Abstract
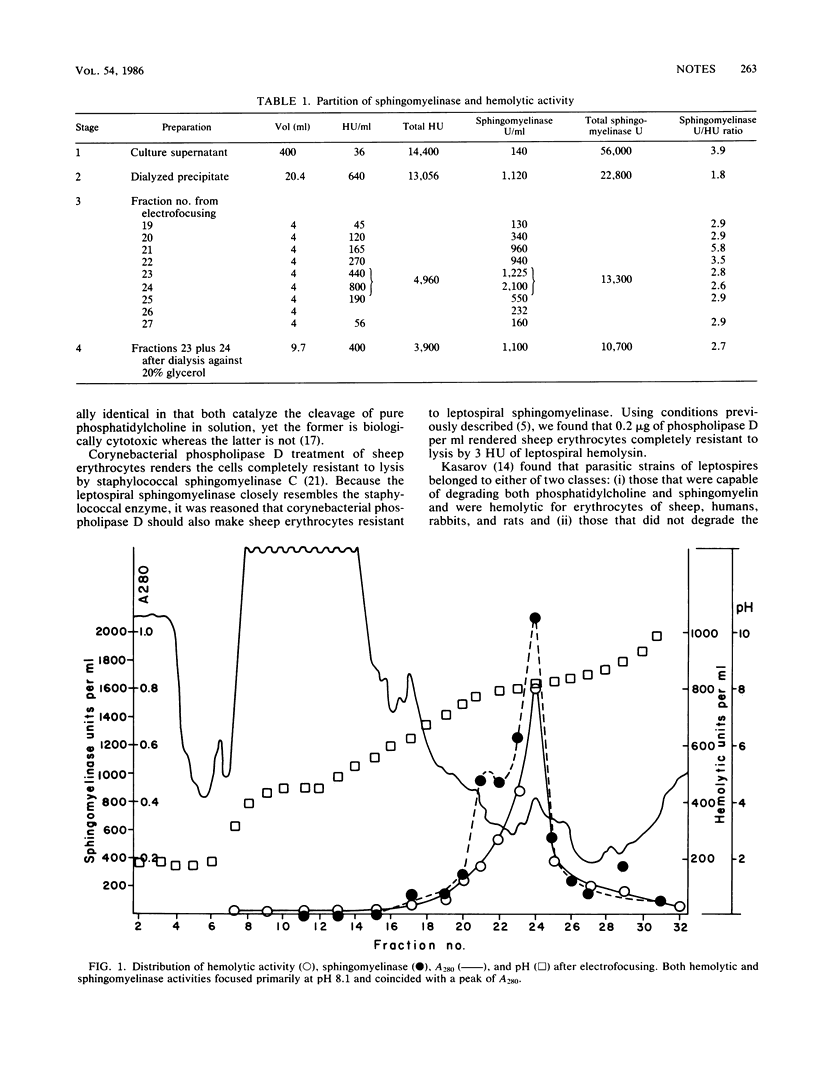
The hemolytic and sphingomyelinase C activities of supernatants of cultures of Leptospira interrogans serovar pomona tended to copurify when isoelectric fractionation was carried out. Both activities focused primarily at pH 8.1. Considered in conjunction with other circumstantial evidence, the results led to the conclusion that sphingomyelinase C is responsible for hemolysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER A. D., SMITH O. H., HIATT C. W., GLEISER C. A. Presence of hemolysin in cultures of pathogenic leptospires. Proc Soc Exp Biol Med. 1956 Feb;91(2):205–211. doi: 10.3181/00379727-91-22214. [DOI] [PubMed] [Google Scholar]
- BAUER D. C., EAMES L. N., SLEIGHT S. D., FERGUSON L. C. The significance of leptospiral hemolysin in the pathogenesis of Leptospira pomona infections. J Infect Dis. 1961 Mar-Apr;108:229–236. doi: 10.1093/infdis/108.2.229. [DOI] [PubMed] [Google Scholar]
- Bazovská S. Sfingomyelinázova aktivita leptospírových kultúr. Cesk Epidemiol Mikrobiol Imunol. 1978 Jun;27(3):137–143. [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S., Kim K. S. Staphylococcal sphingomyelinase (beta-hemolysin). Ann N Y Acad Sci. 1974 Jul 31;236(0):292–306. doi: 10.1111/j.1749-6632.1974.tb41499.x. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S. Properties of a toxin from the sea anemone Stoichacis helianthus, including specific binding to sphingomyelin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):467–471. doi: 10.1073/pnas.73.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey R. F., Auran N. E., Johnson R. C. Immunogenicity of whole cell and outer envelope leptospiral vaccines in hamsters. Infect Immun. 1974 Nov;10(5):1051–1056. doi: 10.1128/iai.10.5.1051-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey R. F., Johnson R. C. Protein-free and low-protein media for the cultivation of Leptospira. Infect Immun. 1978 Feb;19(2):562–569. doi: 10.1128/iai.19.2.562-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOERY H. M., MAGNUSSON B. J., CHEYNE I. M., SULASEKHARAM J. A phospholipase in staphylococcal toxin which hydrolyses sphingomyelin. Nature. 1963 Jun 15;198:1091–1092. doi: 10.1038/1981091a0. [DOI] [PubMed] [Google Scholar]
- Gal A. E., Brady R. O., Barranger J. A., Pentchev P. G. The diagnosis of type A and type B Niemann Pick disease and detection of carriers using leukocytes and a chromogenic analogue of sphingomyelin. Clin Chim Acta. 1980 May 21;104(1):129–132. doi: 10.1016/0009-8981(80)90143-6. [DOI] [PubMed] [Google Scholar]
- Gal A. E., Brady R. O., Hibbert S. R., Pentchev P. G. A practical chromogenic procedure for the detection of homozygotes and heterozygous carriers of Niemann-Pick disease. N Engl J Med. 1975 Sep 25;293(13):632–636. doi: 10.1056/NEJM197509252931304. [DOI] [PubMed] [Google Scholar]
- Kasărov L. B. Degradiation of the erythrocyte phospholipids and haemolysis of the erythrocytes of different animal species by leptospirae. J Med Microbiol. 1970 Feb;3(1):29–37. doi: 10.1099/00222615-3-1-29. [DOI] [PubMed] [Google Scholar]
- Kojima T., Yanagihara Y., Mifuchi I. Characterization of inhibitor to leptospiral hemolysin present in bovine serum. Microbiol Immunol. 1984;28(3):291–302. doi: 10.1111/j.1348-0421.1984.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Levina L. F., Volina E. G., Soboleva G. L., Arutiunova G. A. Sravnitel'noe izuchenie fosfolipazmoi aktivnosti patogennykh i saprofitnykh leptospir pri kul'tivirovanii na syvorotochno-letsitinovom agare. Zh Mikrobiol Epidemiol Immunobiol. 1983 Aug;(8):27–31. [PubMed] [Google Scholar]
- RUSSELL C. M. A hemolysin associated with leptospirae. J Immunol. 1956 Dec;77(6):405–409. [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucková A., Soucek A. Inhibition of the hemolytic action of and lysins of Staphylococcus pyogenes by Corynebacterium hemolyticum, C. ovis and C. ulcerans. Toxicon. 1972 Aug;10(5):501–509. doi: 10.1016/0041-0101(72)90176-6. [DOI] [PubMed] [Google Scholar]
- Trowbridge A. A., Green J. B., 3rd, Bonnett J. D., Shohet S. B., Ponnappa B. D., McCombs W. B., 3rd Hemolytic anemia associated with leptospirosis. Morphologic and lipid studies. Am J Clin Pathol. 1981 Oct;76(4):493–498. doi: 10.1093/ajcp/76.4.493. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Wadström T., Vesterberg K., Svensson H., Malmgren B. Studies on extracellular PROTEINS FROM Staphylococcus aureus. I. Separation and characterization of enzymes and toxins by isoelectric focusing. Biochim Biophys Acta. 1967 Apr 11;133(3):435–445. doi: 10.1016/0005-2795(67)90547-8. [DOI] [PubMed] [Google Scholar]
- Yanagihara Y., Kojima T., Mifuchi I. Hemolytic activity of Leptospira interrogans serovar canicola cultured in protein-free medium. Microbiol Immunol. 1982;26(7):547–556. [PubMed] [Google Scholar]


