Summary
We explore cellular and molecular mechanisms of nasal adjuvant of a combination of a plasmid encoding the Flt3 ligand cDNA (pFL) and CpG oligodeoxynucleotides (CpG ODN). The double DNA adjuvant given with OVA maintained prolonged OVA-specific secretory IgA (S-IgA) Ab responses in external secretions for more than twenty-five weeks after the final immunization. Further, both Th1- and Th2-type cytokine responses were induced by this combined adjuvant regimen. The frequencies of plasmacytoid DCs (pDCs) and CD8+ DCs were significantly increased in nasopharyngeal-associated lymphoreticular tissue (NALT) of mice given the combined adjuvant. Importantly, when we examined adjuvanticity of pFL plus CpG ODN in 2-year-old mice, significant levels of mucosal IgA Ab responses were also induced. These results demonstrate that nasal delivery of a combined DNA adjuvant offers an attractive possibility for the development of an effective mucosal vaccine for the elderly.
Keywords: Cytokines, Dendritic cells, Nasal immunization
Introduction
Nasal delivery has been shown to preferentially induce antigen (Ag)-specific antibody (Ab) responses in the upper respiratory tract as well as other mucosal lymphoid tissues, whereas oral immunization is more limited to induction of immunity in the gastrointestinal (GI) tract [1–6]. In order to elicit maximal levels of Ag-specific immune responses in both mucosal and systemic lymphoid tissue compartments, it is essential to employ an appropriate mucosal adjuvant [2, 7, 8]. Although native cholera toxin (nCT) is an effective mucosal adjuvant in animal models, its innate toxicity limits its use in humans. In this regard, several mucosal adjuvants have been developed [5, 9–14]. Despite these reports, the development of effective and reliable mucosal adjuvants that can be safely co-administered with vaccine Ag is of central importance in new generation vaccines.
It is well known that a variety of molecules such as Ags derived from bacteria, viral products, growth factors, cytokines, and chemokines can promote dendritic cell (DC) activation, expansion, and maturation [15, 16]. Flt3 ligand (FL), a type 1 transmembrane protein, binds to either fetal liver kinase 2 (flk2) or fms-like tyrosine kinase 3 (flt3) receptors. It has been demonstrated that daily administration of FL to mice resulted in dramatic increases in DCs in the bone marrow, the peritoneal cavity, spleens, and thymus [17–19]. Interestingly, it was reported that pretreatment with FL facilitated the induction of systemic unresponsiveness through expansion of immature DCs when protein Ag was subsequently administered via the oral route [20]. In contrast, our previous studies demonstrated that nasal delivery of a plasmid encoding the FL cDNA (pFL) as the mucosal adjuvant preferentially expanded CD8+ DCs and subsequently induced Ag-specific mucosal immune responses mediated by IL-4-producing CD4+ T cells [21]. These results clearly demonstrate that nasal pFL prevented mucosal tolerance and exhibited effective mucosal adjuvanticity.
Recent studies have clearly shown that conserved signature molecules of microbes, termed pathogen-associated molecular patterns (PAMPs), are also involved in the regulation of DC activation [22, 23]. These PAMPs interact with pattern recognition receptors, which are toll-like receptor (TLR) family members, and subsequently induce innate and acquired immunity [23]. Of these PAMPs, synthetic oligodeoxynucleotides that contain one or more unmethylated cytosine-guanine dinucleotide (CpG ODN) motifs exhibit mucosal adjuvant activity through direct activation of TLR9-expressing plasmacytoid DCs (pDCs) [24–29]. The pDCs can be identified by their expression of CD11c+, B220+, and CD11b− [30–32]. When pDCs are activated through TLR9, they secrete a variety of cytokines, including IFN-α, IL-6, IL-12, and TNF-α [25, 26]. In this regard, it has been shown that Th1-type cytokine-mediated Ag-specific IgG2a Ab responses were induced when CpG ODN was co-administered as an adjuvant [27–29].
The development of nasal vaccines may also contribute to better preventive medicine in elderly persons when compared with oral immunization. Although age-associated changes in the mucosal immune system are less understood when compared with immunosenescence in systemic immunity, it has been shown that the mucosal immune system is also altered by aging because the elderly are much more susceptible to infections of the GI tract [33, 34]. Our previous study revealed that Ag-specific mucosal and systemic immune responses were diminished in 1-year-old mice immunized orally with OVA and nCT, whereas significant immune responses were observed in orally immunized young adult mice in both mucosal and systemic lymphoid compartments [35]. In contrast, our recent study demonstrated that nasal administration of OVA and nCT revealed significant systemic as well as mucosal Ag-specific immune responses that provided effective protection of one-year-old mice [36]. These results clearly indicate that the regulation of the NALT-mediated mucosal immune system is distinct from the gut-associated lymphoid tissue (GALT)-directed system and that the route of immunization is a critical factor for effective induction of protective mucosal immunity in the elderly. To further investigate the efficacy of nasal vaccination, two-year-old mice were nasally immunized with OVA and nCT; however, nCT failed to induce Ag-specific mucosal S-IgA Ab responses despite the induction of plasma IgG Ab responses [36]. Thus, it will be essential to develop new generation of mucosal adjuvants which are effective in the elderly.
In this study, we examined whether a combination of pFL and CpG ODN as a combined DC-targeting mucosal adjuvant would elicit enhanced Ag-specific Ab responses with balanced Th1 and Th2 cytokine responses. Further, we investigated the role of this double adjuvant system in the modification of DC subsets in NALT, an important inductive site for mucosal immunity. Finally, the efficacy of the combined adjuvant was examined in aged mice in order to continue any efforts to develop a more effective nasal vaccine not only for young adults but also for the elderly.
Materials and Methods
Mice
Young adult (six- to eight-week-old) female BALB/c mice were purchased from the Frederick Cancer Research Facility (Frederick, MD). The retired BALB/c female breeders (eight-month-old) were obtained from the Jackson Laboratory (Bar Harbor, ME). OVA TCR-transgenic mice on a BALB/c background, clone DO11.10, that recognizes the 323–339 peptide fragment of ovalbumin (OVA) were also used in some experiments [37]. Upon arrival, all mice were immediately transferred to microisolators, maintained in horizontal laminar flow cabinets, and provided sterile food and water. Experiments were performed using young adult BALB/c mice between 6 and 8 weeks of age or mice over 2 years of age. The health of these mice was tested semiannually, and mice of all ages used in these experiments were free of bacterial and viral pathogens.
Nasal adjuvants and immunization
The plasmid pORF9-mFLt3L (pFL), comprising the pORF9-mcs vector (pORF) and the full-length mouse FL cDNA gene (InvivoGen, San Diego, CA), was used as a nasal adjuvant [21]. This plasmid DNA was purified using QIAGEN Plasmid Giga Kits (QIAGEN, Valencia, CA). The limulus amebocyte lysate assay (BioWhittaker Inc., Walkersville, MD) resulted in < 0.1 endotoxin units of lipopolysaccharide (LPS) per µg of plasmid. A synthetic oligodeoxynucleotide (ODN) containing CpG motif 1826 (CpG ODN) was also used as a nasal adjuvant (Coley Pharmaceutical Group, Wellesley, MA) [29]. Mice were nasally immunized with different preparations of Ag three times at weekly intervals. Each group of mice was given 6.5 µl of PBS containing 100 µg of OVA/nostril (fraction V; Sigma-Aldrich, St. Louis, MO) and 50 µg of pFL and/or 10 µg of CpG ODN. In some experiments, mice were immunized three times at weekly intervals with nasal doses of 100 µg of OVA and 0.5 µg of nCT (List Biological Laboratories, Campbell, CA).
Sample collection
Plasma, saliva, and vaginal washes (VWs) were collected on days 0, 7, 14, and 21. In some experiments, these samples were collected at weekly intervals for a total of 27 times after the final immunization. Nasal washes (NWs) were obtained by instillation of one ml of PBS on three occasions into the posterior opening of the nasopharynx with a 30-gauge hypodermic needle [3].
Cell isolation
Spleen and cervical lymph nodes (CLNs) were removed aseptically, and mononuclear cells were isolated by a mechanical dissociation method using gentle teasing through stainless steel screens as described previously [6]. For isolation of mononuclear cells from NALT, we used a modified dissociation method based on a previously described protocol [38, 39]. In brief, the peeled palates including NALT were scratched with 26-gauge needles under a dissection microscope, and the NALT fragment was then teased gently with frosted slide glasses. Mononuclear cells from nasal passages (NPs) and submandibular glands (SMGs) were isolated by an enzymatic dissociation procedure with collagenase type IV (0.5 mg/ml; Sigma-Aldrich) followed by discontinuous Percoll® (Amersham Biosciences, Uppsala, SWEDEN) gradient centrifugation.
OVA-specific Ab assays
OVA-specific Ab levels in plasma and mucosal secretions were determined by ELISA as previously described [3, 6, 21]. Endpoint titers were expressed as the last dilution yielding an optical density at 414 nm (OD414) of > 0.1 units above background control values. Mononuclear cells obtained from mucosal and systemic lymphoid tissues were subjected to an ELISPOT assay to detect numbers of OVA-specific antibody-forming cells (AFCs) [3, 6, 21].
OVA-specific CD4+ T-cell Responses
CD4+ T cells from spleen and CLNs were purified by using an automated magnetic activated cell sorter (AutoMACS) system (Miltenyi Biotec, Auburn, CA) as described previously [3, 5]. This purified T cell fraction (> 97 % CD4+ and > 99 % viable) was cultured with T cell-depleted irradiated (3000 rads) splenic APCs obtained from naïve mice in the presence of one mg/ml OVA. The supernatants of identically treated T-cell cultures were then subjected to a cytokine-specific ELISA [3, 32]. The detection limits for each cytokine were as follows: 9.8 pg/ml for IFN-γ, 2.0 pg/ml for IL-2; 1.5 pg/ml for IL-4; 4.9 pg/ml for IL-5; 4.9 pg/ml for IL-6; and 24.4 pg/ml for IL-10.
Quantitative analysis of cytokine-specific mRNA
For evaluation of cytokine-specific mRNA levels in OVA-stimulated CD4+ T cells, real-time PCR was used with a LightCycler® (Roche Applied Sciences, Indianapolis, IN). The CD4+ T cells were harvested after five days of incubation and the total RNA was isolated by the TRIZOL® extraction procedure (Invitrogen Corporation, Carlsbad, CA). The concentration of sample cDNA was determined using external standards diluted linearly obtained by an identical PCR protocol with the LightCycler®.
Flow cytometry analysis
Aliquots of mononuclear cells (0.2–1.0 × 106 cells) isolated from various tissues as previously described were stained with FITC-conjugated anti-mouse CD11b, CD8, or CD40 mAbs; PE-labeled anti-mouse CD8, CD11b, I-Ad, CD80, or CD86 mAbs; biotinylated anti-mouse CD11c mAbs followed by streptavidin-PerCP-Cy5.5; and allophycocyanin (APC)-labeled anti-mouse B220 mAb (all conjugates were obtained from BD PharMingen, San Jose, CA). The samples were then subjected to FACS analysis (FACSCalibur™; BD Biosciences, San Jose, CA).
Immunohistochemistry of NALT
Hearts of sacrificed mice were injected with 4 % paraformaldehyde for perfusion fixation [40]. The skin, lower jaw, and incisors of the upper jaw were removed from the heads of the decapitated mice, and the remaining tissue was fixed in 1 % paraformaldehyde for 24 h. The skull was decalcified in 10 % EDTA in PBS for 7 days at 4 °C. Upon decalcification, the tissues were saturated with 30 % sucrose in PBS for 2 h to avoid tissue separation, embedded in OCT, and snap-frozen at −160 °C. We stained 4-µm cryostat sections with PE-conjugated anti-B220 (RA3-6B2), biotin-labeled anti-CD11c (HL3), or biotin-labeled anti-CD3 (145-2C11) mAbs (BD PharMingen). Biotin-labeled mAb was followed by HRP-conjugated streptavidin-Alexa Fluor 488® (Molecular Probes, Eugene, OR). Sections were examined with a fluorescence microscope (BX50/BXFLA; Olympus, Tokyo, JAPAN) equipped with a digital image capture system (Olympus, Tokyo, JAPAN) or an all-in-one type fluorescence microscope (BZ-8000; KEYENCE, Tokyo, JAPAN).
In vitro APC functional analysis
To assess whether NALT DCs possess Ag presenting cell (APC) function, DCs were isolated two weeks after the final immunization from NALT, NPs and CLNs of mice immunized with OVA plus a combination of pFL and CpG as adjuvant. Mononuclear cells isolated from NALT and NPs were stained with PE- labeled CD11c (HL3) mAbs (BD PharMingen, San Jose, CA) and DCs from NALT and NPs were then sorted by FACSAria™ (BD Biosciences). CD11c+ DCs from CLNs were purified by use of CD11c microbeads (Miltenyi Biotec, Auburn, CA) and the AutoMACS system. These purified DC fractions were > 97 % CD11c+ and the cells were > 99 % viable. Splenic cells from DO11.10 Tg mice were stained with FITC conjugated anti-CD4 and PE- labeled anti-KJ1.26 mAbs. OVA-specific naïve CD4+ T cells were then purified by FACSAria™. NALT DCs (1 × 104 cells) as well as DCs isolated from CLNs and NPs (2 × 104 cells) were incubated with the same cell numbers of OVA-specific CD4+ T cells for five days without OVA. The wells containing splenic OVA-specific CD4+ T cells from naïve DO11.10 Tg mice with or without OVA (one mg /ml) were used as positive and negative controls, respectively. To assess T cell proliferative responses, an aliquot of 0.5 mCi of tritiated-thymidine [3H]TdR (Amersham Biosciences, Arlington Heights, IL) was added during the final 18 h of incubation, and the amount of [3H]TdR incorporation was determined by scintillation counting.
Statistical analysis
All results are expressed as the mean ± the standard error of the mean (SEM), and experimental groups were compared with controls using an unpaired non-parametric Mann-Whitney U test with Statview software (Abacus Concepts, Berkley, CA) designed for Macintosh computers. Values of p of < 0.05 or < 0.01 were considered significant.
Results
A combination of pFL and CpG ODN as nasal adjuvant enhanced Ag-specific mucosal and plasma Ab responses
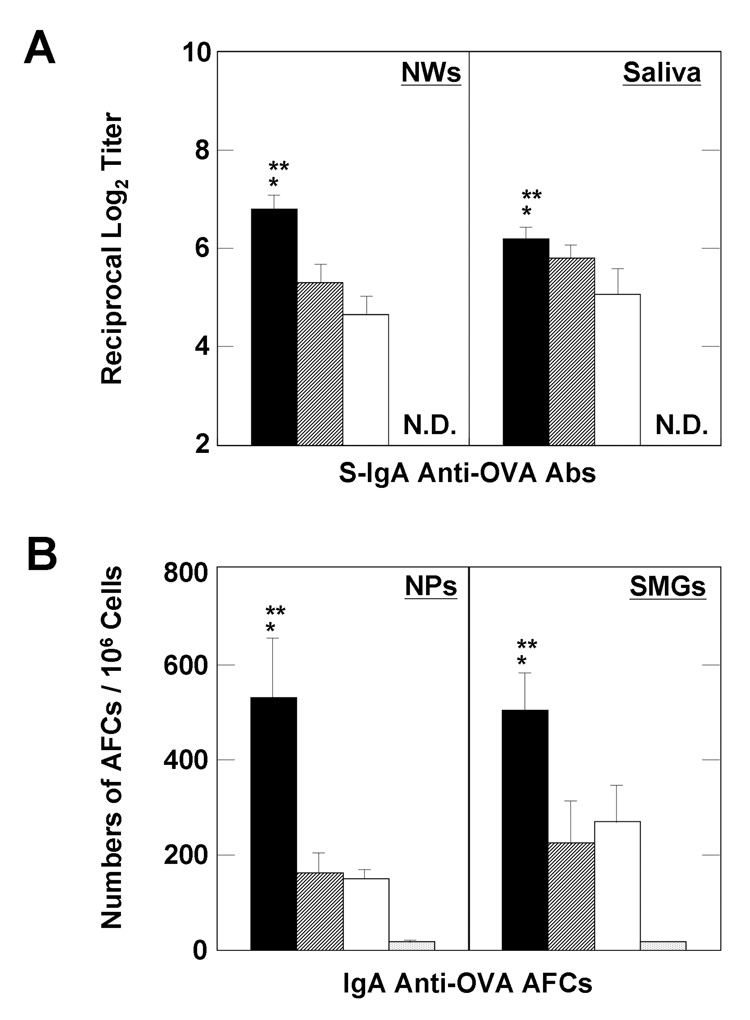
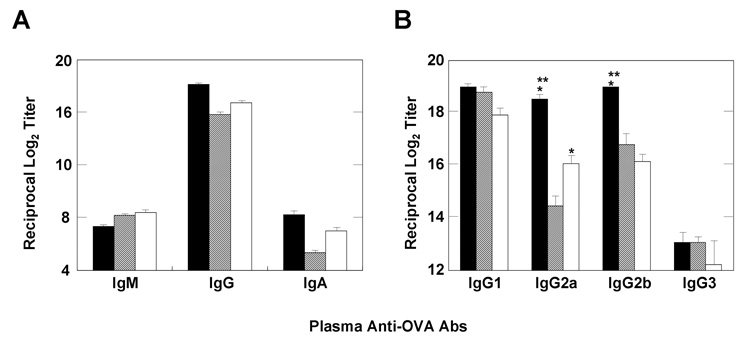
We first examined whether the combination of pFL and CpG ODN as a nasal adjuvant would enhance OVA-specific Ab responses or result in suppressed immune responses in both mucosal and systemic lymphoid tissues. Young adult mice given nasal OVA and a combination of pFL and CpG ODN exhibited OVA-specific S-IgA Ab responses in saliva and NWs. In contrast, mice immunized with OVA alone did not produce OVA-specific S-IgA Ab responses (Figure 1A). Each of these OVA-specific S-IgA Ab responses in external secretions was similar to those of mice given OVA and either pFL or CpG ODN alone (Figure 1A). To further support these findings, mice given nasal OVA and the combined adjuvant exhibited significantly higher numbers of OVA-specific IgA AFCs in SMGs and NPs than those administered OVA alone. Interestingly, these AFC numbers were also significantly higher when compared with those of mice given OVA and pFL or CpG ODN (Figure 1B). A similar pattern of enhanced OVA-specific Ab responses were also seen in plasma. Thus, elevated levels of OVA-specific plasma IgG Ab responses were observed in mice nasally administered OVA and a combination of pFL and CpG ODN as well as mice given OVA nasally with either pFL or CpG ODN adjuvant (Figure 2A). Ab responses of both IgG1 and IgG2b, but not IgG2a subclasses were increased in mice immunized with OVA and pFL (Figure 2B). In contrast, Ab levels of not only IgG1 and IgG2b but also IgG2a were increased in mice immunized with OVA and CpG ODN. Moreover, significantly higher levels of IgG2a and IgG2b were seen in mice immunized with OVA and the double DNA adjuvant, when compared with mice given nasal OVA and either pFL or CpG ODN as adjuvant (Figure 2B). These data suggest that pFL and CpG ODN as a combined nasal adjuvant induced both Th1- and Th2-type, cytokine-mediated OVA-specific immune responses. Taken together, these results indicated that nasal immunization with the double DNA adjuvant effectively induced a more diverse OVA-specific Ab response in both mucosal and systemic immune compartments than that observed in mice given OVA and a single adjuvant.
Figure 1.
OVA-specific Ab responses in mucosal lymphoid tissues. Groups of BALB/c mice were nasally immunized weekly for three consecutive weeks with OVA and a combination of plasmid encoding the Flt3 ligand cDNA (pFL) and CpG oligodeoxynucleotides (CpG ODN) (solid pattern), pFL alone (striped pattern), or CpG ODN alone (open pattern) as nasal adjuvants. In the control groups, mice were nasally immunized with OVA alone (dotted pattern). The levels of S-IgA anti-OVA Abs in nasal washes (NWs) and saliva were determined by OVA-specific ELISA seven days after the final immunization (A). To determine the numbers of IgA Ab-forming cells (AFCs), mononuclear cells isolated from submandibular glands (SMGs) and nasal passages (NPs) were subjected to an OVA-specific ELISPOT assay seven days after the last immunization (B). The values are presented as the mean ± SEM of 25 mice in each group. When compared with mice immunized with OVA and pFL, *p < 0.01. When compared with mice immunized with OVA and CpG ODN, **p < 0.01. N.D. means that O. D. values were not detected.
Figure 2.
Comparison of OVA-specific Ab responses in systemic lymphoid tissues. BALB/c mice were immunized weekly for three consecutive weeks with OVA and a combination of plasmid encoding the Flt3 ligand cDNA (pFL) and CpG oligodeoxynucleotides (CpG ODN) (solid pattern), pFL alone (striped pattern), or CpG ODN alone (open pattern) as nasal adjuvants. The levels of plasma anti-OVA IgM, IgG, IgA (A), or IgG subclass (B) Abs were measured by OVA-specific ELISA seven days after the final immunization. The values are presented as the mean ± SEM of 25 mice in each group. The plasma from mice nasally immunized with OVA alone did not contain detectable Ab levels (data not shown). The values are presented as the mean ± SEM of 25 mice in each group. When compared with mice immunized with OVA and pFL, *p < 0.01. When compared with mice immunized with OVA and CpG ODN, **p < 0.01.
A combination of pFL and CpG ODN as nasal adjuvant elicits long-lasting Ag-specific mucosal immunity
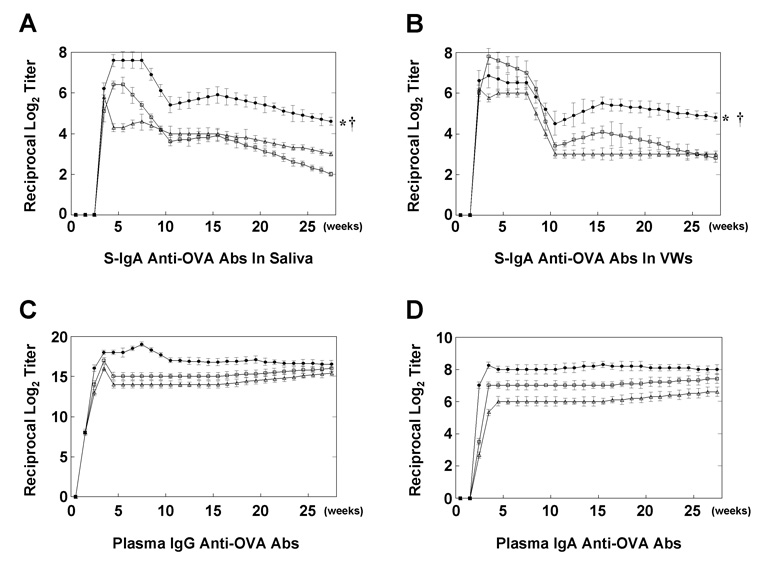
We next investigated the kinetics of OVA-specific Ab responses induced by a combination of DNA adjuvants. Importantly, OVA-specific S-IgA Ab titers in saliva and VWs of young adult mice immunized with OVA and double adjuvant were maintained until at least 25 weeks after the final immunization, although these Ab responses were slightly reduced when compared with peak Ab titers (Figure 3, A and B). Interestingly, OVA-specific S-IgA Abs in saliva differed significantly in mice given OVA and combined adjuvant than in mice given OVA and either pFL or CpG ODN (Figure 3A). Similarly, OVA-specific S-IgA Abs in VWs obtained from mice given OVA and combined adjuvant were significantly higher when compared with mice given OVA and either pFL or CpG ODN, although there were no significant differences between each group at one week after the final immunization (Figure 3B). In contrast, although prolonged OVA-specific plasma IgG and IgA Ab responses were observed in mice immunized with OVA and combined adjuvant, these levels were similar to those of mice immunized with OVA and either pFL or CpG ODN (Figure 3, C and D).
Figure 3.
Long-term analysis of OVA-specific Ab responses in external secretions. BALB/c mice were immunized weekly for three consecutive weeks with OVA and a combination of plasmid encoding the Flt3 ligand cDNA (pFL) and CpG oligodeoxynucleotides (CpG ODN) (filled boxes), pFL alone (open triangles), or CpG ODN alone (open boxes) as nasal adjuvants. Saliva (A), vaginal washes (VWs) (B) and plasma (C, D) were collected weekly from the first day of immunization until 27 weeks afterwards. The levels of OVA-specific Ab responses were determined by OVA-specific ELISA. The values are presented as the mean ± SEM of 20 mice for each group. When compared with mice immunized with OVA and pFL, *p < 0.01. When compared with mice immunized with OVA and CpG ODN, †p < 0.01.
Nasal administration of OVA with a combination of pFL and CpG ODN induce both Th1- and Th2-type cytokine responses
We next assessed Th1- and Th2-type cytokine profiles induced by a combination of the 2 adjuvants or single application of either pFL or CpG ODN with OVA via the nasal route. OVA-stimulated CD4+ T cells purified from CLNs of young adult mice given nasal OVA and pFL exhibited significantly higher IL-2 and IL-4 production levels when compared with mice given OVA and CpG ODN as nasal adjuvant. In contrast, significantly higher levels of IFN-γ and IL-6 were produced by CD4+ T cells from CLNs of mice administered OVA and CpG ODN when compared with those given OVA and pFL. Interestingly, the combination of pFL and CpG ODN exhibited significantly higher levels of IFN-γ production by CD4+ T cells when compared with pFL as a nasal adjuvant. Further, mice given nasal pFL and CpG ODN exhibited significantly higher levels of IL-4 production by CD4+ T cells than those from mice given nasal CpG ODN (Table 1). These results were further confirmed at the mRNA level by quantitative real-time PCR analysis. Thus, OVA-stimulated CD4+ T cells from CLNs of mice immunized with OVA and combined adjuvant exhibited increased levels of both IFN-γ- and IL-4-specific mRNA when compared with those given OVA and either pFL or CpG ODN as nasal adjuvants (Table 1). Taken together, these results clearly indicate that the double DNA adjuvant elicits a balanced IFN-γ (Th1) and IL-4 (Th2) immune response.
Table 1.
OVA-induced CD4+ Th1- and Th2-type cytokine profiles in cervical lymph nodes (CLNs) of young adult mice immunized with OVA plus a combination of plasmid encoding the Flt3 ligand cDNA (pFL) and CpG oligodeoxynucleotides (CpG ODN), and either pFL or CpG ODNa
| Th1- And Th2-Type Cytokineb | Levels Of Cytokine-Specific cDNAc | |||||||
|---|---|---|---|---|---|---|---|---|
| (pg/ml) | (Attomol /1 ug total cDNA) | |||||||
| Nasal adjuvant | IFN-γ | IL-2 | IL-4 | IL-5 | IL-6 | IL-10 | IFN-γ | IL-4 |
| pFL and CpG ODN | 2881e (± 141)d | 26 (± 8) | 19f (± 2) | 26 (± 6) | 181 (± 59) | 321 (± 45) | 0.11 (± 0.08) | 0.17 (± 0.06) |
| pFL | 37 (± 9) | 206f (± 6) | 42f (± 12) | 38 (± 8) | 292 (± 65) | 88 (± 10) | 0.00 (± 0.00) | 0.35f (± 0.08) |
| CpG ODN | 4609e (± 197) | 6 (± 1) | 0 (± 0) | 62 (± 4) | 103 (± 5) | 686 (± 12) | 0.64e (± 0.02) | 0.08 (± 0.03) |
Each group of young adult mice were nasally immunized three times at weekly intervals with OVA (100 µg) plus pFL (50 µg), CpG ODN (10 µg) or a combination of pFL (50 µg) and CpG ODN (10 µg). The CD4+ T cells (4 × 106 cells/ml) in CLNs were cultured with one mg/ml of OVA in the presence of T cell-depleted and irradiated splenic feeder cells (8 × 106 cells/ml).
Culture supernatants were harvested after five days of incubation and analyzed by the respective cytokine-specific ELISA.
Total RNA was extracted from these cells and subjected to quantitative RT-PCR analysis by use of a Lightcycler™.
The values shown are the mean ± SEM of three independent experiments. Each group consisted of five BALB/c mice.
p<0.05 compared with mice immunized with OVA plus pFL (unpaired non-parametric Mann-Whitney U test).
p<0.05 compared with mice immunized with OVA plus CpG ODN (unpaired non-parametric Mann-Whitney U test).
A combination of pFL and CpG ODN selectively expands activated pDCs and CD8+ DCs in NALT
We next investigated the frequency and phenotypes of CD11c+ DCs in various mucosal inductive and effector tissues by flow cytometric analysis. Our results revealed that a combination of nasal pFL and CpG ODN significantly increased the frequencies of CD11c+ DCs in NALT and NPs, when compared with naïve mice (Table 2). Further, CD11c+ DCs were also increased in CLNs and spleen to some extent (Table 2). Although pFL given alone as nasal adjuvant increased the frequencies of CD11c+ DCs in NALT, nasal CpG ODN failed to do so, and the DC frequencies were essentially the same as those observed in non-immunized mice (Table 2). These expanded DCs expressed higher frequencies of costimulatory molecules (CD40, CD80, and CD86) and MHC class II when compared with DCs from NALT and NPs of naïve mice. Interestingly, increased CD11c+ DCs in NALT and NPs expressed either B220 or CD8. Thus, absolute cell numbers of CD8− B220+ pDC and CD8+ B220− non-pDC populations were preferentially increased by the combined adjuvant regimen as well as the pFL nasal adjuvant (Table 3). DC profiles in NPs were similar to those observed in NALT (data not shown). These results indicate that the presence of pFL in the vaccine construction is essential for the activation and expansion of pDCs and CD8+ DCs, although an additional nasal CpG ODN is required for the induction and maintenance of prolonged high levels of mucosal immune responses, as well as the production of Th1-type cytokine-mediated OVA-specific Ab responses.
Table 2.
Comparison of the frequencies of CD11c+ dendritic cells (DCs) in various lymphoid tissuesa.
| Percentage Of CD11c+ DCs In Total Lymphocyteb |
||||
|---|---|---|---|---|
| Nasal Adjuvant | NALT | NPs | CLNs | Spleen |
| pFL+CpG ODN | 5.5c (± 0.7) | 11.5c (± 0.4) | 3.0 (± 0.2) | 4.4 (± 0.1) |
| pFL | 5.9c (± 0.4) | 13.2c (± 0.7) | 1.7 (± 0.5) | 3.2 (± 0.8) |
| CpG ODN | 2.7 (± 0.2) | 6.2 (± 0.3) | 1.5 (± 0.5) | 3.9 (± 0.3) |
| (Non-immunized) | 1.7 (± 0.3) | 5.1 (± 0.2) | 1.4 (± 0.3) | 2.7 (± 0.2) |
Mononuclear cells were isolated from NALT, nasal passages (NPs), cervical lymph nodes (CLNs) and spleen of young adult mice immunized with OVA plus a combination of plasmid encoding the Flt3 ligand cDNA (pFL) and CpG oligodeoxynucleotide (CpG ODN), or either pFL or CpG ODN. These cells were stained with fluorescence-conjugated anti-CD11c mAbs and subjected to flow cytometry analysis by FACSCalibur™.
The values are presented as the mean ± SEM of three independent experiments. Each experiment consisted of five BALB/c mice.
p < 0.01 when compared with non-immunized naïve mice (unpaired non-parametric Mann-Whitney U test).
Table 3.
Comparison of the frequencies of CD11c+ dendritic cells (DCs), and absolute cell numbers of costimulatory molecule expression by subsets of CD11c+ DCs in NALTa
| % Total Lymphocytes |
Absolute Cell Numbersb (×103) |
Absolute Cell Numbers (×103) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B220+ |
B220−CD11c+ |
CD11c+ |
|||||||
| Nasal Adjuvant | CD11c+ | CD11c+ | CD8+ | CD11b+ | CD8−CD11b− | CD40+ | I-Ad+ | CD80+ | CD86+ |
| pFL and CpG ODN | 5.5d (± 0.7)c | 21.8d (± 2.6) | 1.3d (± 0.1) | 1.1 (± 0.4) | 2.9 (± 0.0) | 24.8d (± 2.6) | 24.8d (± 2.3) | 9.7d (± 0.7) | 16.2d (± 2.0) |
| pFL | 5.9d (± 0.4) | 21.6d (± 3.4) | 1.9d (± 0.4) | 1.4 (± 0.3) | 7.1 (± 2.7) | 25.3d (± 3.7) | 23.8e (± 4.1) | 11.6d (± 2.2) | 16.5e (± 3.2) |
| CpG ODN | 2.7 (± 0.2) | 7.8 (± 1.9) | 0.9 (± 0.1) | 0.7 (± 0.2) | 2.5 (± 0.9) | 10.6 (± 1.7) | 8.5 (± 2.3) | 2.8 (± 1.6) | 6.3 (± 1.3) |
| (Non-immunized) | 1.7 (± 0.3) | 5.8 (± 1.9) | 0.5 (± 0.1) | 0.6 (± 0.2) | 1.4 (± 0.4) | 3.4 (± 1.2) | 5.4 (± 1.0) | 1.4 (± 0.7) | 3.0 (± 1.1) |
Mononuclear cells were isolated from NALT of young adult mice immunized with OVA plus a combination of plasmid encoding the Flt3 ligand cDNA (pFL) and CpG oligodeoxynucleotide (CpG ODN), or either pFL or CpG ODN. These cells were stained with fluorescence-conjugated mAbs and subjected to flow cytometry analysis by FACSCalibur.
Total cell number isolated from NALT of one mouse is ~0.8 × 106.
The values are presented as the mean ± SEM of three independent experiments. Each experiment consisted of five BALB/c mice.
p < 0.01
p < 0.05 when compared with non-immunized naïve mice (unpaired non-parametric Mann-Whitney U test).
NALT DCs accumulate in NALT T cell area
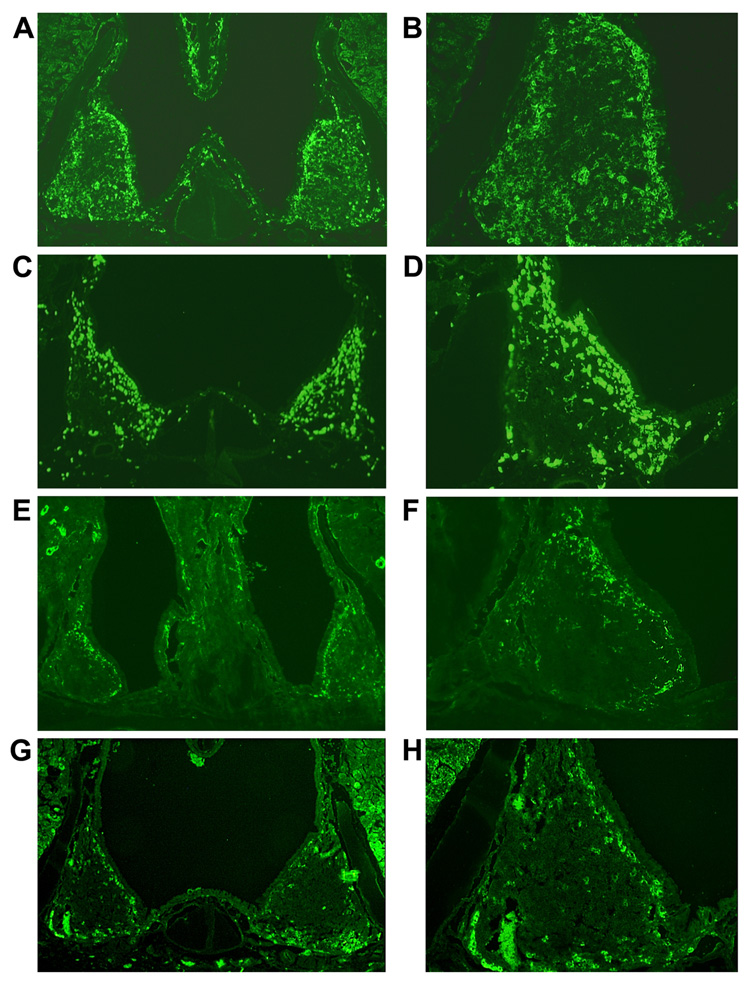
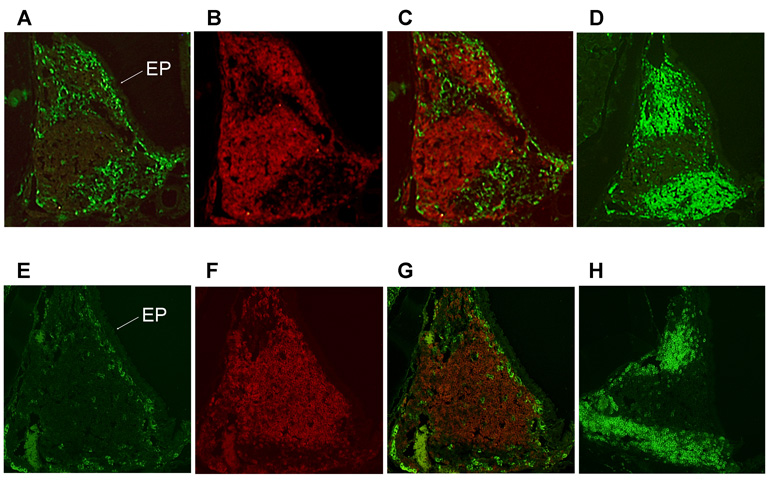
Based on the above flow cytometric analysis results, immunohistochemical analysis confirmed the markedly increased numbers of CD11c+ DCs in the NALT of young adult mice immunized with OVA and a combination of pFL and CpG ODN (Figure 4, A and B) or that of mice given nasal pFL (Figure 4, C and D) when compared with those of naïve mice (Figure 4, G and H). The numbers of CD11c+ DCs in NALT of naïve mice or mice given nasal CpG ODN alone were essentially unchanged (Figure 4, E and F). These results were confirmed by flow cytometric analysis illustrating the expansion of NALT DCs (Table 2). Since NALT plays important roles in the initial induction of mucosal immune responses, it was important to establish the precise tissue location of these expanded DCs in NALT. Thus, immunofluorescence staining revealed that CD11c+ DCs were mainly located beneath the nasal epithelium surrounding NALT (Figure 5, E and G). In contrast, the expanded numbers of CD11c+ DCs were observed within CD3+ T cell zones in addition to the subepithelial region of mice given a combination of nasal pFL and CpG ODN (Figure 5, A and C). These results suggest that increased numbers of activated CD11c+ DCs, which are most probably pDCs and CD8+ DCs, cross-talk with T cells to initiate Ag-specific immune responses.
Figure 4.
Immunofluorescence staining of CD11c+ DCs in NALT. BALB/c mice were immunized with OVA and plasmid encoding Flt3 ligand cDNA (pFL) and CpG oligodeoxynucleotides (CpG ODN) (A, B), pFL (C, D), or CpG ODN (E, F) as nasal adjuvants. NALT taken from naïve mice were also stained as controls (G, H). Frozen sections of NALT were stained with biotin-conjugated, anti-CD11c (HL3) mAbs followed by HRP-conjugated streptavidin-Alexa Fluor 488®. The original magnification was × 40 (A, C, E, G) and ×100 (B, D, F, H). The picture is a typical example of results of immunofluorescence analysis of over 20 samples.
Figure 5.
Immunofluorescence analysis of localization of CD11c+ dendritic cells (DCs) in NALT. BALB/c mice were administered OVA and a combination of plasmid encoding the Flt3 ligand cDNA (pFL) and CpG oligodeoxynucleotides (CpG ODN) (A, B, C, D) as nasal adjuvants. NALT taken from naïve mice were also stained as controls (E, F, G, H). Frozen sections of NALT were stained with biotin-conjugated anti-CD11c mAb followed by Alexa Fluor 488® and PE-conjugated anti-B220 mAb (A and B, or E and F). These two images were acquired from the same slide and merged to determine the precise location of the DCs in NALT (C, G). Serial sections were also stained with biotin-conjugated anti-CD3 mAb followed by Alexa Fluor 488® (D, H). Cells stained with Alexa Fluor 488® developed a green color, while those stained with PE-conjugated anti-B220 are observed in red. The original magnification is ×100. Arrow labeled as EP indicates the epithelium of NALT.
NALT DCs play a key role in the induction of Ag-specific immunity
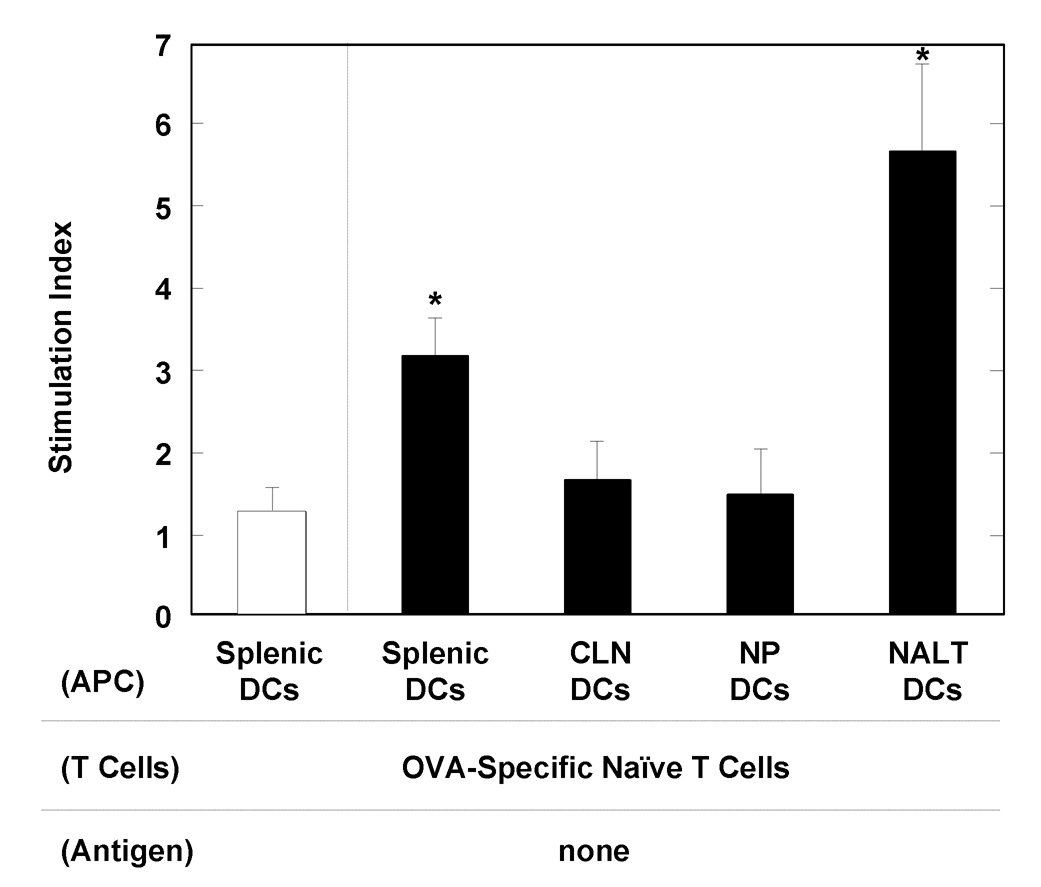
To assess the functional roles of NALT DCs in the induction of mucosal and systemic immune responses in mice nasally-immunized with OVA plus a combination of pFL and CpG ODN, we next examined whether NALT DCs were able to activate CD4+ T cells. The NALT DCs were isolated from mice given OVA plus a combination of pFL and CpG ODN two weeks after the last immunization. These purified DCs were cultured with naïve CD4+ T cells taken from non-immunized, DO11.10 OVA-specific TCR-Tg mice without any Ag stimulation. Five days later, CD4+ T cells co-cultured with NALT DCs showed significantly higher proliferative responses when compared with control cultures containing splenic DCs isolated from naïve mice (Figure 6). However, DCs from CLNs and NPs of mice given nasal OVA plus double adjuvant failed to support OVA-specific CD4+ T cell proliferative responses (Figure 6). When CD4+ T cells from DO11.10 mice were cultured with OVA, significantly elevated proliferative responses were seen (approximately 25 – 50 of stimulation index) (data not shown). In contrast, CD4+ T cells without DCs or with DCs isolated from CLN, NPs and NALT in naïve mice showed essentially no proliferative responses (data not shown). Since this culture system does not contain any OVA as stimulus, these results show that NALT DCs from mice given nasal OVA plus combined adjuvants are responsible for stimulating OVA-specific CD4+ T cells. Taken together, our findings clearly show that NALT DCs from mice immunized with OVA plus a combination of pFL and CpG ODN play a central role in NALT APC functions.
Figure 6.
Analysis of APC activity by NALT DCs. DC activities for induction of CD4+ T cell proliferation were analyzed. BALB/c mice were nasally immunized with OVA plus a combination of pFL and CpG ODN (filled pattern) three times at weekly intervals. Two weeks after the last immunization, DCs were purified from NALT, CLNs and NPs. The DCs from CLNs, NPs (4 × 105 cells/ml) or NALT (2 × 105 cells/ml) were cultured with the same numbers of naïve CD4+ T cells isolated from non-immunized, DO11.10 OVA TCR transgenic mice for five days. The wells containing OVA-specific CD4+ T cells with or without OVA served as positive and negative controls, respectively. An aliquot of 0.5 mCi of tritiated [3H]TdR was added during the final 18 h of incubation. The stimulation index was determined as “cpm of wells with DCs / cpm of wells without DCs”. Splenic DCs isolated from naïve mice were also used as controls (open pattern). The values are presented as the mean ± SEM of 20 mice for each group. *p < 0.01 when compared with splenic DCs isolated from naïve mice.
A combination of pFL and CpG ODN as nasal adjuvant elicits both plasma and mucosal Abs in 2-year-old mice
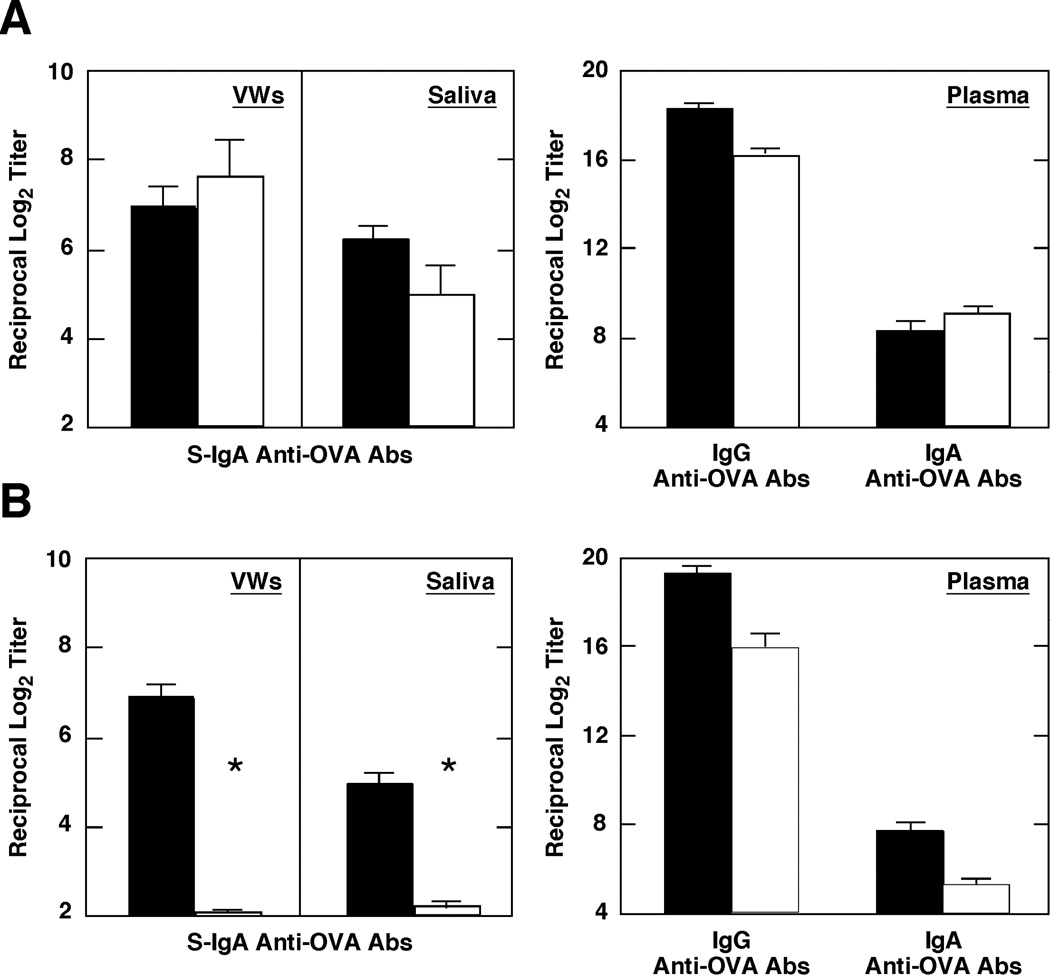
We next examined whether a combination of pFL and CpG ODN as nasal adjuvant were capable of eliciting mucosal S-IgA Ab responses in two-year-old mice, since our previously studies showed that nCT (which is the most potent nasal adjuvant known) failed to induce Ag-specific S-IgA Ab responses in these old mice [36]. When two-year-old mice were nasally immunized with 100 µg of OVA and a combination of 50 µg of pFL and 10 µg of CpG ODN, significant S-IgA Ab responses were noted in both saliva and VWs, which were essentially equivalent to those observed in young adult mice. In addition, plasma IgG and IgA Ab responses were also noted in two-year-old mice immunized with OVA and double DNA adjuvants (Figure 7A). In contrast, 0.5 µg of nCT as a nasal adjuvant failed to induce Ag-specific S-IgA Ab responses in saliva and VWs of two-year-old mice although significant level of anti-OVA IgG Ab response was noted (Figure 7B). These results clearly indicate that the combination of pFL and CpG ODN as a nasal adjuvant is superior to nCT in the induction of mucosal Ab responses in aged mice.
Figure 7.
Comparison of OVA-specific IgA and IgG Ab responses in vaginal washes (VWs), saliva, and plasma obtained from young adult (filled pattern) and two-year-old (open pattern) mice. Each mouse group was nasally immunized weekly for 3 consecutive weeks with 100 µg of OVA and a combination of 50 µg of plasmid encoding the Flt3 ligand cDNA (pFL) and 10 µg of CpG oligodeoxynucleotides (CpG ODN) (A), or 100 µg of OVA and 0.5 µg of nCT (B). IgA Ab levels in VWs and saliva and IgG and IgA Ab responses in plasma were determined seven days later by OVA-specific ELISA. Values presented are the mean ± SEM of 5 mice in each experimental group. *p < 0.05 when compared with young adult mice.
Discussion
In the present study, we have shown that nasal delivery of a combination of pFL and CpG ODN enhances co-administered Ag, e.g., OVA-specific mucosal and systemic immune responses similar to those induced by a single nasal adjuvant regimen (either pFL or CpG ODN). Each of these DNA adjuvant regimen could elicit long-lasting mucosal and systemic OVA-specific Ab responses for up to 25 weeks after the last immunization. Of note, the levels of prolonged mucosal immunity induced by the combined adjuvant displayed significantly higher responses when compared with nasal administration with OVA and either pFL or CpG ODN as individual adjuvants. In addition, the combination of pFL and CpG ODN as nasal adjuvants induced both CD4+ Th1- and Th2-type cytokine-mediated immune responses. Induction of prolonged Ag-specific immune responses was associated with elevated numbers of activated pDCs and CD8+ DCs in NALT. Importantly, significant levels of mucosal and systemic Abs responses were elicited even in two-year-old mice by nasal delivery of the combined adjuvant. This is the first study to show the effectiveness of DCs-targeting nasal DNA adjuvants for the induction of not only memory T cell responses but also Ag-specific S-IgA Abs in aged mice.
Our previous study demonstrated that nasal administration of pFL as a mucosal adjuvant induced Th2-type cytokine production, especially IL-2 and IL-4, by CD4+ T cells [21]. Our present study confirmed these findings and exhibited essentially identical results, i.e., increased levels of IL-2 and IL-4 and lower levels of IFN-γ production by Ag-specific CD4+ T cells when pFL was employed as the nasal adjuvant. In contrast, nasal administration of CpG ODN as the mucosal adjuvant resulted in increased levels of IFN-γ and IL-6, but not IL-4, by CD4+ T cells. The results of our studies and those of others have shown that nasal administration of CpG ODN as mucosal adjuvant most probably induced Th1-type cytokine responses based upon Ag-specific plasma IgG subclass profiles [28, 29]. Although it is well known that CpG ODN as a systemic adjuvant induces Th1-dominant immune responses that mainly consist of IFN-γ production [24–27], our findings are the first direct evidence that nasal CpG ODN elicits a dominant Th1-type cytokine responses by Ag-specific CD4+ T cells. When a combination of pFL and CpG ODN is used as the nasal adjuvant, both IFN-γ and IL-4 production by CD4+ T cells, as well as Ab responses of IgG1, IgG2a and IgG2b were significantly upregulated when compared with mice immunized with OVA and either pFL or CpG ODN as the nasal adjuvant. Taken together, these results clearly show that a combination of pFL and CpG ODN as a combined nasal adjuvants can elicits both Th1- and Th2-type cytokine-mediated Ag-specific Ab responses.
Induction of both Th1- and Th2-type responses is the major goal for the development of mucosal vaccines in the elderly since these responses would provide protective immunity against viral and bacterial infections by maximizing Ag-specific Ab and CTL responses an immunocompromised situation. Since a combined pFL and CpG ODN adjuvant elicited balanced Th1- and Th2-type responses, we examined its adjuvant activity in two-year-old mice. Of importance, significant Ag-specific S-IgA Ab responses, which were comparable to those of young adult mice were induced in mucosal secretions of two-year-old mice. These findings are the first to show that nasal adjuvant successfully elicits mucosal S-IgA Ab responses in two-year-old mice. Indeed, our current and previous studies showed that the potent mucosal adjuvant, nCT failed to induce mucosal S-IgA Ab responses, although Ag-specific systemic IgG Ab responses were essentially identical to those observed in young adult mice [36]. Thus, CD4+ T cell proliferation as well as Th1 and Th2 cytokine responses in the spleens of two-year-old mice was comparable to those of young adult mice when nCT was used as the nasal adjuvant [36]. These findings agree well with our previous report that mucosal immunosenescence occurs prior to systemic immune dysregulation [35]; however, the present studies clearly showed that a combination of pFL and CpG ODN overcomes mucosal immunosenescence in two-year-old mice. In this regard, the mechanism for a combination of pFL and CpG ODN as the nasal adjuvant in aged mice could be due to the expansion of pDCs and CD8+ DCs in NALT. Taken together, our results indicate that NALT DCs targeting adjuvants would be a key for the induction of mucosal and systemic immunity in the elderly.
In case of naïve mice, NALT DCs are mainly located underneath the nasal epithelium. When mice were immunized with OVA and pFL alone or with a combination of pFL and CpG ODN, an increased frequency of DCs could be detected within the T-cell zones but not the B-cell zones in addition to the subepithelial region of NALT. This pattern of DC population is similar in nature to the location of DCs in Peyer’s patches (PPs) [41]. Thus, CD11b+ myeloid-type DCs were observed in the subepithelial dome (SED) region, whereas CD8α+ lymphoid-type DCs are present in the T cell-rich interfollicular region (IFR). It has been shown that PP DCs in the SED are key players in Ag presentation to CD4+ T cells in the IFR. Indeed, DCs in the SED process reovirus strain type 1 Lang (T1L) Ags from infected apoptotic epithelial cells and subsequently present Ag information to CD4+ T cells [42]. In this regard, it is logical to predict that NALT DCs would possess similar immunobiological activity since NALT DCs in naïve mice reside under the nasal epithelium where they are constantly exposed to environmental Ags, allergens, and pathogens present in inhaled air. Indeed, based upon our immunohistochemical analysis, an increased frequency of NALT DCs in this T-cell-rich area of mice given pFL and CpG ODN clearly indicates that Ag-loading SED DCs migrate into the T cell area in order to stimulate naïve CD4+ T cells. Since identical phenotypes of DCs are increased in NPs, one could predict that some of these DC subsets migrate from NALT in addition to DCs expanded within the NPs. In addition to NALT and NPs, the numbers of DCs were increased in CLNs and spleen to some extent. These results indicate that DCs in CLNs and spleen also play important roles in the induction of Ag-specific immune responses. We predict that these DCs are originated from either NALT or NPs and they play significant roles in the initiation of Ag-specific mucosal and systemic immune responses. To support this notion, our most recent studies showed that a specific DC subset initially increased in NALT of mice given nasal OVA plus adenovirus-expressing FL as mucosal adjuvant, and subsequently migrated into CLNs [43]. Since the frequencies of DCs in various lymphoid tissues were examined on day 21 in the present study, we are currently investigating the numbers of DCs in early phase (day 7 or 14) in order to show NALT DC migration and its cellular nd molecular mechanisms.
It is well known that FL is the major cytokine for the development of pDCs from hematopoietic stem cells in both humans and mice [44, 45]. In this regard, FL-transgenic mice have more pDCs, whereas FL-deficient mice have fewer pDCs [45]. Freshly isolated murine pDCs express lower levels of MHC and costimulatory molecules than the myeloid CD11c+ CD11b+ subset (mDC), which suggests a poor stimulatory capacity by these naïve pDCs for allogeneic and naïve T cells [46, 47]. Further, other reports have shown that the classical definition of DCs as cells with the unique function of inducing naïve T cells to enter the cell cycle is not applicable to non-activated pDCs [32]. Upon in vitro activation with CpG ODN, however, pDCs can develop a dendritic morphology and acquire the capacity to stimulate T cell proliferation [46]. These reports clearly suggest distinct roles of pDCs in the induction of innate and adaptive immunity. Thus, pre-activated innate-type pDCs possess the ability to rapidly respond to pathogens with production of enormous amounts of type I IFN and a poor ability to activate naïve T cells whereas activated acquired-type pDCs are able to stimulate naïve CD4+ T cells for the induction of adaptive immune responses [30, 32, 46]. In this regard, CD40 expressing NALT pDCs induced by nasal pFL and CpG ODN are most probably activated pDCs that are distinct from conventional pDCs. In addition to NALT pDC expansion, the numbers of CD8+ DCs is also increased when pFL and CpG ODN were administered nasally. This was due to the nasal application of pFL since our previous and current studies demonstrated that numbers of CD8+ DCs in NALT were selectively increased when pFL was used as a nasal adjuvant [21]. However, it is also possible that these expanded CD8+ DCs may be derived from pDCs since it was reported that pDCs were normally long lived and able to differentiate into CD8+ DCs after microbial infections [32]. Thus, it is also possible that long-lasting adaptive immune responses induced by a combination of pFL and CpG ODN may be attributable to long-lived pDCs and their progressing CD8+ DCs. Taken together, expansion of activated pDCs would correlate with the expansion of CD8+ DCs in NALT, which would result in long-lasting mucosal and systemic immune responses induced by a combination of pFL and CpG ODN as the nasal adjuvant.
In summary, the present study has shown that the combined adjuvant is an effective vaccination regimen in terms of maintaining long-lasting Ag-specific immune responses. The mechanisms of this DNA adjuvant were clearly mediated by increased pDCs and CD8+ DCs in NALT. Subsequently, these NALT DCs induced both IFN-γ-producing Th1- and IL-4-producing Th2-type CD4+ T cells for the induction of mucosal S-IgA and systemic IgG Ab responses. Thus, this combined adjuvant strategy successfully elicited mucosal immune responses in aged mice. Application of a combination of pFL and CpG ODN as a combined nasal adjuvant targeting NALT DC activation will help our efforts for mucosal vaccine development that will ultimately contribute to the prevention of infectious diseases of the upper respiratory tract, which is more serious in the elderly rather than in young adults.
Acknowledgments
This work is supported by NIH grants AG 025873, DE 12242, AI 18958, and AI 043197 and the Japanese Grant-in-Aid for Scientific Research 19791217.
We thank Ms. Annette M. Pitts for excellent technical assistance in the immunohistochemistry assays.
Abbreviations
- AFCs
antibody forming cells
- APCs
antigen presenting cells
- CLNs
cervical lymph nodes
- CpG ODN
synthetic oligodeoxynucleotides that contain one or more unmethylated cytosine-guanine dinucleotides
- DCs
dendritic cells
- FL
Flt3 ligand
- mDCs
myeloid DCs
- NALT
nasopharyngeal-associated lymphoreticular tissue
- NP
nasal passage
- NW
nasal wash
- pFL
plasmid encoding FL cDNA
- pDCs
plasmacytoid DCs
- PPs
Peyer’s patches
- SMGs
submandibular glands
- SPs
spleens
- TLRs
Toll-like receptors
- VWs
vaginal washes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authers have no financial conflict of interest.
References
- 1.Mestecky J, Blumberg RS, Kiyono H, McGhee JR. The mucosal immune system. In: Paul William E., editor. Fundamental Immunology. 5th edition. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 965–1020. [Google Scholar]
- 2.Imaoka K, Miller CJ, Kubota M, McChesney MB, Lohman B, Yamamoto M, et al. Nasal immunization of nonhuman primates with simian immunodeficiency virus p55gag and cholera toxin adjuvant induces Th1/Th2 help for virus specific immune responses in reproductive tissues. J Immunol. 1998;161(11):5952–5958. [PubMed] [Google Scholar]
- 3.Kurono Y, Yamamoto M, Fujihashi K, Kodama S, Suzuki M, Mogi G, et al. Nasal immunizaton induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J Infect Dis. 1998;180(1):122–132. doi: 10.1086/314827. [DOI] [PubMed] [Google Scholar]
- 4.McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10(2):75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel FW, et al. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94(10):5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujihashi K, McGhee JR, Kweon MN, Cooper MD, Tonegawa S, Takahashi I, et al. γδT-cell deficient mice have impaired mucosal IgA responses. J Exp Med. 1996;183(4):1929–1935. doi: 10.1084/jem.183.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vadolas J, Davies JK, Wright PJ, Strugnell RA. Intranasal immunization with liposomes induces strong mucosal immune responses in mice. Eur J Immunol. 1995;25(4):969–975. doi: 10.1002/eji.1830250417. [DOI] [PubMed] [Google Scholar]
- 8.Langermann S, Palaszynski S, Sadziene A, Stover CK, Koenig S. Systemic and mucosal immunity induced by BCG vector expressing outer-surface protein A of Borrelia burgdorferi. Nature. 1994;372(6506):552–555. doi: 10.1038/372552a0. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Briles DE, Yamamoto S, Ohmura M, Kiyono H, McGhee JR. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J Immunol. 1998;161(8):4115–4121. [PubMed] [Google Scholar]
- 10.Ohmura M, Yamamoto M, Kiyono H, Fujihashi K, Takeda Y, McGhee JR. Highly purified mutant E112K of cholera toxin elicits protective lung mucosal immunity to diphtheria toxin. Vaccine. 2001;20(5–6):756–762. doi: 10.1016/s0264-410x(01)00412-1. [DOI] [PubMed] [Google Scholar]
- 11.Kweon MN, Yamamoto M, Watanabe F, Tamura S, Van Ginkel FW, Miyauchi A, et al. A nontoxic chimeric enterotoxin adjuvant induces protective immunity in both mucosal and systemic compartments with reduced IgE antibodies. J Infect Dis. 2002;186(9):1261–1269. doi: 10.1086/344526. [DOI] [PubMed] [Google Scholar]
- 12.Hagiwara Y, McGhee JR, Fujihashi K, Kobayashi R, Yoshino N, Kataoka K, et al. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J Immunol. 2003;170(4):1754–1762. doi: 10.4049/jimmunol.170.4.1754. [DOI] [PubMed] [Google Scholar]
- 13.Yoshino N, Lu FX, Fujihashi K, Hagiwara Y, Kataoka K, Lu D, et al. A novel adjuvant for mucosal immunity to HIV-1 gp120 in nonhuman primates. J Immunol. 2004;173(11):6850–6857. doi: 10.4049/jimmunol.173.11.6850. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi R, Kohda T, Kataoka K, Ihara H, Kozaki S, Pascual DW, et al. A novel neurotoxoid vaccine prevents mucosal botulism. J Immunol. 2005;174(4):2190–2195. doi: 10.4049/jimmunol.174.4.2190. [DOI] [PubMed] [Google Scholar]
- 15.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 16.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 17.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 Ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184(5):1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson E, Westrich GM, Viney JL. Modulating dendritic cells to optimize mucosal immunization protocols. J Immunol. 1999;163(7):3668–3675. [PubMed] [Google Scholar]
- 19.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci. USA. 1999;96(3):1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viney JL, Mowat AM, O'Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160(12):5815–5825. [PubMed] [Google Scholar]
- 21.Kataoka K, McGhee JR, Kobayashi R, Fujihashi K, Shizukuishi S, Fujihashi K. Nasal Flt3 ligand cDNA elicits CD11c+CD8+ dendritic cells for enhanced mucosal immunity. J Immunol. 2004;172(6):3612–3619. doi: 10.4049/jimmunol.172.6.3612. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Rev Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 23.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 24.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 25.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon-γ. Proc Natl Acad Sci USA. 1996;93(7):2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-γ production by stimulation of interleukin-γ and tumor necrosis factor-α. Cell Immunol. 1996;167(1):72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 27.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186(10):1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCluskie MJ, Davis HL. CpG DNA as mucosal adjuvant. Vaccine. 1999;18(3–4):231–237. doi: 10.1016/s0264-410x(99)00194-2. [DOI] [PubMed] [Google Scholar]
- 29.Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective mucosal immunity to anthrax: Neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J Immunol. 2003;170(11):5636–5643. doi: 10.4049/jimmunol.170.11.5636. [DOI] [PubMed] [Google Scholar]
- 30.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5(12):1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 31.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5(12):1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J Exp Med. 2002;196(10):1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powers DC. Immunological principles and emerging strategies of vaccination for the elderly. J Am Geriatr Soc. 1992;40(1):81–94. doi: 10.1111/j.1532-5415.1992.tb01835.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmucker DL, Heyworth MF, Owen RL, Daniels CK. Impact of aging on gastrointestinal mucosal immunity. Dig Dis Sci. 1996;41(6):1183–1193. doi: 10.1007/BF02088236. [DOI] [PubMed] [Google Scholar]
- 35.Koga T, McGhee JR, Kato H, Kato R, Kiyono H, Fujihashi K. Evidence for early aging in the mucosal immune system. J Immunol. 2000;165(9):5352–5359. doi: 10.4049/jimmunol.165.9.5352. [DOI] [PubMed] [Google Scholar]
- 36.Hagiwara Y, McGhee JR, Fujihashi K, Kobayashi R, Yoshino N, Kataoka K, et al. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J Immunol. 2003;170(4):1754–1762. doi: 10.4049/jimmunol.170.4.1754. [DOI] [PubMed] [Google Scholar]
- 37.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250(4988):1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 38.Asanuma H, Inaba Y, Aizawa C, Kurata T, Tamura S. Characterization of mouse nasal lymphocytes isolated by enzymatic extraction with collagenase. J Immunol Meth. 1995;187(1):41–51. doi: 10.1016/0022-1759(95)00165-7. [DOI] [PubMed] [Google Scholar]
- 39.Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M, Kiyono H. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur J Immunol. 1998;28(10):3346–3353. doi: 10.1002/(SICI)1521-4141(199810)28:10<3346::AID-IMMU3346>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 40.Harmsen A, Kusser K, Hartson L, Tighe M, Sunshine MJ, Sedgwick JD, et al. Cutting edge: organogenesis of nasal-associated lymphoid tissue (NALT) occurs independently lymphotoxin-alpha (LT-α) and retinoic acid receptor-related orphan receptor-gamma, but the organization of NALT is LT-αdependent. J Immunol. 2002;168(3):986–990. doi: 10.4049/jimmunol.168.3.986. [DOI] [PubMed] [Google Scholar]
- 41.Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β and secondary lymphoid organ chemokine. J Exp Med. 2000;191(8):1381–1393. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleeton M, Contractor N, Leon F, Wetzel JD, Dermody TS, Kelsall BL. Peyer’s patch dendritic cells process viral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J Exp Med. 2004;200(2):235–245. doi: 10.1084/jem.20041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekine S, Kataoka K, Fukuyama Y, Adachi Y, Davydova J, Yamamoto M, et al. A novel adenovirus expressing flt3 ligand enhances mucosal immunity by inducing mature nasopharyngeal-associated lymphoreticular tissue dendritic cell migration. J Immunol. 2008;180(12):8126–8134. doi: 10.4049/jimmunol.180.12.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O'Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by Flt3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195(7):953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brawand P, Fitzpatrick DR, Greenfield BW, Brasel K, Maliszewski CR, De Smedt T. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J Immunol. 2002;169(12):6711–6719. doi: 10.4049/jimmunol.169.12.6711. [DOI] [PubMed] [Google Scholar]
- 46.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2(12):1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 47.Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194(8):1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]