Abstract
Specific identification of Entamoeba histolytica in clinical specimens is an essential confirmatory diagnostic step in the management of amebiasis. Here, we report an unusual case of amebic colitis in a 20-year old female immigrant from South China. The patient had experienced diarrhea, crampy abdominal pain and fever for approximately 3 weeks prior to admission to hospital, and had treated herself at home with metronidazole. On admission, stool microscopy and serology for E. histolytica were negative. Because the clinical findings raised the suspicion of Clostridium difficile fulminant colitis, she underwent a subtotal colectomy. Histopathology revealed flask-shaped ulcers characteristic of amebic colitis. Consequently, E. histolytica DNA was detected by a sensitive small-subunit rRNA polymerase chain reaction (SSU-rRNA-PCR) from feces, and the patient was successfully treated for amebiasis with metronidazole. This case exemplifies the relative insensitivity of serologic tests for the diagnosis of amebiasis, and the difficulties encountered in detecting the parasite antigen in a patient partially treated with metronidazole. We conclude that when the possibility of invasive intestinal amebiasis is suspected, detecting the parasite DNA directly in the stool sample by PCR using E. histolytica-specific primers may be an alternative, non-invasive and reliable tool for the specific diagnosis of the disease.
1. Introduction
The World Health Organization (WHO) estimates that amebiasis is one of the three most frequent causes of death from parasitic diseases, responsible for up to 100,000 deaths per annum (World Health Organization, 1997). Infections by the protozoan parasite E. histolytica result in diverse clinical manifestations. Although most infections with E. histolytica are asymptomatic, up to 20% of infections result in either amebic colitis or liver abscess (Tanyuksel and Petri, 2003; Solaymani-Mohammadi et al., 2006)
Although microscopic identification remains the most common routine means of diagnosis used in areas where amebiasis is endemic, it is both insensitive and nonspecific (E. histolytica cannot be distinguished from Entamoeba dispar and Entamoeba moshkovskii microscopically alone without the presence of phagocytosed erythrocytes in E. histolytica trophozoites). The specific diagnosis of these species is of the utmost clinical importance, since the two latter are considered non-pathogenic species and no treatment is warranted.
Serologic tests may be helpful in diagnosis of amebic colitis, since more than 65% of patients at the time of presentation have demonstrable serum antibodies (World Health Organization, 1997; Solaymani-Mohammadi et al., 2006). Serum antiamebic IgG antibodies are generally found after 1 week of symptoms due to invasive amebiasis (Abd-Alla et al., 1992; Haque et al., 1998; McCarthy et al., 2002). Detection of parasite antigen in stool is more than 90% sensitive (World Health Organization, 1997; Abd-Alla et al., 1992). In addition, a number of PCR-based techniques offer the means to identify E. histolytica in an array of clinical specimens, including stool, tissue, liver abscess aspirate, and cerebrospinal fluid (Solaymani-Mohammadi et al, 2007). Therefore to augment the clinical laboratory diagnosis of amebiasis, a combined approach of serological tests with detection of the parasite (by antigen detection or PCR) has been suggested to offer the best approach to diagnosis the infection (World Health Organization, 1997; McCarthy et al., 2002). Here, we report a case that demonstrates the benefit of this combined diagnostic approach.
2. Patient
A 20-year old female university student without any significant past medical history traveled from Southeastern China to Boston to study in September 2006. In January 2007, she developed watery diarrhea several times a day associated with cramping. She noted that there was blood in the toilet towards the end of the bowel movement. At that time, she self-medicated with metronidazole brought with her from China, although it was not clear what dose she took. Initially, she experienced some relief, but diarrhea resumed soon afterwards. By early March 2007, the diarrhea began increasing and the patient experienced malaise; she was treated with ciprofloxacin by a family physician. She did not respond and described diffuse crampy abdominal pain, vomiting and chills the day prior to admission. On admission, she was febrile (101° F), BP 103/68, P 130 RR 16. On physical examination, she had diffuse abdominal tenderness without rebound or guarding. Bowel sounds were present and her stool was Guaiac positive. Her Hgb was 9.9, WBC 17,100 with 21% bands, platelets 366,000, and PT/INR of 13.9/1.4. Electrolytes and liver function tests were normal, as was the chest x-ray. Abdominal CT showed moderate ascites, normal liver, spleen, pancreas, and adrenals. There was diffuse thickening and dilation of the colon and rectum, with pneumatosis intestinalis in the left colon. Stool exams for ova and parasites were negative, as was the serum ELISA for E. histolytica antibodies.
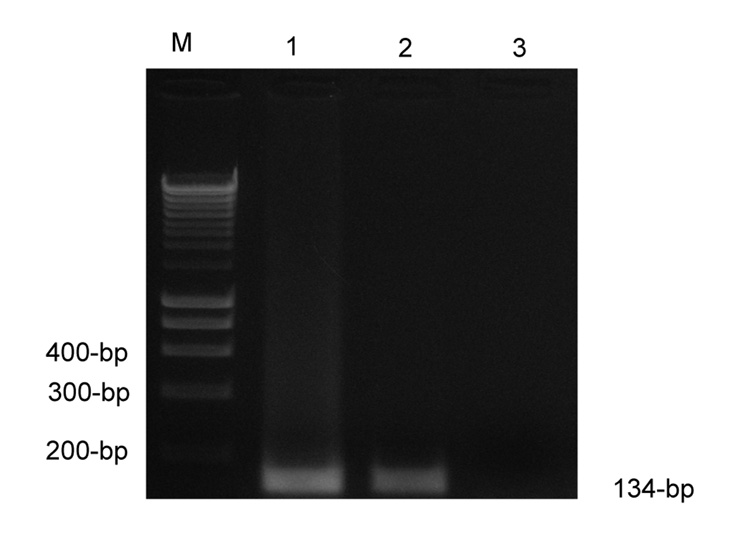
Toxic megacolon from C. difficile was suspected and a subtotal colectomy performed. In the OR, the surgeons noted murky ascites, a distended colon with areas of crepitus and diffuse inflammation of the serosal surfaces. The pathology revealed scant atypical E. histolytica trophozoites, probably because of the partial treatment with metronidazole, and the classic flask-shaped ulcers consistent with invasive amebiasis. Post-operatively E. histolytica DNA was detected in stool by a sensitive PCR (Fig. 1) and the patient was treated with metronidazole.
Figure 1.
SSU-rRNA-based analysis of E. histolytica. M, Molecular size marker(100-bp); Lane 1, Positive control(HM1:IMSS); Lane 2, Patient sample; Lane 3, Negative control (no DNA).
3. Materials and Methods
3.1. Stool antigen detection
Initially, stool samples were tested for the parasite antigen using ProSpecT® Entamoeba histolytica microplate assay (Remel, Lenexa, Kansas) according to manufactures’ instructions. This kit is a direct qualitative enzyme immunoassay (EIA) for detection of E. histolytica specific antigen in fresh, frozen, or preserved fecal specimens. Postoperatively, the stool samples were examined by using the Techlab E. histolytica II antigen detection kit (Techlab Inc., Blacksburg, Virginia) following the instructions provided by the manufacturer. The Techlab kit is a monoclonal antibody-based ELISA, which detects E. histolytica antigens, but not E. dispar, specifically in faecal samples.
3.2. DNA extraction
DNA was isolated from stool using the QIAamp DNA stool mini kit (QIAGEN, Inc.,Valencia, CA, USA) according to the manufacturer’s instructions except that the suspension was incubated in the kit’s stool lysis buffer (ASL buffer) at 95° C for 10 min and a 3-min incubation with the InhibitEx tablets at room temperature was used (Solaymani-Mohammadi et al., 2007). Finally, the DNA was eluted in 0.2 ml AE buffer (supplied with the QIAGEN kit) and stored at −20° C until PCR amplification.
3.3. Oligonucleotide primers and PCR
DNA was subjected to PCR using primers (QIAGEN, Inc., Valencia, CA, USA) Ehf (5’-AAC AGT AAT AGT TTC TTT GGT TAG TAA AA-3’) and Ehr (5’-CTT AGA ATG TCA TTTCTC AAT TCAT-3’) to specifically amplify a 134-bp fragment inside the 16S-like small subunit rRNA gene of E. histolytica (Roy et al., 2005; Solaymani-Mohammadi et al., 2007). The PCR products were size-fractioned on an ethidium bromide 2.5% agarose gel and UV visualized (Fig. 1).
4. Discussion
The instructive aspect of this case report was the application of molecular methods to rapidly validate the features indicative of amebiasis observed at histopathology, in a case initially suspected as C. difficile colitis. In particular, SSU-rRNA-PCR was a sensitive means for diagnosis, even in this patient where partial self-treatment with metronidazole made identification of the parasite by stool microscopy difficult.
The sensitivity and specificity of conventional microscopy on a single stool specimen for Entamoeba is notoriously poor, and in one study less than 10% specific (Pillai et al., 1999). The E. histolytica antibody diagnostic test in intestinal disease is also suboptimal, with a sensitivity of about 65% (World Health Organization, 1997; Tanyuksel and Petri, 2003). Antigen detection in stool is greater than 95% sensitive and specific compared to amebic culture as a gold standard (Haque et al., 1998). However, PCR detection of parasite DNA in stool may even be more sensitive than antigen detection, especially in a situation where the infection has been partially treated, as in this case (Roy et al., 2005). The combination of a serological test with detection of the parasite (by antigen detection or PCR) thus may offer the best approach to diagnosis (World Health Organization, 1997; Haque et al., 1998).
To conclude, the rapid identification of E. histolytica colitis using sensitive molecular methods permitted an accurate diagnosis and a specific therapy. This approach offered a good alternative to the conventional serologic and microscopic assays. Direct detection of the parasite (by PCR and antigen detection) should be considered whenever the clinical symptoms raised the suspicion of invasive amebiasis in patients with negative serology and negative stool antigen detection for E. histolytica. Although human amebiasis is uncommon in the U.S., the combined approach may be particularly attractive for the well-equipped reference laboratories in developing countries that already use conventional serology and microscopy, and wish to use PCR as an alternate confirmatory method for E. histolytica diagnosis.
Acknowledgements
This study was supported by the National Institutes of Health grant AI-43596 to W.A.P. S.S.-M was a visiting researcher at the Division of Infectious Diseases and International Health, University of Virginia Health System, Charlottesville, and his stay in UVa was partly funded by a scholarship from the Iranian Ministry of Health and Medical Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd-Alla MD, el-Hawey AM, Ravdin JI. Use of an enzyme-linked immunosorbent assay to detect anti-adherence protein antibodies in sera of patients with invasive amebiasis in Cairo, Egypt. Am J Trop Med Hyg. 1992;47:800–804. doi: 10.4269/ajtmh.1992.47.800. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Som I, Bhattacharya A. The ribosomal DNA plasmids of Entamoeba. Parasitol Today. 1998;4:181–185. doi: 10.1016/s0169-4758(98)01222-8. [DOI] [PubMed] [Google Scholar]
- Haque R, Ali IK, Akther S, Petri WA., Jr Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 1998;36:449–452. doi: 10.1128/jcm.36.2.449-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr Current Concepts: Amebiasis. New Engl J Med. 2003;348:1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- McCarthy JS, Peacock D, Trown KP, Bade P, Petri WA, Jr, Currie BJ. Endemic invasive amoebiasis in northern Australia. Med J Aust. 2002;177:570. doi: 10.5694/j.1326-5377.2002.tb04957.x. [DOI] [PubMed] [Google Scholar]
- Pillai DR, Keystone JS, Sheppard DC, MacLean JD, MacPherson DW, Kain KC. Entamoeba histolytica and Entamoeba dispar: epidemiology and comparison of diagnostic methods in a setting of nonendemicity. Clin Infect Dis. 1999;29:1315–1318. doi: 10.1086/313433. [DOI] [PubMed] [Google Scholar]
- Roy S, Kabir M, Mondal D, Ali IK, Petri WA, Jr, Haque R. Real-time-PCR assay for diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 2005;43:2168–2172. doi: 10.1128/JCM.43.5.2168-2172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Guillén MC, Velázquez-Rojas M, Salgado-Rosas H, Torres-Rasgado E, Pérez-Fuentes R, Martínez-Munguía J, Talamás-Rohana P. Seroprevalence of anti-Entamoeba histolytica antibodies by IHA and ELISA assays in blood donors from Puebla, Mexico. Arch Med Res. 2000;31 Suppl. 1:53–54. doi: 10.1016/s0188-4409(00)00178-8. [DOI] [PubMed] [Google Scholar]
- Solaymani-Mohammadi S, Rezaian M, Babaei Z, Rajabpour A, Meamar AR, Pourbabai AA, Petri WA., Jr Comparison of a stool antigen detection kit and PCR for diagnosis of Entamoeba histolytica and Entamoeba dispar infections in asymptomatic cyst passers in Iran. J Clin Microbiol. 2006;44:2258–2261. doi: 10.1128/JCM.00530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaymani-Mohammadi S, Lam MM, Zunt JR, Petri WA., Jr Entamoeba histolytica encephalitis diagnosed by PCR of cerebrospinal fluid. Trans R Soc Trop Med Hyg. 2007;101:311–313. doi: 10.1016/j.trstmh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Tanyuksel M, Petri WA., Jr Laboratory diagnosis of amebiasis. Clin Microbiol Rev. 2003;16:713–729. doi: 10.1128/CMR.16.4.713-729.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Amoebiasis. Wkly Epidemiol Rec. 1997;72:97–99.