Abstract
Muscle atrophy with aging or disuse is associated with deregulated iron homeostasis and increased oxidative stress likely inflicting damage to nucleic acids. Therefore, we investigated RNA and DNA oxidation, and iron homeostasis in gastrocnemius muscles. Disuse atrophy was induced in 6- and 32-month old male Fischer 344/Brown Norway rats by 14 days of hind limb suspension (HS). We show that RNA, but not DNA, oxidative damage increased 85% with age and 36% with HS in aged muscle. Additionally, non-heme iron levels increased 233% with aging and 83% with HS at old age, while staining for free iron was strongest in the smallest fibers. Simultaneously, the mRNA abundance of transferrin receptor-1 decreased by 80% with age and 48% with HS for young animals, while that of the hepcidin regulator hemojuvelin decreased 37% with age, but increased about 44% with disuse, indicating a dysregulation of iron homeostasis favoring increased intracellular free iron in atrophied muscles. RNA and DNA concentrations increased with age and were negatively correlated with muscle mass, whereas protein concentrations decreased with aging, indicating a preferential loss of protein compared to nucleic acids. Furthermore, xanthine oxidase activity increased with age, but not with HS, while mRNA abundance of the Y box-binding protein-1, which has been suggested to bind oxidized RNA, did not change with age or HS. These results suggest that RNA oxidation, possibly mediated by increased non-heme iron, might contribute to muscle atrophy due to disuse particularly in aged muscle.
Keywords: gastrocnemius, hemojuvelin (HJV), RNA oxidation, sarcopenia, transferrin receptor 1 (TfR1), Y box binding protein 1 (YB-1), xanthine oxidase (XOD)
Introduction
Aging is associated with a progressive loss of muscle mass and strength (Brooks and Faulkner, 1994; Frontera et al., 2000; Lexell, 1993), known as sarcopenia, which represents an important risk factor for disability and mortality (Metter et al., 2002). In addition, muscles from older individuals display an impaired capability to recover from atrophy induced by disuse (Chakravarthy et al., 2000; Gallegly et al., 2004; Zarzhevsky et al., 2001), which poses them at an even higher risk for impaired function in case of prolonged inactivity. One common phenomenon associated with age- and disuse-induced muscle atrophy is an increase in apoptosis and oxidative stress (Alway et al., 2003; Arbogast et al., 2007; Dirks and Leeuwenburgh, 2002; Lawler et al., 2003; Leeuwenburgh et al., 2005; Powers et al., 2005). It has been shown that atrophy induced by hind limb suspension (HS) or immobilization is associated with a disruption of antioxidant status (Lawler et al., 2003), and it has been suggested that iron plays an important role in this process (Kondo et al., 1992; Powers et al., 2005).
In some diseases and with aging, the level of oxidants and transition metals rise, resulting in cellular toxic stress. The balance between the generation and removal or repair of oxidative damage may be impaired with age- and disuse-induced muscle atrophy resulting in a total increase in reactive oxygen species (ROS) mediated damage. Even if ROS levels remain constant with age, damage to biomolecules may still accumulate, because the removal of these molecules is likely impaired with advancing age. In this context, nucleic acids, being negatively charged, chelate transition metals to a certain degree (Wacker and Vallee, 1959), making them more susceptible to oxidation. In fact, a small amount of oxidizing intermediates such as H2O2 is constantly present and when in contact with trace amounts of reduced transition metals, can oxidize RNA and DNA (Hofer, 2001; Hofer et al., 2005). This produces miscoding or mutagenic adducts such as 8-oxo-7,8-dihydroguanosine (8-oxoGuo) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) in RNA and DNA, respectively (Hofer, 2001; Hofer et al., 2005; Hofer and Moller, 1998). DNA oxidation can give rise to mutations during replication (Cheng et al., 1992; Kuchino et al., 1987) or can lead to faulty transcription of messenger RNA (mRNA). While DNA is constantly checked for mistakes and errors by repair enzymes, it is still unclear how and to what extent damaged RNA is removed.
Excess catalytic iron (Fe2+) loosely bound to biomolecules inflicts oxidative damage and iron homeostasis is therefore tightly controlled. However, conditions such as aging may be associated with less perfect iron control, favoring toxicity. Proteins playing a central role in iron homeostasis are the transferrin receptor-1 (TfR1) and hemojuvelin (HJV), which is mainly expressed in heart and skeletal muscle (Rodriguez et al., 2007).
The total cellular RNA pool consists of cytoplasmic ribosomal RNA (rRNA) (70–80%), transfer (tRNA, 15%), nuclear rRNA precursors (4%), and messenger (mRNA, 1–5%), and the functional consequences of oxidation of the different RNA subsets are largely unknown (Bregeon and Sarasin, 2005; Dirks et al., 2006). A recent study indicates that oxidative damage to mRNA reduces translational fidelity and results in proteins that are readily removed by proteolytic degradation (Tanaka et al., 2007). Little is known about the consequences of tRNA or nuclear rRNA oxidation, but it has recently been shown that oxidized rRNA was associated with decreased protein synthesis (Honda et al., 2005). Interestingly, rRNA has been found to be significantly oxidized in age-related degenerative diseases (Honda et al., 2005; Liu et al., 2002; Zhang et al., 1999) and to have a particularly high affinity towards iron (higher than that shown by tRNA) (Zhang et al., 1999). Our previous studies showed increased oxidation of total RNA in aged rat livers (24 months) compared to young counterparts (6 months) (Seo et al., 2006), but to our knowledge, RNA oxidation in skeletal muscles atrophied due to aging or disuse has not yet been investigated. RNA oxidation may play a particularly important role in skeletal muscles with aging, because it may affect the balance between protein degradation and synthesis which ultimately determines total muscle mass and therefore sarcopenia (Honda et al., 2005; Tanaka et al., 2007).
Hence, the goal of this study was to investigate the levels of RNA and DNA oxidation in atrophying gastrocnemius muscles with aging and HS, and to identify possible sources of oxidative stress. We hypothesized that RNA oxidation would be increased in aged atrophied muscles and that this was associated with changes in iron homeostasis. Disuse atrophy was induced in young and old rats by HS and molecules involved in ROS and iron homeostasis were measured.
Materials and Methods
Animals and experimental procedures
All procedures were performed in accordance with institutional guidelines for the care and use of laboratory animals. Male Fischer 344 × Brown Norway rats (6 and 32 months of age) were purchased from the National Institute on Aging. This strain of rat was chosen because it has increased longevity and decreased cumulative lesion incidence compared to other strains; therefore, aging aspects can be studied in the relative absence of disease (Lipman et al., 1996). The different ages were chosen to reflect a mature rat (young = 6 months) and an aged rat at about 50% mortality (old = 32 months). Rats of both ages were divided into 2 groups (n = 6 per group): non-suspended control and hind limb suspended for 14 days. Rats were allowed free access to food and water. Animals were housed in a 12:12 hr light:dark cycle and were allowed free access to food and water. HS was performed as previously described (Gallegly et al., 2004; Leeuwenburgh et al., 2005). Briefly, a tail device containing a hook was attached with gauze and cynoacrylate glue while the animals were anesthetized with ketamine and xylazine (60 and 10 mg/kg body weight, respectively, by intraperitoneal injection). After the animal regained consciousness, the tail device was connected via a thin cable to a pulley sliding on a vertically adjustable stainless steel bar running longitudinally above a high-sided cage. The system was designed in such a way that the rats could not rest their hind limbs against any side of the cage. After 14 days of control housing or HS, rats were euthanized with an overdose of sodium pentobarbital (100 mg/kg). Gastrocnemius muscles were carefully dissected, weighed, frozen in liquid nitrogen, and stored in −80°C until analysis. Muscle weights are reported as the average between the two legs. Gastrocnemius muscles were selected for analysis, because they contain a variety of muscle fiber types and the results may therefore be generalized to other muscles. Also, the substantial amount of tissue in gastrocnemius muscle allowed us to perform all the analyses on the same muscle type.
Measurement of RNA and DNA oxidation and yields using HPLC-ECD
Total RNA and DNA oxidation levels, nucleic acid yields, and RNA/DNA ratios of gastrocnemius muscles were analyzed simultaneously using a novel HPLC-ECD method (Hofer et al., 2006). This procedure is based on high-salt nucleic acid release from proteins, followed by removal of proteins/fats by organic solvents at neutral pH, all in the presence of the metal chelator deferoxamine mesylate (DFOM; affinity constant for Fe(III): log K = 30.8) at 0°C. Briefly, muscles were dissected, frozen in liquid N2, stored at −80°C, thawed, stripped for tendons on ice, weighed (200–230 mg), minced, and homogenized on slush ice in using a glass-glass Duall homogenizer in 4 ml (1:20 w:v) guanidine thiocyanate (GTC) buffer (3 M GTC, 0.2% (w/v) N-lauroylsarcosinate, 20 mM Tris, pH 7.5) containing 10 mM freshly dissolved DFOM. After transferring the solution into phase-lock gel (PLG) tubes, an equal volume of phenol/chloroform/isoamyl alcohol (25:24:1, pH 6.7) was added and the samples were immediately vortexed, followed by a 10 min vortexing period at 0°C to completely release nucleic acids as previously described (Hofer et al., 2006). After centrifugation (4,500 × g, 5 min, 0°C), the aqueous phase was transferred into a new PLG tube and extracted against an equal volume of chloroform/isoamyl alcohol (24:1). The samples were hand-shaken, centrifuged, and the aqueous phase was collected and nucleic acids precipitated with an equal amount of isopropanol at −80°C over night. After centrifugation (10,000 × g, 10 min, 0°C), nucleic acids were washed in 70% (v/v) ethanol (0°C), dried, dissolved in water containing 30 µM DFOM, and hydrolyzed using 4 U nuclease P1 and 5 U alkaline phosphatase in buffer (30 mM sodium acetate, 20 µM ZnCl2, pH 5.3, final volume 100 µl) at 50°C for 60 min. After filtration, the samples were analyzed by high-performance liquid chromatography coupled to electrochemical and UV detection (HPLC-ECD) as described (Hofer et al., 2006).
Non-heme iron determination
The level of non-heme iron was measured in gastrocnemius muscles, because it appears to contribute to the generation of oxidative stress in immobilized skeletal muscle (Kondo et al., 1992; Powers et al., 2007). The gastrocnemius non-heme iron content was measured as described by Rebouche et al. (Rebouche et al., 2004). Briefly, muscle pieces (~50 mg) were homogenized in water (1:10 w:v) using a glass-glass Duall homogenizer on slush ice. An equal amount of an iron releasing and protein precipitating solution (1 N HCl and 10% (v/v) trichloroacetic acid) was added to an aliquot (80 µl) of the homogenates, a blank (water), or the iron standards, and the samples were incubated at 95°C for 60 min. Following centrifugation (10,000 × g, 10 min, room temperature) to remove heme-containing proteins, the supernatant was collected and divided into two fractions: one to which an equal amount of sample blank solution (1.5 M sodium acetate and 0.1% (v/v) thioglycolic acid) was added, and one to which a chromogen solution (0.508 mM ferrozine, 1.5 M sodium acetate, 0.1% (v/v) thioglycolic acid) was added. The samples were incubated at room temperature for 30 min for color development, and the absorbance was read at 562 nm in a quartz cuvette using a Beckman DU 640 spectrophotometer. A commercially available iron standard (High-Purity Standards, Charleston, SC, cat. no. 100026-1) was diluted to 2, 4, 6, 8, 10 µg Fe/ml in deionized water. After correction for sample blanks, the iron content was calibrated against the iron standard curve and the tissue non-heme iron content calculated as µg iron per g of muscle.
In addition, free iron content was visualized histologically to identify which fibers displayed the highest iron content, using an iron detection kit as specified by the manufacturer’s instructions (Stressgen Biotechnologies, Victoria, BC, Canada). Gastrocnemius muscle cross sections were cut on a cryostat at 7 µm thickness, air dried, and stored at −20°C until analysis. Sections were rehydrated in phosphate buffered saline and fixed in methacarn (60% absolute methanol, 30% chloroform, 10% glacial acetic acid (v/v)) for 20 min at room temperature. After washes, the iron III reagent was added to the sections and incubated for 2 h at 37°C, followed by 3 rinses in Wash Buffer 1 and one rinse in Wash Buffer 2 (solutions supplied by Stressgen). The substrate solution was added to sections and incubated for 10 min. Sections were then washed once in Wash Buffer 2, dehydrated with alcohol and xylene, and cover slipped. Pictures were taken at the same magnification for the different groups with a Zeiss AxioCam camera (Peabody, MA).
Determination of mRNA abundance
The mRNA abundance of genes associated with iron homeostasis was measured to investigate whether this process was affected by age and HS, and mRNA abundance of Y-box protein (YB-1) was measured because it has been shown to bind oxidized RNA. Gastrocnemius muscle samples (~100 mg) were taken from liquid N2, directly immersed into ice-cold TriReagent™ (Sigma, St. Louis, MO), and immediately homogenized using a glass-glass Duall homogenizer on slush ice. Total RNA was isolated from the homogenate by using TriReagent™ according to the manufacturer’s protocol. Residual DNA was removed by using the Turbo DNA-free kit (Ambion, Austin, TX). Integrity of the isolated and purified RNA was confirmed by denaturing agarose gel electrophoresis. Transcript abundance of rat transferrin receptor 1 (TfR1), hemojuvelin (HJV), YB-1, and 18S was determined by using quantitative RT-PCR as described previously (Dupont-Versteegden et al., 2004). Briefly, RNA was converted to cDNA by using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). PCR was performed by using the cDNA plus gene-specific primers: YB-1 (sense: 5’ AAGATGGCAAAGAGACAAAAGCA 3’; antisense: 5’CGGGAGCGGACGAATTC 3’), TfR1 (sense: 5’ GAATACGTTCCCCGTTGTTGA 3’; antisense: 5’ATCCCCAGTTCCTAGATGAGCAT 3’), HJV (sense: 5’ GGGAGCCACGTGGAGATTC 3’; antisense: 5’ GCTGTCTGACGAACGATTATAGTT 3’), 18S (sense: 5’ CGAGGAATTCCCAGTAAGTGC 3’; antisense: 5’CCATCCAATCGCTAGTAGCG 3’). For PCR, Power SYBR Green PCR Master Mix (Applied Biosystems) was used with universal cycling conditions on an Applied Biosystems 7300 Real-Time PCR System. Dissociation curve analysis of PCR products revealed single peaks, indicating specific amplification products. Relative quantitation of TfR1 and YB-1 was performed using standard curves with 18S rRNA as the normalizer for each sample. No differences in 18S rRNA were found between the groups. Average values for the 6-month control group were set to 1 and values of the other groups were calculated relative to the 6-month old control group.
Xanthine Oxidase (XOD) activity measurements
Since XOD was suggested to be involved in the increase in ROS in atrophied muscles we measured its activity in the muscles (Kondo et al., 1993). Cleaned gastrocnemius muscles were homogenized in 1 mM EDTA, 1 mM PMSF, and 100 mM tris, pH 8.1 (1:5 w:v), with a homogenizer on slush ice. After centrifugation (15,000 × g, 20 min, +4°C) the supernatant was collected and analyzed for superoxide production (30 min, 37°C) using xanthine as substrate according to the kit instructions from the Amplex® Red Xanthine Oxidase Assay kit (Molecular Probes, Eugene, OR prod. nr. A22182). Data were calibrated against commercial XOD (Sigma) activity, reading the absorbance at 560 nm using a Synergy HT microplate reader from BIO-TEK Instruments (Winooshi, VT). H2O2 was used as a positive control. The XOD activity was normalized to total protein content as determined by the Bradford method.
Analysis of myeloperoxidase (MPO) activity
We measured whether oxidation was a consequence of increased inflammation often seen in old animals and individuals, by quantifying muscle MPO activity. Analysis of MPO activity was performed as described by Bradley et al. (Bradley et al., 1982). Briefly, 40–50 mg gastrocnemius muscle was homogenized in buffer (1:10, w:v, 50 mM potassium phosphate, pH 6.0) to which 0.5% (w/v) HTAB (hexadecyltrimethylammonium bromide) had been freshly added) using a Duall glass-glass homogenizer on slush ice. The homogenate was transferred to a vacuum-sealable tube and centrifuged at 41,400 × g using a Beckman ultracentrifuge for 10 min at +4°C. After removing the supernatant, the pellet was resuspended in homogenization buffer using half of the initial volume and transferred into a cryovial. To release MPO from neutrophils’ granules, the samples were sonicated on ice for 10 s and freeze-thawed in liquid nitrogen three times, then sonicated on ice again for 10 s. After centrifugation at 18,200 × g for 60 min, +4°C, the supernatant was transferred to a new tube and the MPO activity was immediately assessed using the chlorination assay described in the EnzChek® MPO Activity Assay kit from Molecular Probes (Eugene, OR) reading the emission at 530 nm after excitation at 485 nm using a Synergy HT microplate reader.
Statistical analysis
To test for statistically significant differences between the groups, two-way ANOVA was used. When significant F-ratios were observed, a Bonferroni multiple comparisons test was applied to test individual means. Pearson’s correlation test was used to determine significant correlations between data sets. Statistical significance was assumed at p < 0.05 and results are expressed as mean ± SEM.
Results
Muscle atrophy associated with age and HS
Aging was associated with a decrease in gastrocnemius muscle weight (p < 0.0001) and muscle to body weight ratio (p < 0.0001) by 42% and 53% respectively, indicating the occurrence of sarcopenia in these animals (Table 1). HS was associated with a 36% decrease in muscle mass (p < 0.001) and 23% in muscle to body weight ratio (p < 0.001) at 6 months of age, and at 32 months of age muscle mass decreased 23% (p < 0.05), while muscle weight to body weight ratio was 15% lower (p < 0.05) (Table 1). Therefore, muscles at both ages responded similarly to disuse (HS) as had been previously shown for soleus muscle (Leeuwenburgh et al., 2005).
Table 1.
Changes in muscle size with age and hind limb suspension
| 6 months | 32 months | |||
|---|---|---|---|---|
| Control | HS | Control | HS | |
| muscle weight (mg) | 2077.6 ± 94.8 | 1332.9 ± 39.0* | 1214.9 ± 84.0# | 940.6 ± 26.5* # |
| MW/BW (mg/g) | 5.222 ± 0.039 | 4.083 ± 0.050* | 2.473 ± 0.169# | 2.121 ± 0.085*# |
Values are means ± SE.
Significant difference from control at the same age.
Significant difference from 6 months within the experimental group. Significance assumed at p < 0.05. MW/BW = muscle weight to body weight ratio.
RNA, but not DNA oxidation increases with atrophy
RNA oxidation was elevated by 85% in gastrocnemius muscles from old rats compared to young rats (p < 0.0001). Two weeks of disuse had a significant treatment effect (p=0.011), and the old HS rats displayed a 36% increase in RNA oxidation (p < 0.05) over the old control rats (Figure 1a). Aging was associated with a small (21% for control groups) increase in DNA oxidation, which was not statistically significant, while HS had no measurable effect on DNA oxidation (Figure 1b).
Figure 1. Total RNA oxidation increases with atrophy in rat gastrocnemius muscle.

RNA (A) and DNA (B) oxidation in gastrocnemius muscles of 6 and 32 month (mo) old control (unfilled bars) and hind limb suspended (HS; hatched bars) rats is shown. For RNA (A), a main effect for age and HS was observed. The * indicates a significant difference for HS within the age group.
The methodology used for measuring RNA and DNA oxidation also produces data on the total RNA and DNA yields of the tissue samples. We found that the RNA and DNA yield from gastrocnemius muscles was significantly elevated by 34% (p < 0.001) and 211% (p < 0.0001), respectively, in old compared to young control rats, but no effect of HS was observed on RNA yield (Table 2). However, the DNA yield was elevated in gastrocnemius muscles from young rats in response to HS (p < 0.05). These data indicate that the loss of protein in muscles from aged and HS animals may exceed that of nuclei. We therefore measured total protein concentration and found that it was indeed 9.5% lower in gastrocnemius muscle from old compared to young rats (p < 0.0001) (Table 2). This confirms a previous report showing that during atrophy the loss of protein and nuclei is not directly proportional and muscles have a decreased protein-to-DNA ratio or smaller nuclear domain following a period of disuse (Gallegly et al., 2004).
Table 2.
Nucleic acid and total protein concentration in gastrocnemius muscle
| 6 months | 32 months | |||
|---|---|---|---|---|
| Control | HS | Control | HS | |
| Total protein concentration (mg/ml) | 48.1 ± 0.38 | 49.0 ± 1.0 | 44.1 ± 0.98# | 43.7 ± 0.78# |
| RNA yield (µg/mg muscle) | 0.689 ± 0.039 | 0.712 ± 0.058 | 0.926 ± 0.070# | 0.961 ± 0.057# |
| DNA yield (µg/mg muscle) | 0.108 ± 0.0092 | 0.179 ± 0.012* | 0.228 ± 0.024# | 0.260 ± 0.019# |
| YB-1/18S | 1.000 ± 0.072 | 0.943 ± 0.12 | 1.007 ± 0.074 | 1.290 ± 0.089 |
Values are means ± SE.
Significant difference from control at the same age.
Significant difference from 6 months within the experimental group. Significance assumed at p < 0.05.
Intracellular iron homeostasis is altered with atrophy
Elevated levels of iron, which is both an essential nutrient and a potential toxin if in excess (Cairo et al., 2002; Ponka and Lok, 1999), are associated with increased oxidative stress in cells (Halliwell and Gutteridge, 1999; Honda et al., 2005; Kruszewski, 2003), and this could be a possible cause of the observed elevation in RNA oxidation. We therefore measured tissue non-heme iron, which mainly represents iron storage in cytosolic ferritin. We also determined transferrin receptor 1 (TfR1) mRNA levels, which vary inversely with the level of intracellular labile iron (Ponka and Lok, 1999), and hemojuvelin (HJV) mRNA abundance, since it has been shown to modulate the expression of hepcidin (Lin et al., 2005), the master regulator of whole-body iron homeostasis (Nemeth and Ganz, 2006). Non-heme iron levels in gastrocnemius muscles were increased by 233% with age (p < 0.0001) and, interestingly, with HS for old, but not young rats (p < 0.01) (Figure 2a). Increased levels of intracellular iron are associated with decreased levels of TfR1 mRNA and we found that mRNA abundance of TfR1 in gastrocnemius muscles was indeed decreased by 80% with age (p < 0.001) and by 48% with HS in young rats (p < 0.01) (Figure 2b). Moreover, HJV mRNA decreased by 37% with age in the controls (p < 0.01), but increased by 36% and 52% with HS in young and old rats, respectively (p < 0.01) (Figure 2c).
Figure 2. Changes in non-heme iron, TfR1, and HJV mRNA levels with atrophy.
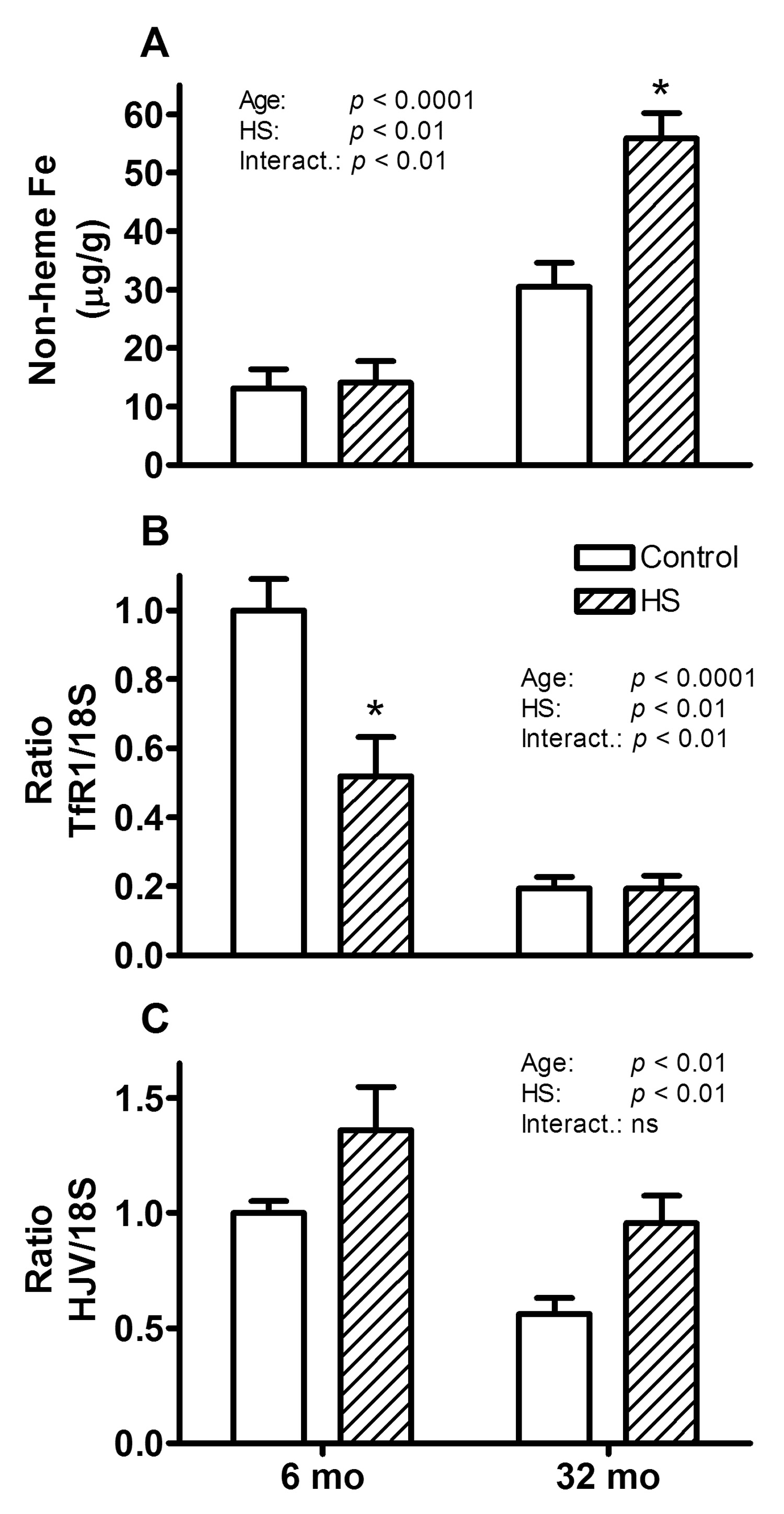
Non-heme iron concentration (A) and relative TfR1 (B) and HJV (C) mRNA levels of gastrocnemius muscles from 6 and 32 month (mo) old control (open bars) and HS (hatched bars) rats are shown. For all three parameters, a main effect for age and HS was observed. The * indicates a significant difference for HS within the age group.
Significant correlations point to possible link between iron-induced RNA oxidation and atrophy
The most relevant correlation observed was that between RNA oxidation levels and muscle mass (Figure 3a; r = − 0.84; p < 0.0001). This indicates that RNA oxidation is strongly associated with muscle atrophy due to either age or disuse, and that the causality of this relationship warrants further investigation. Further, non-heme iron concentrations were also strongly correlated with muscle mass due to either aging or disuse (Figure 3b: r = − 0.78; p < 0.0001), leading to a very strong correlation between RNA oxidation and non-heme iron concentration (Figure 3c; r = 0.85; p < 0.0001).
Figure 3. Muscle mass is correlated with RNA oxidation and iron levels.

Correlation between muscle weight to body weight ratio (MW/BW) and RNA oxidation in gastrocnemius muscle (A): Pearson’s coefficient: r = − 0.84 (p < 0.0001). Correlation between muscle weight to body weight ratio (MW/BW) and non-heme iron (Fe) in gastrocnemius muscles (B); Pearson’s correlation: r = − 0.78 (p < 0.0001). Correlation between non-heme iron (Fe) and RNA oxidation in gastrocnemius muscles (C); Pearson’s coefficient: r = 0.85 (p < 0.0001).
To visualize whether iron content was indeed increased in atrophied fibers, gastrocnemius muscle cross sections were stained for free iron. Smaller fibers were observed following HS in gastrocnemius muscles from both young and old muscles (Figure 4B and 4D, respectively). Interestingly, in smaller fibers, mainly in muscles from old animals, content of free iron was elevated and this was particularly true for muscles from old HS rats (Figure 4D). These findings compliment the free iron measurements above and suggest a correlation between fiber size and free iron content.
Figure 4. Free iron content in muscle fibers.

Representative histology pictures showing free iron content and muscle atrophy of the four groups. Gastrocnemius muscle cross sections of young (6 mo: A and B) and old (32 mo: C and D) control (A and C) and hind limb suspended (HS: B and D) rats were stained for free iron. Bar in D for all pictures 25µm.
Changes in sources of oxidants and removal mechanisms of RNA oxidation
XOD is an enzyme that is capable of producing ROS possibly resulting in increased RNA oxidation and therefore we measured its activity. XOD activity was significantly higher in gastrocnemius muscles from old (p < 0.0001) compared to young rats, while HS had no effect at either age (Figure 5). Therefore, increased XOD activity could play a role in increased oxidation with age, but not with disuse.
Figure 5. Increased XOD activity with age.
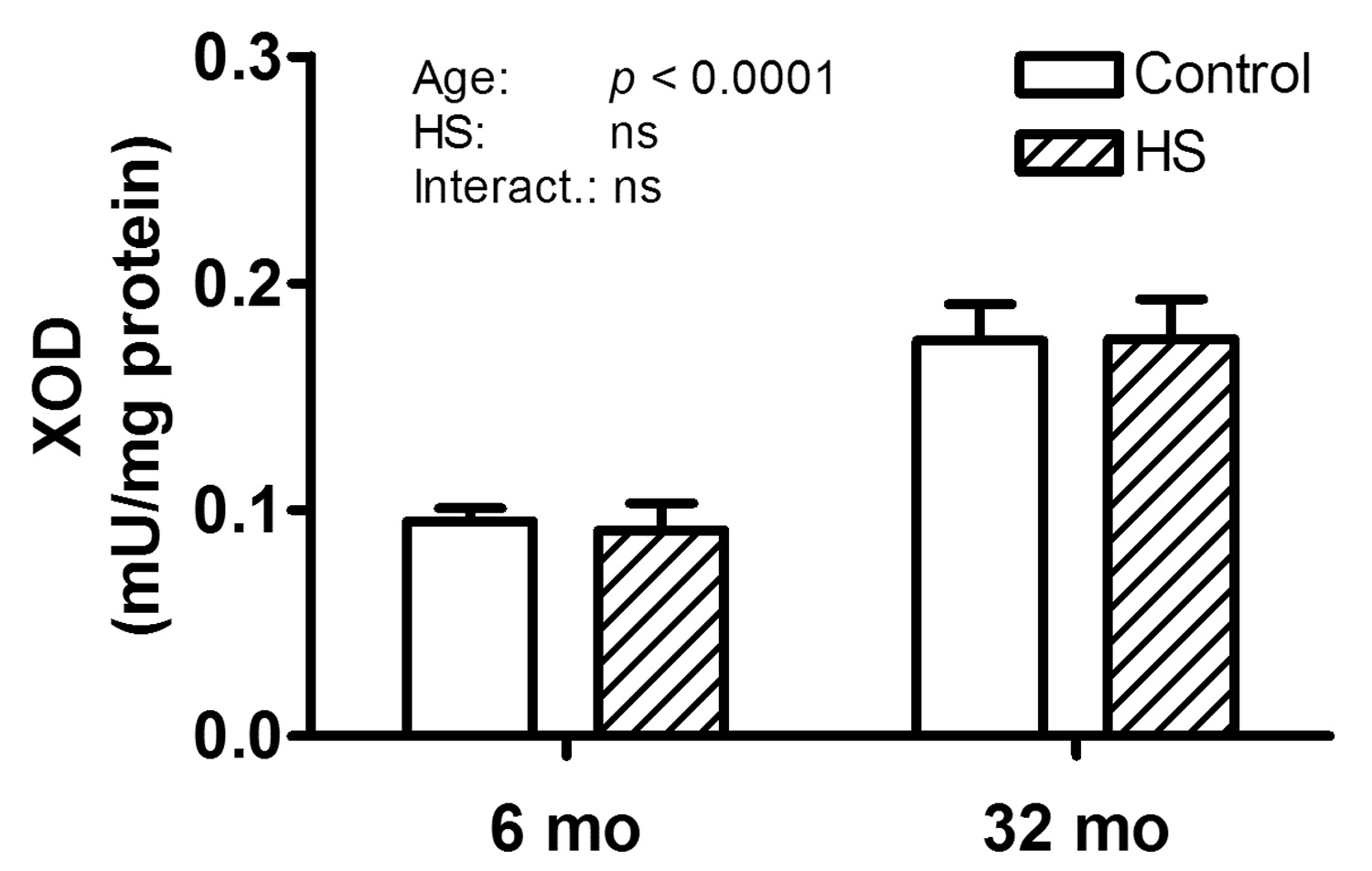
The XOD activity normalized to total protein concentration in gastrocnemius muscles of 6 and 32 month (mo) old control (open bars) and hind limb suspended (HS; hatched bars) rats is shown. A main effect for age was observed.
Increases in ROS levels in skeletal muscle are observed in circumstances where inflammation is increased, due to MPO activity of infiltrated leucocytes. However, in this study no detectable MPO activity was found in any of the groups, and therefore ROS production in response to inflammation does not seem to play a role in age-related or disuse-induced muscle atrophy. It is currently unknown how oxidized RNA is removed from the cell, but the mammalian Y box-binding protein 1 (YB-1) protein was found to bind oxidized RNA (Hayakawa et al., 2002), and has also been suggested to have a role in iron regulation (Kohno et al., 2003). YB-1 gene expression levels, however, were not changed with either age or HS (Table 2) and therefore YB-1 may not be involved in the removal of oxidized RNA in these muscles or is possibly failing to respond appropriately. The role of other RNA-binding proteins remains to be investigated.
Discussion
This study shows for the first time that RNA oxidative damage and levels of non-heme iron in skeletal muscle were elevated in muscles of aged animals, particularly after a period of disuse. In addition, we show that RNA, but not DNA, oxidative damage increased significantly in muscles at advanced age, even though the levels were similar in young rats. The higher levels of oxidative damage in RNA than in DNA support our previous observation that oxidative damage to RNA is generally greater than that to DNA (Hofer et al., 2005; Hofer et al., 2006; Seo et al., 2006). The higher susceptibility of RNA to oxidative damage could be due to a greater exposure of RNA to ROS and iron, or to differences in protection from ROS, repair or turnover mechanisms (Hofer et al., 2005; Hofer et al., 2006). These results complement previous studies showing that RNA oxidation is increased in brain and liver of old animals, and may therefore constitute a general phenomenon with aging (Honda et al., 2005; Liu et al., 2002; Seo et al., 2006; Zhang et al., 1999).
The functional consequences of increased RNA oxidative damage in skeletal muscles could be significant. Recently it was shown that oxidized mRNA resulted in decreased translational fidelity and that the produced proteins from oxidized RNA were readily degraded (Tanaka et al., 2007). If the same phenomenon applies to skeletal muscles, it would likely be associated with muscle atrophy. Also, oxidative damage to rRNA has been shown to result in decreased protein synthesis (Honda et al., 2005; Tanaka et al., 2007), which in skeletal muscles will eventually result in muscle atrophy. Therefore, the increased RNA oxidation could be causative to the observed muscle atrophy, which would explain the very high correlation between muscle mass and RNA oxidation observed in our study. The fact that RNA oxidation increased particularly in muscles from old rats after disuse indicates that aged animals may be more susceptible to RNA damage, possibly because of the concomitant elevation in iron. Alternatively, it could indicate that removal mechanisms of oxidized RNA are impaired with aging and disuse. We therefore assessed the gene expression of YB-1 as a potential protein involved in recognition of oxidized RNA (Hayakawa et al., 2002), but no significant changes were detected with either age or disuse. Hence, YB-1 may not be involved in the removal of oxidized RNA in our experimental model or its ability to respond appropriately to increased levels of RNA oxidation may be impaired with aging. However, alterations in the removal of oxidized RNA with age remain to be investigated.
Changes in iron homeostasis may play a role in the development of skeletal muscle atrophy with aging, since non-heme iron increased, and TfR1 and HJV mRNA abundance decreased with age. This increase in non-heme iron with advancing age is similar to what has been observed in liver, kidney and brain of aged rats (Cook and Yu, 1998). The decrease in HJV mRNA levels in gastrocnemius muscle of old rats is consistent with a recent report showing decreased HJV expression in iron-loaded skeletal muscle from male and female Balb/c mice (Rodriguez et al., 2007). The cellular labile iron pool (LIP: Fe2+ and Fe3+) constitutes 3–5% of the total cellular iron and has been suggested to induce oxidative stress (Kruszewski, 2003). This would explain the strong correlation between non-heme iron and RNA oxidation found in this study. However, the role of non-heme iron in muscle atrophy due to disuse is more complicated. We observed a change in non-heme iron with disuse in old, but not in young rats, even though TfR1 was decreased significantly in the young hind limb suspended rats. It is possible that the TfR1 was so low in the old controls already that it could not decrease further, which is supported by the very high levels of non-heme iron in the older suspended rats. This increase, rather than a decrease, in HJV mRNA levels in HS rats indicates that HJV expression responds to factors other than non-heme iron levels. Another possibility is that HJV was increased with HS as a compensatory response. These observations highlight the need for further studies to address how changes in HJV expression affect skeletal muscle iron homeostasis. Interestingly, the highest levels of non-heme iron in the muscles from old animals were mostly observed in the smallest fibers, particularly after HS. This strengthens our observation that non-heme iron is correlated with muscle size, even at the level of single fibers. Based on our studies, a cause-effect relationship between increased free iron, RNA oxidation and muscle atrophy can not be inferred. However, our observations suggest that interventions aimed at decreasing non-heme iron are worth investigating for attenuation of muscle atrophy due to disuse. Indeed, it has previously been shown that the iron chelator deferoxamine attenuated muscle atrophy due to immobilization in vivo in young rats (Kondo et al., 1992), but its effect in old animals remains to be investigated. It is possible that the iron buffering systems are better adapted in young animals and therefore interventions that decrease the free iron pool during muscle atrophy may be particularly useful in aged subjects and should be investigated in future studies.
In addition to age-related changes in removal mechanisms of damaged RNA, it is also possible that the generation of ROS with disuse is different in muscles from aged animals. XOD has previously been suggested as one cause of increased ROS during immobilization-induced muscle atrophy (Kondo et al., 1993). We found elevated XOD activity with age, but not with HS, suggesting that this enzyme does not play a significant role in disuse atrophy, although it could still contribute to the age-associated increase in oxidative stress. Also, MPO activity was not detected in the muscles, indicating that ROS production from infiltrating leukocytes is very unlikely in our model. Even though the source of the oxidative damage during disuse-induced atrophy remains to be determined it has been shown that anti-oxidant treatment is beneficial in attenuating muscle loss (Arbogast et al., 2007). In a recent mouse study using hind limb unloading, soleus muscle weight losses correlated with increased cytosolic oxidant generating activity and muscle weight and force losses could be prevented by dietary supplementation of the soy protein Bowman-Birk inhibitor, a protease inhibitor with strong anti-oxidant properties (Arbogast et al., 2007).
In relation to the observed increases in iron levels in aged or exposed muscle, it should be pointed out that this constitutes a challenge for measurements of oxidative damage. As 'free iron' can catalyze reactions during sample processing (Hofer and Moller, 1998), differences in iron levels may have biased measurements of oxidative damages in previous studies (Fano et al., 2001; Marzani et al., 2005; Mecocci et al., 1999), unless iron was well controlled during sample processing (in this report we used 10 mM DFOM). Thus, earlier reports showing elevated DNA oxidative damage in muscles at advanced age (Fano et al., 2001; Hamilton et al., 2001; Mecocci et al., 1999) might have been biased by the lack of controlling such ‘free iron’ during sample processing.
In summary, our data suggest that RNA oxidation plays a role in the development of atrophy with disuse, particularly in aged animals. Our results further suggest that interventions that counteract intracellular iron accumulation or that can increase the control of iron (such as chelators) in skeletal muscle may be especially beneficial in attenuating disuse-induced muscle atrophy in aged animals. Future research should be directed towards investigating the processes responsible for the increase in RNA oxidation with atrophy and the mechanisms involved in removal of RNA damage.
Acknowledgements
This research was supported by grants from the National Institute on Aging (AG17994 and AG21042 to CL and AG20407 and AG028925 to ED), an American Heart Postdoctoral Fellowship to TH (0525346B), and an American Heart Fellowship to AYS (0615256B). EM is supported by the Claude D. Pepper Older Americans Independence Center (OAIC) (1 P30 AG028740-01). We would like to thank Cathy Gurley for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alway SE, Degens H, Krishnamurthy G, Chaudhrai A. Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J Gerontol A Biol Sci Med Sci. 2003;58:687–697. doi: 10.1093/gerona/58.8.b687. [DOI] [PubMed] [Google Scholar]
- Arbogast S, Smith J, Matuszczak Y, Hardin BJ, Moylan JS, Smith JD, Ware J, Kennedy AR, Reid MB. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol. 2007;102:956–964. doi: 10.1152/japplphysiol.00538.2006. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Bregeon D, Sarasin A. Hypothetical role of RNA damage avoidance in preventing human disease. Mutat Res. 2005;577:293–302. doi: 10.1016/j.mrfmmm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26:432–439. [PubMed] [Google Scholar]
- Cairo G, Recalcati S, Pietrangelo A, Minotti G. The iron regulatory proteins: targets and modulators of free radical reactions and oxidative damage. Free Radic Biol Med. 2002;32:1237–1243. doi: 10.1016/s0891-5849(02)00825-0. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Davis BS, Booth FW. IGF-1 restores satellite cell proliferative potential in immobolized old skeletal muscle. J Appl Physiol. 2000;89:1365–1379. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mech Ageing Dev. 1998;102:1–13. doi: 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev. 2006;5:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Houlé JD, Dennis RA, Zhang J, Knox M, Wagoner G, Peterson CA. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- Fano G, Mecocci P, Vecchiet J, Belia S, Fulle S, Polidori MC, Felzani G, Senin U, Vecchiet L, Beal MF. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22:345–351. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE. Satellite cell regulation of muscle mass is altered at old age. J Appl Physiol. 2004;97:1082–1090. doi: 10.1152/japplphysiol.00006.2004. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J. Free Radicals in Biology and Medicine. 3rd Edition. Oxford: Oxford university Press; 1999. [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa H, Uchiumi T, Fukuda T, Ashizuka M, Kohno K, Kuwano M, Sekiguchi M. Binding capacity of human YB-1 protein for RNA containing 8-oxoguanine. Biochemistry. 2002;41:12739–12744. doi: 10.1021/bi0201872. [DOI] [PubMed] [Google Scholar]
- Hofer T. Oxidation of 2′-deoxyguanosine by H2O2-ascorbate: evidence against free OH. and thermodynamic support for two-electron reduction of H2O2. J Chem Soc Perkin Trans. 2001;2:210–213. [Google Scholar]
- Hofer T, Badouard C, Bajak E, Ravanat JL, Mattsson A, Cotgreave IA. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol Chem. 2005;386:333–337. doi: 10.1515/BC.2005.040. [DOI] [PubMed] [Google Scholar]
- Hofer T, Moller L. Reduction of oxidation during the preparation of DNA and analysis of 8-hydroxy-2′-deoxyguanosine. Chem Res Toxicol. 1998;11:882–887. doi: 10.1021/tx980041x. [DOI] [PubMed] [Google Scholar]
- Hofer T, Seo AY, Prudencio M, Leeuwenburgh C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol Chem. 2006;387:103–111. doi: 10.1515/BC.2006.014. [DOI] [PubMed] [Google Scholar]
- Honda K, Smith MA, Zhu X, Baus D, Merrick WC, Tartakoff AM, Hattier T, Harris PL, Siedlak SL, Fujioka H, Liu Q, Moreira PI, Miller FP, Nunomura A, Shimohama S, Perry G. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J Biol Chem. 2005;280:20978–20986. doi: 10.1074/jbc.M500526200. [DOI] [PubMed] [Google Scholar]
- Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays. 2003;25:691–698. doi: 10.1002/bies.10300. [DOI] [PubMed] [Google Scholar]
- Kondo H, Miura M, Kodama J, Ahmed SM, Itokawa Y. Role of iron in oxidative stress in skeletal muscle atrophied by immobilization. Pflugers Arch. 1992;421:295–297. doi: 10.1007/BF00374844. [DOI] [PubMed] [Google Scholar]
- Kondo H, Nakagaki I, Sasaki S, Hori S, Itokawa Y. Mechanism of oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol. 1993;265:E839–E844. doi: 10.1152/ajpendo.1993.265.6.E839. [DOI] [PubMed] [Google Scholar]
- Kruszewski M. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res. 2003;531:81–92. doi: 10.1016/j.mrfmmm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Kuchino Y, Mori F, Kasai H, Inoue H, Iwai S, Miura K, Ohtsuka E, Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987;327:77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1288–R1296. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- Lexell J. Ageing and human muscle: observations from Sweden. Can J Appl Phys. 1993;18:2–18. doi: 10.1139/h93-002. [DOI] [PubMed] [Google Scholar]
- Lin L, Goldberg YP, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106:2884–2889. doi: 10.1182/blood-2005-05-1845. [DOI] [PubMed] [Google Scholar]
- Lipman R, Chrisp E, Hazzard D, Bronson R. Pathologic characterization of Brown Norway, Brown Norway × Fischer 344, and Fischer 344 × Brown Norway rats with relation to age. Journal of Gerontology: Biological Sciences. 1996;51A:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzani B, Felzani G, Bellomo RG, Vecchiet J, Marzatico F. Human muscle aging: ROS-mediated alterations in rectus abdominis and vastus lateralis muscles. Exp Gerontol. 2005;40:959–965. doi: 10.1016/j.exger.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, Cherubini A, Vecchiet J, Senin U, Beal MF. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–308. doi: 10.1016/s0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102:2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ, Wilcox CL, Widness JA. Microanalysis of non-heme iron in animal tissues. J Biochem Biophys Methods. 2004;58:239–251. doi: 10.1016/j.jbbm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Hilvo M, Kytomaki L, Fleming RE, Britton RS, Bacon BR, Parkkila S. Effects of iron loading on muscle: genome-wide mRNA expression profiling in the mouse. BMC Genomics. 2007;8:379. doi: 10.1186/1471-2164-8-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo AY, Hofer T, Sung B, Judge S, Chung HY, Leeuwenburgh C. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8:529–538. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Chock PB, Stadtman ER. Oxidized messenger RNA induces translation errors. Proc Natl Acad Sci U S A. 2007;104:66–71. doi: 10.1073/pnas.0609737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker W, Vallee B. Nucleic acids and metals. I. Chromium, manganese, nickel, iron, and other metals in ribonucleic acid from diverse biological sources. J Biol Chem. 1959;234:3257–3262. [Google Scholar]
- Zarzhevsky N, Carmeli E, Fuchs D, Coleman R, Stein H, Reznick AZ. Recovery of muscles of old rats after hindlimb immobilisation by external fixation is impaired compared with those of young rats. Exp Gerontol. 2001;36:125–140. doi: 10.1016/s0531-5565(00)00189-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


