Thyroid hormone (TH) is essential for normal brain development (1, 2). The absence of TH during the critical period of neurogenesis leads to multiple irreversible morphological abnormalities due, in large part, to defects in neuronal migration and neuronal process outgrowth. Up until the late 1970s, work had focused on the influence of TH on the cytoskeleton of the developing neuron (3–6), but this avenue was dropped with the discovery that the thyroxine metabolite, 3′,3,5-triiodothyronine (T3), interacted with a transcription factor that regulated specific target genes (7). These TH dependent transfactors belong to the c-erbA family of gene products that encode receptors for the steroid and thyroid hormones (8). More recently, the discovery that the brain developments normally in animals lacking these chromatin bound thyroid receptors, once again has raised the possibility that non-genomic actions of TH participate in the morphogenic actions of this hormone, especially in the brain. In this brief review, I will focus on the recent experimental support resurrecting the concept that TH dependent regulation of the cytoskeleton plays a key role in the developmental program of the brain.
The rat cerebellum completes its developmental program during the first 2–3 weeks of life and was one of the first identified targets for TH action. It is widely used to study the developmental effects of TH (9–11). In the euthyroid rat cerebellum, granule cell precursor neurons located in the External Granular Layer (EGL) undergo intense proliferation during the first week of life. Following a 24 hour period of lateral migration along the interface between the EGL and the Molecular Layer (ML) (Fig. 1, Stage 1), the granule cell sends a single radial projection through molecular layer into the Internal Granular Layer (IGL) (Fig. 1, Stage 2), and the cell body then migrates down this projection into the IGL (Fig. 1, Stage 3), where it synapses with axons in the climbing fibers from higher brain centers (12). Radial migration of granule cells reaches a maximum between postnatal days 10–14 and by 21 days of life the EGL has all but disappeared. During granule cell migration, there is extensive process outgrowth and dendritic arborization by the Purkinje cells, the target for granule neuron axonal projections. In the hypothyroid rat cerebellum, the EGL is retained for up to 24 days and there is increased granule cell death in the IGL presumably due to delayed granule cell migration from the EGL and a failure of the maturing granule cell to make connections (13, 14). As a result, the dendritic arbor of the Purkinje cell is markedly blunted in the hypothyroid rat (15) due, in large part, to decreased synaptogenesis (16). Importantly, TH replacement, specifically T4 or rT3, during the first two weeks of life rescues granule cell migration and normalizes Purkinje cell arborization and the cerebellum to complete its maturation according to the euthyroid developmental program. While the consequences of hypothyroidism on cerebellar development are well recognized, the biochemical and molecular bases for the effects of this morphogenic hormone on neuronal integration remain elusive.
Figure 1. Developmental Fate of the maturing Granule Neuron in the Cerebellum.
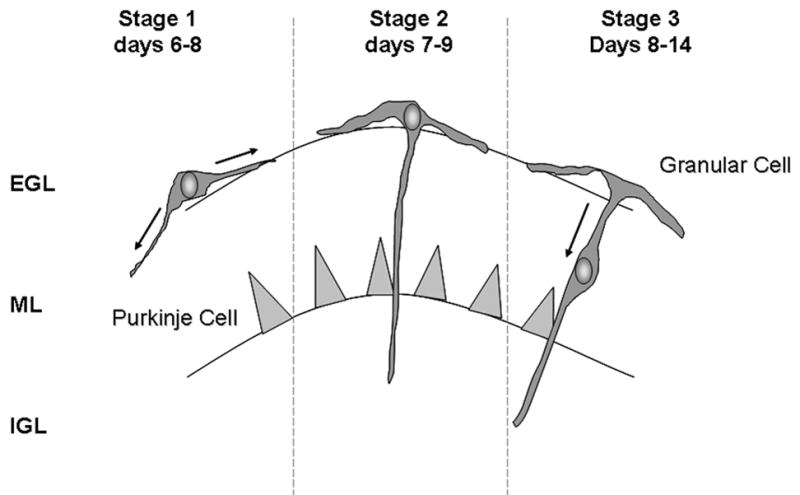
EGL, external granule layer; ML, molecular layer, IGL, internal granule layer.
Transcriptional actions of TH on the brain developmental program
The major TH produced by the thyroid gland is thyroxine (T4) which is deiodinated in peripheral tissues to the transcriptionally active iodothyronine, 3,5,3′-triiodothyronine (T3) (17) or to the transcriptionally inert metabolite, 3,3′,5′-trioodothyronine (reverse T3, rT3), which is the predominant iodothyronine produced during fetal life (18–20). Many of the actions of TH are initiated by the binding of T3 to specific chromatin-bound, ligand-activated receptors (thyroid receptors, TRs) (21–23). There are two TR genes that encode at least 10 gene products—only three are chromatin bound, T3 binding receptors (24). While the predominant T3-binding TRs, TRβ1 and TRα1, are developmentally expressed in the rat brain, the most abundant TR isoform expressed throughout development is the non-T3-binding TRα2 (25, 26). TRα1 does not show developmental changes in expression and is the predominant isoform in cerebellar granule cells (27). On the other hand, TRβ1 is the predominant isoform in the Purkinje cells (28) and shows a marked increase in abundance just prior to birth and remains elevated until postnatal day 10–12; a pattern that coincides with, rather than precedes, TH-dependent neurite integration (25, 29). While differential display and microchip analysis have identified a number of cerebellar gene products that are altered by thyroid status, few genes have been shown to be directly regulated by T3 (ie contain an identified thyroid hormone-response element) (for review see (26, 30). Thus, the identification of developmentally important, T3-responsive genes in the brain has been difficult at best.
Recent work done in transgenic mice suggests that T3-induced transcriptional regulation is not the sole contributor to the brain developmental program. Mice lacking one or more of the TR isoforms show abnormalities in the auditory system, the thyroid-pituitary axis, the heart, the skeletal system and the small intestine, but few, if any, abnormalities in brain development (31, 32). This is true for both single receptor knockout mice (33, 34) and for knockout mice devoid of all known TRs (35, 36).
Since the developmental deficits observed when nuclear TRs are missing are markedly less than those observed in hypothyroid neonates, it has been proposed that the unliganded TRs participate in this developmental program (32, 37). Because TRs bind to the thyroid response element of target genes in the unliganded state and in vitro studies have shown that the unliganded receptor represses transcription (38), some have proposed that during brain development T3 acts on target genes to release gene repression, rather than to activate gene expression (32). However, this hypothesis cannot account for the developmental defects observed in hyperthyroid neonates, and ignores the presence of the most abundant TR transcriptional repressor that can not bind T3, TRα2 (25, 39). Importantly, there is no direct in vivo demonstration of regulated gene repression by either the unliganded T3-binding TRs or by TRα2; instead all in vivo data has been inferential and based on the presence of a more benign phenotype than predicted. Thus, it seems likely that TH-dependent processes other than gene regulation contribute to the developmental program of the brain.
Non-genomic actions of TH in the brain: Regulation of actin polymerization
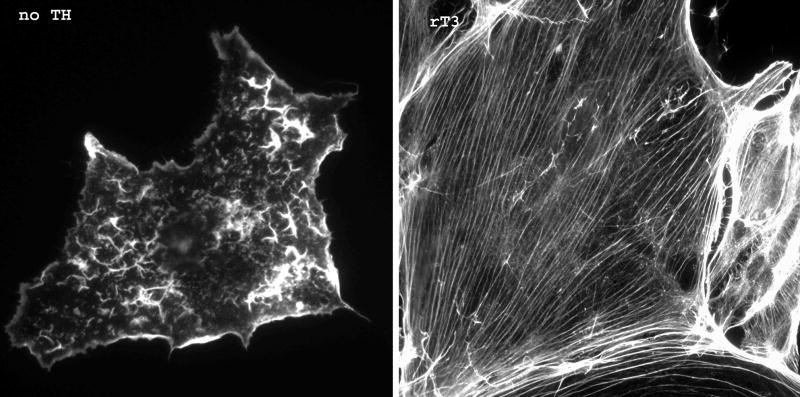
One well-recognized non-genomic action of TH is the T4-dependent regulation of actin polymerization. In astrocytes and the neuronal processes of granular neurons grown in the absence of TH only 40–60% of the cellular actin is polymerized (F-actin) and the ctin cytoskeleton is disorganized (Figure 2). Both T4 and rT3, but not T3, rapidly (~10–20 minutes) increase the F-actin content to 90–95% of the cellular actin and restore the microfilaments without altering total actin content or gene expression (40–42).
Figure 2. Effects of rT3 on microfilament organization in astrocytes.
Astrocytes were grown on p-lysine coated coverslips for 48 h in DMEM containing 10% calf serum and antibiotics. Twenty-four hours before staining, growth medium was replaced with serum-free DMEM containing 1 mg/ml BSA ± 10 nM rT3. After 24 hrs, coverslips were then washed, fixed with 4% paraformylaldehyde and the actin cytoskeleton visualized with alexafluor488-phallacidin as described in ref (41).
These cell culture findings mimic those observed during cerebellar development in vivo, where the F-actin content of the hypothyroid rat cerebellum remains significantly lower than that of the euthyroid cerebellum until postnatal day 15–16 (43). A single injection of either T4 or rT3 to the hypothyroid neonate on day 14 will normalize the F-actin content in the cerebellum within 3 h without altering the total cerebellar actin content (42). As observed in cell culture, T3 has no effect on either F-actin or total actin content in the hypothyroid rat cerebellum. These data indicate that TH initiated changes in actin polymerization during brain development is one action of TH that does not require the TRs and is an especially attractive mechanism of action because the two effector hormones, T4 and rT3, are the two predominant thyroid hormones produced during fetal life (17–20).
Actin polymerization, microfilament organization and neuronal migration
During normal brain development developing neurons migrate over long distances and project axons along specific pathways towards target cells using a cohort of transmembrane sensors tethered in place by the actin cytoskeleton (44, 45). Thus, the ability of the developing neuron to regulate actin polymerization in neurites is essential for the interpretation of extracellular guidance cues (45–48). Chemical disruption of neuronal actin cytoskeleton markedly impairs neuronal growth cone motility and pathfinding ability in vitro (48, 49). In light of the effects of TH on actin polymerization, we found that the lack of TH led to a marked reduction in granule cell migration from cerebellar explants and a dramatic decrease in neurite outgrowth and process formation. Replacement with T3 did not correct these defects; however, physiological replacement with either the T4 or rT3 normalized granule cell migration and neurite formation (50). Direct analysis of the actin cytoskeleton in the cells migrating from cerebellar explants revealed a large decrease in F-actin content in the cellular projections when grown in TH-deficient or T3-treated medium and that both T4 or rT3 restored the F-actin content of granule neurons to normal (50). These data illustrate the ability of transcriptionally inert iodothyronines such as T4 and rT3 to regulate neuronal cell migration by modulating the organization of the neuronal actin cytoskeleton.
Regulation of the actin cytoskeleton in the supporting astrocytes is also essential for the organization of extracellular neuronal guidance molecules during brain development. One such guidance molecule is laminin, an astrocyte-derived extracellular matrix protein that appears on the astrocyte surface during neuronal migration (51–53). Secreted laminin binds to integrins and is organized in specific patterns on the astrocyte surface by macromolecular focal contacts (54, 55). Focal contacts then transduce mechanical forces through their associated microfilaments. Microfilament disassembly in the TH-deficient or T3-treated astrocyte prevents focal contact formation on laminin coated surfaces, while abundant focal contacts are found on T4-treated cells (56). The loss of focal contacts prevents newly secreted laminin from binding to the astrocyte surface and leads to the loss of this guidance molecule from the cell surface. In the presence of T4 or rT3, focal contacts form normally leading to the accumulation of secreted laminin in macromolecular arrays of the astrocyte’s cell surface (50, 57, 58). These TH-dependent alterations in laminin organization on the astrocyte surface occur in the absence of any changes in laminin γ chain mRNA expression, laminin protein synthesis, or the rate of laminin secretion (57).
These in vitro findings also have an in vivo counterpart in the developing cerebellum of hypothyroid neonates where the appearance of laminin derived migration pathways are both delayed and diminished in quantity (58). These data provide the essentials to construct a physiological pathway where TH-dependent regulation of the polymerization state of actin in the astrocyte and the neuron modulates the production and recognition of guidance cues—cues that if disrupted lead to abnormal neuronal migration and neuronal process formation—and lead to the morphological deficits observed in the cretinous brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Santisteban P, Bernal J. Thyroid development and effect on the nervous system. Rev Endocr Metab Disord. 2005;6:217–28. doi: 10.1007/s11154-005-3053-9. [DOI] [PubMed] [Google Scholar]
- 2.Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95–122. doi: 10.1016/S0083-6729(05)71004-9. [DOI] [PubMed] [Google Scholar]
- 3.Legrand J, Rabie A. Influence of thyroid hormones on the size of the synaptosomal fraction of the cerebellum of young rats. Comptes Rendus Hebdomadaires Des Seances de L Academie Des Sciences. D: Sciences Naturelles. 1972;274:922–4. [PubMed] [Google Scholar]
- 4.Rabie A, Favre C, Clavel MC, Legrand J. Sequential effects of thyroxine on the developing cerebellum of rats made hypothyroid by propylthiouracil. Brain Research. 1979;161:469–479. doi: 10.1016/0006-8993(79)90676-0. [DOI] [PubMed] [Google Scholar]
- 5.Mareck A, Fellous A, Francon J, Nunez J. Changes in composition and activity of microtubule-associated protiens during brain development. Nature. 1980;284:353–355. doi: 10.1038/284353a0. [DOI] [PubMed] [Google Scholar]
- 6.Nunez J, Couchie D, Aniello F, Bridoux AM. Regulation by thyroid hormone of microtubule assembly and neuronal differentiation. Neurochem Res. 1991;16:975–82. doi: 10.1007/BF00965840. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheimer JH, Schwartz HL, Surks MI, Koerner D, Dillmann WH. Nuclear receptors and the initiation of thyroid hormone action. Recent Progress in Hormone Research. 1976;32:529–65. doi: 10.1016/b978-0-12-571132-6.50029-4. [DOI] [PubMed] [Google Scholar]
- 8.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;241:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clos J, Crepel F, Legrand C, Legrand J, Rabie A, Vigouroux E. Thyroid physiology during the postnatal period in the rat: a study of the development of thyroid function and of the morphogenetic effects of thyroxine with special reference to cerebellar maturation. General & Comparative Endocrinology. 1974;23:178–92. doi: 10.1016/0016-6480(74)90127-0. [DOI] [PubMed] [Google Scholar]
- 10.Vigouroux E. Dynamic study of post-natal thyroid function in the rat. Acta Endocrinologica. 1976;83:752–762. doi: 10.1530/acta.0.0830752. [DOI] [PubMed] [Google Scholar]
- 11.Koibuchi N, Jingu H, Iwasaki T, Chin WW. Current perspectives on the role of thyroid hormone in growth and development of cerebellum. Cerebellum. 2003;2:279–89. doi: 10.1080/14734220310011920. [DOI] [PubMed] [Google Scholar]
- 12.Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72:295–339. doi: 10.1016/j.pneurobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Rabie A, Patel AJ, Clavel MC, Legrand J. Effect of thyroid deficiency on the growth of the hippocampus in the rat. A combined biochemical and morphological study. Developmental Neuroscience. 1979;2:183–94. doi: 10.1159/000112453. [DOI] [PubMed] [Google Scholar]
- 14.Dubuis JM, Sanchez-Menegay C, Burger AG. Effects of thyroxine, triiodothyronine and reverse triiodothyronine on the neonatal hypothyroid rat cerebellum. Acta Med Austriaca. 1992;19 Suppl 1:106–9. [PubMed] [Google Scholar]
- 15.Legrand J. Morphogenic actions of thyroid hormones. Trends in Neurological Sciences. 1979;2:234–236. [Google Scholar]
- 16.Vincent J, Legrand C, Rabie A, Legrand J. Effects of thyroid hormone on synaptogenesis in the molecular layer of the developing rat cerebellum. Journal de Physiologie. 1982;78:729–38. [PubMed] [Google Scholar]
- 17.Leonard JL, Koehrle J. Intracellular pathways of iodothyronine metabolism. In: Braverman LE, Utiger RD, editors. The Thyroid. Lippincott-Raven; Philadelphia: 1996. pp. 125–161. [Google Scholar]
- 18.Chopra IJ, Sack J, Fisher DA. 3,3′,5′-Triiodothyronine (reverse T3) and 3,3′,5-triiodothyronine (T3) in fetal and adult sheep: studies of metabolic clearance rates, production rates, serum binding, and thyroidal content relative to thyroxine. Endocrinology. 1975;97:1080–8. doi: 10.1210/endo-97-5-1080. [DOI] [PubMed] [Google Scholar]
- 19.Cooper E, Burke CW. Thyroxine, 3,5,3′-triiodothyronine and 3,3′, 5′-triiodothyronine in human amniotic fluid: relationships between concentrations and turnover. Medical Hypotheses. 1983;12:113–24. doi: 10.1016/0306-9877(83)90073-7. [DOI] [PubMed] [Google Scholar]
- 20.Santini F, Chiovato L, Ghirri P, Lapi P, Mammoli C, Montanelli L, Scartabelli G, Ceccarini G, Coccoli L, Chopra IJ, Boldrini A, Pinchera A. Serum iodothyronines in the human fetus and the newborn: evidence for an important role of placenta in fetal thyroid hormone homeostasis. J Clin Endocrinol Metab. 1999;84:493–8. doi: 10.1210/jcem.84.2.5439. [DOI] [PubMed] [Google Scholar]
- 21.Samuels HH, Forman BM, Horowitz ZD, Ye ZS. Regulation of gene expression by thyroid hormone. Journal of Clinical Investigation. 1988;81:957–967. doi: 10.1172/JCI113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brent G. The molecular basis of thyroid hormone action. N Engl J Med. 1994;331:847–53. doi: 10.1056/NEJM199409293311306. [DOI] [PubMed] [Google Scholar]
- 23.Anderson GW, Mariash CN, Oppenheimer JH. Molecular actions of thyroid hormone. In: Braverman LE, Utiger R, editors. The Thyroid. Lippincott-Williams & Wilkins; Philadelphia: 2000. pp. 174–195. [Google Scholar]
- 24.Flamant F, Baxter JD, Forrest D, Refetoff S, Samuels H, Scanlan TS, Vennstrom B, Samarut J. International Union of Pharmacology. LIX. The pharmacology and classification of the nuclear receptor superfamily: thyroid hormone receptors. Pharmacol Rev. 2006;58:705–11. doi: 10.1124/pr.58.4.3. [DOI] [PubMed] [Google Scholar]
- 25.Strait KA, Schwartz HL, Perez-Castillo A, Oppenheimer JH. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. Journal of Biological Chemistry. 1990;265:10514–10521. [PubMed] [Google Scholar]
- 26.Oppenheimer JH, Schwartz HL. Molecular basis of thyroid hormone-dependent brain development. Endocrine Reviews. 1997;18:462–475. doi: 10.1210/edrv.18.4.0309. [DOI] [PubMed] [Google Scholar]
- 27.Bradley DJ, Young WS, Weinberger C. Differential expression of α and β thyroid hormone receptor genes in rat brain and pituitary. Proceeding of the National Academy of Science, USA. 1989;86:7250–7254. doi: 10.1073/pnas.86.18.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strait KA, Zou L, Oppenheimer JH. Beta 1 isoform-specific regulation of a triiodothyronine-induced gene during cerebellar development. Mol Endocrinol. 1992;6:1874–80. doi: 10.1210/mend.6.11.1282672. [DOI] [PubMed] [Google Scholar]
- 29.Mellstrom B, Naranjo JR, Santos A, Gonzalez AM, Bernal J. Independent expression of the alpha and beta c-erbA genes in developing rat brain. Mol Endocrinol. 1991;5:1339–50. doi: 10.1210/mend-5-9-1339. [DOI] [PubMed] [Google Scholar]
- 30.Bernal J, Guadano-Ferraz A. Thyroid hormone and the development of the brain. Curr Opin Endocrinol Diabetes. 1998;5:296–302. [Google Scholar]
- 31.Forrest D, Vennstrom B. Functions of thyroid hormone receptors in mice. Thyroid. 2000;10:41–52. doi: 10.1089/thy.2000.10.41. [DOI] [PubMed] [Google Scholar]
- 32.Flamant F, Samarut J. Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol Metab. 2003;14:85–90. doi: 10.1016/s1043-2760(02)00043-7. [DOI] [PubMed] [Google Scholar]
- 33.Hsu JH, Brent GA. Thyroid hormone receptor knockouts. Trends in Endocrinology and Metabolism. 1998;9:103–112. doi: 10.1016/s1043-2760(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 34.Morte B, Manzano J, Scanlan T, Vennstrom B, Bernal J. Deletion of the thyroid hormone receptor alpha 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci U S A. 2002;99:3985–9. doi: 10.1073/pnas.062413299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gothe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennstrom B, Forrest D. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes and Development. 1999;13:1329–41. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gauthier K, Plateroti M, Harvey CB, Williams GR, Weiss RE, Refetoff S, Willott JF, Sundin V, Roux JP, Malaval L, Hara M, Samarut J, Chassande O. Genetic analysis reveals different functions for the products of the thyroid hormone receptor alpha locus. Molecular and Cellular Biology. 2001;21:4748–60. doi: 10.1128/MCB.21.14.4748-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brent GA. Tissue-specific actions of thyroid hormone: insights from animal models. Rev Endocr Metab Disord. 2000;1:27–33. doi: 10.1023/a:1010056202122. [DOI] [PubMed] [Google Scholar]
- 38.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–41. [PubMed] [Google Scholar]
- 39.Koenig RJ, Lazar MA, Hodin RA, Brent GA, Larsen PR, Chin WW, Moore DD. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989;337:659–61. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- 40.Siegrist-Kaiser CA, Juge-Aubry C, Tranter MP, Ekenbarger DM, Leonard JL. Thyroxine-dependent modulation of actin polymerization in cultured astrocytes. A novel, extranuclear action of thyroid hormone. J Biol Chem. 1990;265:5296–302. [PubMed] [Google Scholar]
- 41.Farwell AP, Lynch RM, Okulicz WC, Comi AM, Leonard JL. The actin cytoskeleton mediates the hormonally regulated translocation of type II iodothyronine 5′-deiodinase in astrocytes. J Biol Chem. 1990;265:18546–53. [PubMed] [Google Scholar]
- 42.Farwell AP, Dubord-Tomasetti SA, Pietrzykowski AZ, Leonard JL. Dynamic nongenomic actions of thyroid hormone in the developing rat brain. Endocrinology. 2006;147:2567–74. doi: 10.1210/en.2005-1272. [DOI] [PubMed] [Google Scholar]
- 43.Faivre-Sarrailh C, Rabie A. A lower proportion of filamentous to monomeric actin in the developing cerebellum of thyroid-deficient rats. Dev Brain Res. 1988;41:293–7. doi: 10.1016/0165-3806(88)90190-3. [DOI] [PubMed] [Google Scholar]
- 44.Hatten ME, Heinz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annual Review of Neuroscience. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 45.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 46.Lockerbie RO. The neuronal growth cone: A review of its locomotory, navigational and target recognition capabilities. Neuroscience. 1987;20:719–729. doi: 10.1016/0306-4522(87)90235-1. [DOI] [PubMed] [Google Scholar]
- 47.Dodd J, Jessell TM. Axon guidance and the patterning of neuronal projections in vertebrates. Science. 1988;242:692–699. doi: 10.1126/science.3055291. [DOI] [PubMed] [Google Scholar]
- 48.Rivas RJ, Hatten ME. Motility and cytoskeletal organization of migrating cerebellar granule neurons. Journal of Neuroscience. 1995;15:981–9. doi: 10.1523/JNEUROSCI.15-02-00981.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forscher P, Smith SJ. Actions of cytochalasins on the organization of the actin filaments and microtubules in a neuronal growth cone. Journal of Cell Biology. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farwell AP, Dubord-Tomasetti SA, Pietrzykowski AZ, Stachelek SJ, Leonard JL. Regulation of cerebellar neuronal migration and neurite outgrowth by thyroxine and 3,3′,5′-triiodothyronine. Brain Res Dev Brain Res. 2005;154:121–35. doi: 10.1016/j.devbrainres.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 51.Liesi P, Seppala I, Trenkner E. Neuronal migration in cerebellar microcultures is inhibited by antibodies against a neurite outgrowth domain of laminin. Journal of Neuroscience Research. 1992;33:170–6. doi: 10.1002/jnr.490330122. [DOI] [PubMed] [Google Scholar]
- 52.Liesi P, Narvanen A, Soos J, Sariola H, Snounou G. Identification of a neurite outgrowth-promoting domain of laminin using synthetic peptides [published erratum appears in FEBS Lett 1989 Jul 17;251(1–2):283] Febs Letters. 1989;244:141–8. doi: 10.1016/0014-5793(89)81180-9. [DOI] [PubMed] [Google Scholar]
- 53.Liesi P, Hager G, Dodt HU, Seppala I, Zieglgansberger W. Domain-specific antibodies against the B2 chain of laminin inhibit neuronal migration in the neonatal rat cerebellum. Journal of Neuroscience Research. 1995;40:199–206. doi: 10.1002/jnr.490400208. [DOI] [PubMed] [Google Scholar]
- 54.Hynes RO. Integrin: Versitility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 55.Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farwell AP, Tranter MP, Leonard JL. Thyroxine-dependent regulation of integrin-laminin interactions in astrocytes. Endocrinology. 1995;136:3909–3915. doi: 10.1210/endo.136.9.7649099. [DOI] [PubMed] [Google Scholar]
- 57.Farwell AP, Dubord-Tomasetti SA. Thyroid hormone regulates the extracellular organization of laminin on astrocytes. Endocrinology. 1999;140:5014–21. doi: 10.1210/endo.140.11.7114. [DOI] [PubMed] [Google Scholar]
- 58.Farwell AP, Dubord-Tomasetti SA. Thyroid hormone regulates the expression of laminin in the developing rat cerebellum. Endocrinology. 1999;140:4221–7. doi: 10.1210/endo.140.9.7007. [DOI] [PubMed] [Google Scholar]