Abstract
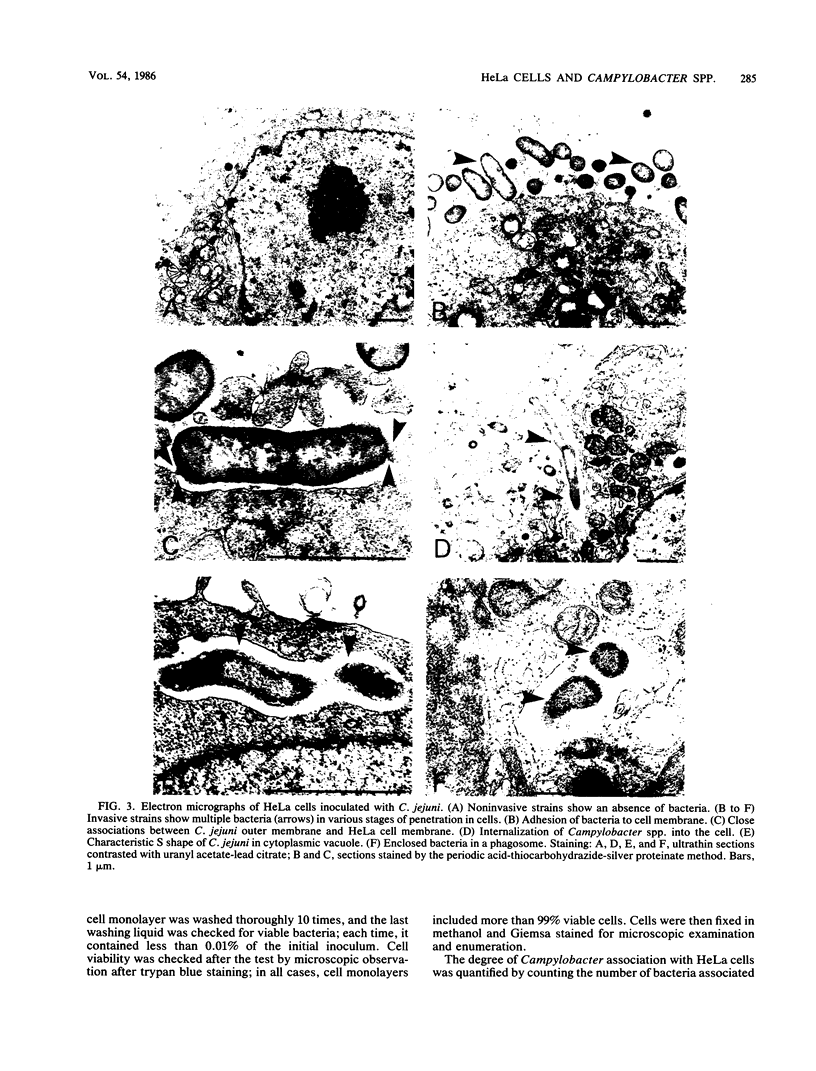
We developed a rapid in vitro test for determining the association of Campylobacter jejuni and C. coli with HeLa cells. Association was expressed as a weighted mean of the number of bacteria associated with one cell in an association index (AI). The reproducibility of the AI was checked by repeating the test six times, using four strains chosen at random. Means and standard deviations of the means were 7.3 +/- 1.2, 6.8 +/- 0.9, 1.8 +/- 1.2, and 0.1 +/- 0.2. The experimental conditions for which the results are reliable have been standardized. Among 42 strains from human feces, two groups appeared: for 22 nonassociative strains (52%), AI values ranged from 0.0 to 2.1 (mean +/- SD, 0.5 +/- 0.6); for 20 associative strains (48%), AI values ranged from 3.5 to 8.3 (mean +/- SD, 6.2 +/- 1.4). Of these 42 strains, 17 were clinically documented. Diarrhea occurred more frequently in patients infected with associative strains than in those infected with noninvasive strains (7/7 versus 3/10, P = 0.01). Fever also occurred more frequently in patients infected with associative strains (6/7 versus 2/10, P = 0.03). Transmission electron microscopy and viable counts made after killing of extracellular bacteria by gentamicin support the fact that associated Campylobacter spp. are adherent to the cell membrane and are internalized into cytoplasmic vacuoles. The described test seems to be a convenient and rapid method for estimating the pathogenicity of a given strain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Duncan D. J., Warren G. H., Wang W. L. Experimental Campylobacter jejuni infection of adult mice. Infect Immun. 1983 Feb;39(2):908–916. doi: 10.1128/iai.39.2.908-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovallius A., Nilsson G. Ingestion and survival of Y. pseudotuberculosis in HeLa cells. Can J Microbiol. 1975 Dec;21(12):1997–2007. doi: 10.1139/m75-287. [DOI] [PubMed] [Google Scholar]
- Brunius G., Bölin I. Interaction between Yersinia pseudotuberculosis and the HeLa cell surface. J Med Microbiol. 1983 Aug;16(3):245–261. doi: 10.1099/00222615-16-3-245. [DOI] [PubMed] [Google Scholar]
- Caldwell M. B., Walker R. I., Stewart S. D., Rogers J. E. Simple adult rabbit model for Campylobacter jejuni enteritis. Infect Immun. 1983 Dec;42(3):1176–1182. doi: 10.1128/iai.42.3.1176-1182.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchère J. L., Véron M., Lellouch-Tubiana A., Pfister A. Experimental infection of gnotobiotic mice with Campylobacter jejuni: colonisation of intestine and spread to lymphoid and reticulo-endothelial organs. J Med Microbiol. 1985 Oct;20(2):215–224. doi: 10.1099/00222615-20-2-215. [DOI] [PubMed] [Google Scholar]
- Fernández H., Neto U. F., Fernandes F., de Almeida Pedra M., Trabulsi L. R. Culture supernatants of Campylobacter jejuni induce a secretory response in jejunal segments of adult rats. Infect Immun. 1983 Apr;40(1):429–431. doi: 10.1128/iai.40.1.429-431.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981 Apr;32(1):137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey C. D., Montag D. M., Pittman F. E. Experimental infection of hamsters with Campylobacter jejuni. J Infect Dis. 1985 Mar;151(3):485–493. doi: 10.1093/infdis/151.3.485. [DOI] [PubMed] [Google Scholar]
- Kihlström E., Edebo L. Association of viable and inactivated Salmonella typhimurium 395 MS and MR 10 with HeLa cells. Infect Immun. 1976 Oct;14(4):851–857. doi: 10.1128/iai.14.4.851-857.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Short H., Schenk E. A. Pathogenic properties of Campylobacter jejuni: assay and correlation with clinical manifestations. Infect Immun. 1985 Oct;50(1):43–49. doi: 10.1128/iai.50.1.43-49.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M. A., Burke V., Chang B. J. Invasion of HEp-2 cells by fecal isolates of Aeromonas hydrophila. Infect Immun. 1985 Mar;47(3):680–683. doi: 10.1128/iai.47.3.680-683.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., O'Rourke J. L., Barrington P. J., Trust T. J. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect Immun. 1986 Feb;51(2):536–546. doi: 10.1128/iai.51.2.536-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., McGrath P. P., Carter P. H., Eide E. L. The ability of some Yersinia enterocolitica strains to invade HeLa cells. Can J Microbiol. 1977 Dec;23(12):1714–1722. doi: 10.1139/m77-247. [DOI] [PubMed] [Google Scholar]
- Manninen K. I., Prescott J. F., Dohoo I. R. Pathogenicity of Campylobacter jejuni isolates from animals and humans. Infect Immun. 1982 Oct;38(1):46–52. doi: 10.1128/iai.38.1.46-52.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäki M., Grönroos P., Vesikari T. In vitro invasiveness of Yersinia enterocolitica isolated from children with diarrhea. J Infect Dis. 1978 Nov;138(5):677–680. doi: 10.1093/infdis/138.5.677. [DOI] [PubMed] [Google Scholar]
- Newell D. G., Pearson A. The invasion of epithelial cell lines and the intestinal epithelium of infant mice by Campylobacter jejuni/coli. J Diarrhoeal Dis Res. 1984 Mar;2(1):19–26. [PubMed] [Google Scholar]
- Niesel D. W., Chambers C. E., Stockman S. L. Quantitation of HeLa cell monolayer invasion by Shigella and Salmonella species. J Clin Microbiol. 1985 Dec;22(6):897–902. doi: 10.1128/jcm.22.6.897-902.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K. B., Winblad S., Bitsch V. Studies on the interaction between different O-serotypes of Yersinia enterocolitica and HeLa cells. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):141–145. doi: 10.1111/j.1699-0463.1979.tb02417.x. [DOI] [PubMed] [Google Scholar]
- Rudoy R. C. Enteroinvasive and enterotoxigenic Escherichia coli. Occurrence in acute diarrhea of infants and children. Am J Dis Child. 1975 Jun;129(6):668–672. doi: 10.1001/archpedi.1975.02120430008004. [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., Escamilla E., Torres N. Experimental Campylobacter diarrhea in chickens. Infect Immun. 1981 Oct;34(1):250–255. doi: 10.1128/iai.34.1.250-255.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., Torres J., Torres N. I., Escamilla E., Ruiz-Palacios B. R., Tamayo J. Cholera-like enterotoxin produced by Campylobacter jejuni. Characterisation and clinical significance. Lancet. 1983 Jul 30;2(8344):250–253. doi: 10.1016/s0140-6736(83)90234-9. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. I., Caldwell M. B., Lee E. C., Guerry P., Trust T. J., Ruiz-Palacios G. M. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]