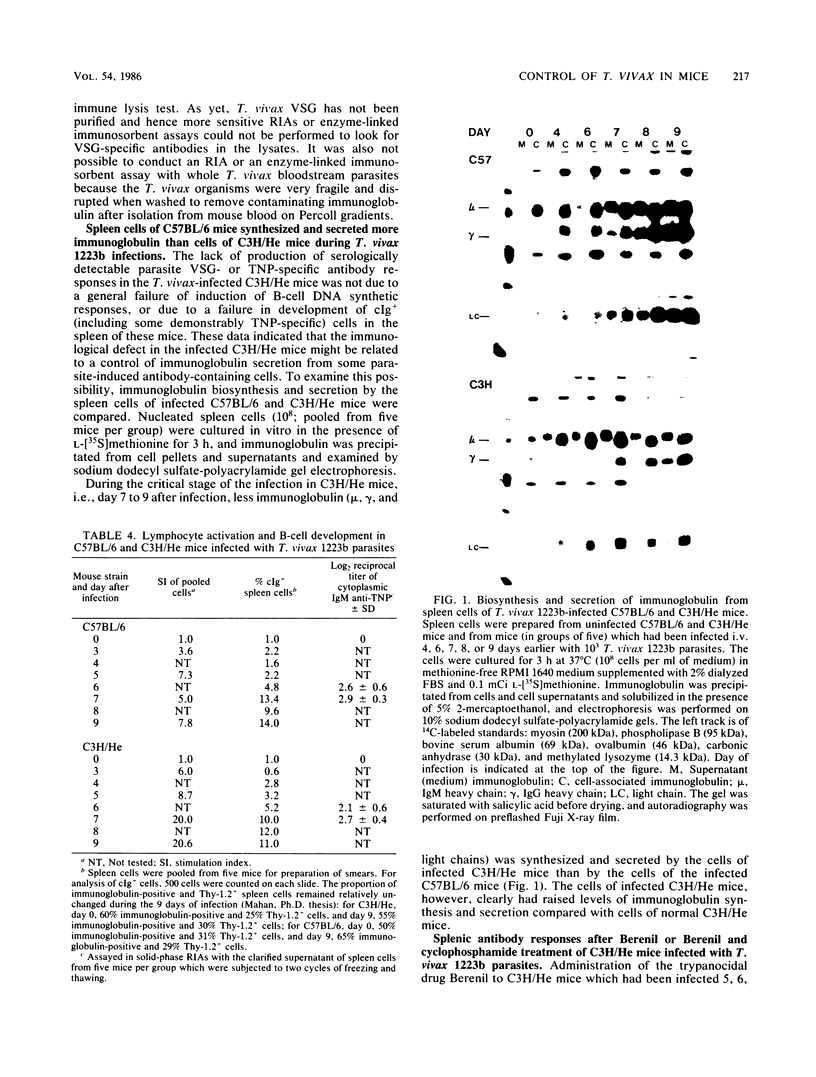
After infection with a cloned population of Trypanosoma vivax, C57BL/6 mice controlled parasitemia during the exponential growth phase and survived, with intermittent parasitemia, for several weeks. In contrast, most mice of the C3H/He strain did not control the first wave of parasitemia and died within 9 to 13 days after infection. Control of parasitemia in C57BL/6 mice was mediated by the production of a variant surface glycoprotein-specific trypanodestructive antibody response which was accompanied by production of antibodies against antigens shared between procyclic and bloodstream T. vivax as well as antibodies against trinitrophenyl (TNP) and sheep erythrocytes. The infected C3H/He mice did not produce trypanodestructive antibodies or antibodies against procyclic antigens or TNP but did produce antibodies against sheep erythrocytes. Although infected C57BL/6 mice produced levels of serum immunoglobulin M four times higher than infected C3H/He mice, their parasite-induced B-cell DNA synthetic responses were similar, and both sets of mice developed similar numbers of spleen cells with cytoplasmic immunoglobulin M, a proportion of which could react with TNP. In vitro biosynthetic labeling studies accompanied by immunoglobulin precipitation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis demonstrated that the immunoglobulin-containing cells of infected C3H/He mice synthesized and secreted less immunoglobulin than similar cells from infected C57BL/6 mice. We concluded that some parasite-induced antibody-forming cells in C3H/He mice, perhaps including parasite-specific and certainly including TNP-specific cells, had an impaired capacity to make and release immunoglobulin. Within 24 h after Berenil-mediated elimination of T. vivax from infected C3H/He mice, a population of cyclophosphamide-sensitive spleen cells produced large amounts of parasite-specific and TNP-specific antibody. We concluded that the defect in terminal B-cell function leading to suppressed parasite-specific and TNP-specific antibody responses was induced either by living trypanosomes or short-lived factors from degenerating trypanosomes or by short-lived parasite-induced host responses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHCROFT M. T. The polymorphism of Trypanosoma brucei and T. rhodesiense, its relation to relapses and remissions of infections in white rats, and the effect of cortisone. Ann Trop Med Parasitol. 1957 Sep;51(3):301–312. doi: 10.1080/00034983.1957.11685819. [DOI] [PubMed] [Google Scholar]
- Balber A. E., Bangs J. D., Jones S. M., Proia R. L. Inactivation or elimination of potentially trypanolytic, complement-activating immune complexes by pathogenic trypanosomes. Infect Immun. 1979 Jun;24(3):617–627. doi: 10.1128/iai.24.3.617-627.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balber A. E. Trypanosoma brucei: fluxes of the morphological variants in intact and X-irradiated mice. Exp Parasitol. 1972 Apr;31(2):307–319. doi: 10.1016/0014-4894(72)90122-1. [DOI] [PubMed] [Google Scholar]
- Black S. J., Sendashonga C. N., Lalor P. A., Whitelaw D. D., Jack R. M., Morrison W. I., Murray M. Regulation of the growth and differentiation of Trypanosoma (Trypanozoon) brucei brucei in resistant (C57Bl/6) and susceptible (C3H/He) mice. Parasite Immunol. 1983 Sep;5(5):465–478. doi: 10.1111/j.1365-3024.1983.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Black S. J., Sendashonga C. N., O'Brien C., Borowy N. K., Naessens M., Webster P., Murray M. Regulation of parasitaemia in mice infected with Trypanosoma brucei. Curr Top Microbiol Immunol. 1985;117:93–118. doi: 10.1007/978-3-642-70538-0_5. [DOI] [PubMed] [Google Scholar]
- Clayton C. E. Trypanosoma brucei: influence of host strain and parasite antigenic type on infections in mice. Exp Parasitol. 1978 Apr;44(2):202–208. doi: 10.1016/0014-4894(78)90099-1. [DOI] [PubMed] [Google Scholar]
- Cox H. W., Hayes M. M., Saleh S. M. Immunoconglutinin and suppression of an induced immune response by plasma from rats infected with Trypanosoma brucei rhodesiense. J Parasitol. 1984 Feb;70(1):57–62. [PubMed] [Google Scholar]
- DESOWITZ R. S., WATSON H. J. C. Studies on Trypanosoma vivax. III. Observations on the maintenance of a strain in white rats. Ann Trop Med Parasitol. 1952 May;46(1):92–100. [PubMed] [Google Scholar]
- Grab D. J., Bwayo J. J. Isopycnic isolation of African trypanosomes on Percoll gradients formed in situ. Acta Trop. 1982 Dec;39(4):363–366. [PubMed] [Google Scholar]
- Grosskinsky C. M., Ezekowitz R. A., Berton G., Gordon S., Askonas B. A. Macrophage activation in murine African trypanosomiasis. Infect Immun. 1983 Mar;39(3):1080–1086. doi: 10.1128/iai.39.3.1080-1086.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Terry R. J. Immunodepression and the course of infection of a chronic Trypanosoma brucei infection in mice. Parasite Immunol. 1979 Winter;1(4):317–326. doi: 10.1111/j.1365-3024.1979.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Jennings F. W., Whitelaw D. D., Urquhart G. M. The relationship between duration of infection with Trypanosoma brucei in mice and the efficacy of chemotherapy. Parasitology. 1977 Oct;75(2):143–153. doi: 10.1017/s0031182000062284. [DOI] [PubMed] [Google Scholar]
- Jones J. F., Hancock G. E. Trypanosomiasis in mice with naturally occurring immunodeficiencies. Infect Immun. 1983 Nov;42(2):848–851. doi: 10.1128/iai.42.2.848-851.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham S. M. Separation of trypanosomes from the blood of infected rats and mice by anion-exchangers. Nature. 1968 Jun 29;218(5148):1273–1274. doi: 10.1038/2181273a0. [DOI] [PubMed] [Google Scholar]
- Leeflang P., Buys J., Blotkamp C. Studies on Trypanosoma vivax: infectivity and serial maintenance of natural bovine isolates in mice. Int J Parasitol. 1976 Oct;6(5):413–417. doi: 10.1016/0020-7519(76)90027-8. [DOI] [PubMed] [Google Scholar]
- Levine R. F., Mansfield J. M. Genetics of resistance to the African trypanosomes. III. Variant-specific antibody responses of H-2-compatible resistant and susceptible mice. J Immunol. 1984 Sep;133(3):1564–1569. [PubMed] [Google Scholar]
- Luckins A. G. Effects of x-irradiation and cortisone treatment of albino rats on infections with Brucei-complex trypanosomes. Trans R Soc Trop Med Hyg. 1972;66(1):130–139. doi: 10.1016/0035-9203(72)90060-0. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mitchell L. A., Pearson T. W. Antibody responses in resistant and susceptible inbred mice infected with Trypanosoma congolense. Immunology. 1986 Feb;57(2):297–303. [PMC free article] [PubMed] [Google Scholar]
- Morrison W. I., Murray M. The role of humoral immune responses in determining susceptibility of A/J and C57BL/6 mice to infection with Trypanosoma congolense. Parasite Immunol. 1985 Jan;7(1):63–79. doi: 10.1111/j.1365-3024.1985.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Morrison W. I., Roelants G. E., Mayor-Withey K. S., Murray M. Susceptibility of inbred strains of mice to Trypanosoma congolense: correlation with changes in spleen lymphocyte populations. Clin Exp Immunol. 1978 Apr;32(1):25–40. [PMC free article] [PubMed] [Google Scholar]
- Murray M., Morrison W. I. Non-specific induction of increased resistance in mice to Trypanosoma congolense and Trypanosoma brucei by immunostimulants. Parasitology. 1979 Dec;79(3):349–366. doi: 10.1017/s0031182000053750. [DOI] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- Roelants G. E., Pearson T. W., Morrison W. I., Mayor-Withey K. S., Lundin L. B. Immune depression in trypanosome-infected mice. IV. Kinetics of suppression and alleviation by the trypanocidal drug Berenil. Clin Exp Immunol. 1979 Sep;37(3):457–469. [PMC free article] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Askonas B. A. Trypanosome-induced suppression of anti-parasite responses during experimental African trypanosomiasis. Eur J Immunol. 1980 Dec;10(12):971–974. doi: 10.1002/eji.1830101216. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Bancroft G., Evans W. H., Askonas B. A. Incubation of trypanosome-derived mitogenic and immunosuppressive products with peritoneal macrophages allows recovery of biological activities from soluble parasite fractions. Infect Immun. 1982 Apr;36(1):160–168. doi: 10.1128/iai.36.1.160-168.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkirk M. E., Sacks D. L. Trypanotolerance in inbred mice: an immunological basis for variation in susceptibility to infection with Trypanosoma brucei. Tropenmed Parasitol. 1980 Dec;31(4):435–438. [PubMed] [Google Scholar]
- Sendashonga C. N., Black S. J. Humoral responses against Trypanosoma brucei variable surface antigen are induced by degenerating parasites. Parasite Immunol. 1982 Jul;4(4):245–257. doi: 10.1111/j.1365-3024.1982.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Van Meirvenne N., Janssens P. G., Magnus E. Antigenic variation in syringe passaged populations of Trypanosoma (Trypanozoon) brucei. 1. Rationalization of the experimental approach. Ann Soc Belg Med Trop. 1975;55(1):1–23. [PubMed] [Google Scholar]
- Watson L. P., Scher I., Lee C. M., McKee A. E. Immunoglobulin determinants on the surface of mouse splenic lymphocytes from Trypanosoma musculi-infected mice. J Parasitol. 1982 Oct;68(5):774–782. [PubMed] [Google Scholar]
- Whitelaw D. D., MacAskill J. A., Holmes P. H., Jennings F. W., Urquhart G. M. Immune mechanisms in C57Bl mice genetically resistant to Trypanosoma congolense infection. I. Effects of immune modulation. Parasite Immunol. 1983 Jan;5(1):85–94. doi: 10.1111/j.1365-3024.1983.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Onodera M., Kato K., Kakinuma M., Kimura T., Richards F. F. Involvement of suppressor cells induced with membrane fractions of trypanosomes in immunosuppression of trypanosomiasis. Parasite Immunol. 1985 Mar;7(2):95–106. doi: 10.1111/j.1365-3024.1985.tb00062.x. [DOI] [PubMed] [Google Scholar]
- de Gee A. L., Shah S. D., Doyle J. J. Trypanosoma vivax: courses of infection with three stabilates in inbred mouse strains. Exp Parasitol. 1982 Aug;54(1):33–39. doi: 10.1016/0014-4894(82)90107-2. [DOI] [PubMed] [Google Scholar]