Abstract
Background
The steady-state redox potential of the cysteine/cystine couple in human plasma provides a measure of oxidative stress, yet available assays are limited by either specificity or speed of assay.
Method
The present study evaluated the use of LC-FTMS for identification based on accurate mass combined with quantification by stable isotopic dilution to rapidly determine cysteine and cystine concentration and cysteine/cystine steady-state redox potential in human plasma.
Results
A simple extraction procedure followed by a rapid LC separation eluted cysteine in 4 min and cystine in 1.5 min with simultaneous measurement of glutathione (GSH) and glutathione disulfide (GSSG). A study of five young (mean age = 25.7) subjects and 5 older (mean age = 67.8 y) subjects showed an increased oxidation with age.
Conclusions
Analysis by LC-FTMS is suitable for high-throughput analysis of plasma cysteine, cystine and cysteine/cystine steady-state redox potential as clinical measures of oxidative stress.
Keywords: FTMS, oxidative stress, human plasma, cysteine, cystine, redox
1. Introduction
Oxidative stress has been associated with aging [1] and a number of age-related diseases such as cardiovascular disease [2], neurodegeneration [3] and cancer [4]. Extensive research has led to a number of biomarkers of oxidative stress [5] although none are in widespread clinical use. The predominant low molecular weight thiol/disulfide system in plasma consists of cysteine (Cys) and cystine (CySS). The steady-state redox potential of this couple has been previously used to measure oxidative stress [1,6–8].
A number of methods are available for determination of Cys and CySS in human plasma. Routine clinical determination utilizes oxidation to cysteic acid [9] to measure total Cys and does not provide information about the steady-state redox potential. Most methods that distinguish Cys and CySS rely upon HPLC with either spectrophotometric [10], fluorometric [11,12] or electrochemical detection [13]. The spectrophotometric and fluorometric assays using reagents targeting thiols depend upon two analyses, one with reduction for detection of disulfides, while assays with reagents targeting amines allow detection of both Cys and CySS directly. The assays dependent upon amine reagents are limited by the ability to obtain detection without interference from other amine compounds, while the electrochemical methods are limited by the stability of Cys during processing and storage. Recently detection by mass spectrometry has been used to determine total Cys without distinguishing between Cys and CySS [14,15].
Assays for simultaneous measurement of Cys and CySS need to prevent artifactual oxidation of Cys. For separation by HPLC, iodoacetic acid is commonly used to prevent oxidation. This approach is useful because the negative charge of the carboxymethyl (CM) product facilitates separation [11]. Maleimides react more rapidly and at a lower pH, thereby providing advantages for trapping steady-state redox potential. However, direct comparison using a maleimide and iodoacetic acid methods showed that there was no significant difference in Cys, CySS or Cys/CySS steady-state redox potential when using millimolar concentration of iodoacetate [16]. The maleimide derivative was less stable, however, suggesting a potential advantage of derivatization with iodoacetic acid.
The purpose of the present study was to test the utility of the high mass accuracy of Fourier-transform ion cyclotron resonance (FT-ICR) for measurement of Cys/CySS steady-state redox potential in human plasma as a basis for high throughput clinical analyses. For this, we used the chromatographic conditions based on the methods of Loughlin et al. [17] for rapid separation of GSH and GSSG, and tested the FT-ICR for identification based upon accurate mass with confirmation by MS/MS. Extraction conditions were optimized to minimize artifactual redox change, and stable isotopic dilution was used for quantification [18]. Results showed that the method reliably measured Cys, CySS and Cys/CySS steady-state redox potential when directly compared to a previously validated method, and application to groups of young (mean age 25.7 y) and older (mean age 67.8 y) adults showed expected oxidation in Cys/CySS steady-state redox potential with age.
2 Materials and methods
2.1 Chemicals
GSH, GSSG, Cys, CySS, iodoacetic acid (IAA), [1,2-13C]-IAA, acetonitrile, HPLC grade water, formic acid (98%) and ammonium bicarbonate were purchased from Sigma Chemical (St. Louis, MO). IAA was recrystallized twice in petroleum ether. [3,3’-13C]-Cystine and [13C2,15N]-glycine-N-FMOC were purchased from Cambridge Isotope Laboratories (Andover, MA). Cys-glutathione disulfide (CySSG) was from Toronto Research Chemicals (North York, ON).
2.2 Synthesis of Standards
[1’,2’-13C]-CM-GSH and [1’,2’-13C]-CM-Cys were prepared respectively by dissolving 2:1 molar ratio of GSH or Cys in [1,2-13C]-IAA (in 1 mol/l NH4OH, 1 mol/l NH3CH2O3, pH 9.2) and incubation for 1 h at room temperature. The resultant isotopic standards were purified by HPLC. [13C2,15N]-GSH was synthesized utilizing FMOC solid-phase synthesis on a 433A Peptide Synthesizer using HOBt/DCC activation for coupling. [1,2-13C,15N]-glycine was coupled to a blank HMP resin using HATU activation for coupling. The remaining residues were added on the 433A Peptide Synthesizer. [13C2,15N]-GSH was cleaved using the reagent K cocktail, precipitated and washed with cold ethyl ether, dissolved with acetic acid/water and lyophilized and oxidized by dissolving the peptide in ammonium bicarbonate (pH ~9) and allowed to shake overnight. [13C4,15N2]-GSSG was purified by reverse-phase HPLC using a Polaris C18 column (4.6 mm × 250 mm, Varian, Inc).
2.3 Solutions
A standard solution containing Cys, CySS, cysteine-GSH disulfide (CySSG), GSH and GSSG was prepared as follows: an appropriate amount of each chemical was added to a 1 mol/l ammonium hydroxide, 1 mol/l ammonium bicarbonate solution (pH 9.2) to a final concentration of 20 mmol/l for each chemical. The standards were then diluted 1:10 into a solution containing 30 mg/ml IAA and let stand at room temperature for 1 h to form the S-carboxymethyl derivative (CM-GSH and CM-Cys). The standard was diluted with acetonitrile 1:1 and stored at −80° C.
Plasma preservation solution contained 90 mmol/l ammonium bicarbonate, 150 mmol/l recrystallized IAA and 2.5 mg/ml heparin (pH 8.3, pH adjusted with ammonium hydroxide).
2.4 Human Subjects
This study was reviewed and approved by the Institutional Review Board of Emory University and performed in accordance with the ethical standards outlined in the 1975 Declaration of Helsinki, as revised in 1983. Each participant gave his/her informed consent prior to inclusion in the study. All participants were recruited at Emory University, including Emory University employees, and were included in the final study based solely on age. A power analysis based on previous results obtained by dansyl chloride fluorescence detection [1] showed that 5 subjects in each age group were sufficient to determine differences in plasma thiols and steady-state redox potential. The data are presented as mean values ± the SE of the mean.
2.5 Plasma sample collection and sample preparation
Blood was collected from the antecubital vein by venipuncture with a 21-gauge butterfly needle [1]. To prevent autoxidation, 1.35 ml of blood was dripped into a microcentrifuge tube containing 150 µl of plasma preservation solution containing iodoacetic acid (IAA) under conditions which rapidly carboxymethylate (CM) thiols at room temperature. The tube was gently inverted twice to mix the contents and centrifuged at 13,000 x g for 2 min. Samples showing any evidence of hemolysis were discarded. One hundred microliter aliquots were stored at −80° C. Aliquots were thawed and 3.0 µL of isotopic standard (500 µmol/l [1’,2’-13C]-CM-Cys, 2.5 mmol/l [3,3’-13C]-Cystine, 75 µmol/l [1’,2’-13C]-CM-GSH, 5 µmol/l [13C4, 15N2]-GSSG) was 4added. Protein was precipitated by adding 2:1 (v:v) of acetonitrile to the plasma and centrifuging at 13,000 x g for 2 min.
2.6 High-performance liquid chromatography
For analysis, 10 µL was injected onto a Hamilton PRP-X110S (2.1×100mm) anion exchange column with a Targa C18 pre-column (Higgins Analytical) for desalting and optimum chromatographic resolution. Solvent A was 0.1% aqueous formic acid/acetonitrile (1:1) and Solvent B was 2.0% aqueous formic acid/acetonitrile (1:1). Analytes were eluted from the HPLC column with a flow of 0.35 ml/min using the following gradient conditions: 0 min, 0% B; 2 min, 0% B; 6 min, 100% B; 8 min, 100% B; 8.1 min, 0% B; 9 min, 0% B.
2.7 Mass spectrometry
Ionization was done by electrospray in positive ion mode. The eluate from the HPLC was connected to a Thermo LTQ-FT mass spectrometer (Thermo-Fisher Scientific, San Jose, CA). The LTQ-FT was operated with a spray voltage of 5 kV, sheath gas setting of 40 (arbitrary units), auxiliary gas setting of 10 (arbitrary units), capillary temperature of 275° C, capillary voltage of 44 V and tube lens of 120 V. Analyses were done using the MS1 mode scanning from m/z 85 to 850 in the FT detector at a resolution of 50,000 with the wide range scan mode and 3 million ions per scan. Maximum ion injection time was 500 ms.
2.8 Analysis
Analytes were quantified based on peak areas calculated from their respective ion chromatograms using Quan Browser™ (Thermo Fisher Scientific) software. All statistical comparisons were done using the two-tailed student t-test with a significance level of p<0.05. Although plasma redox systems are not in equilibrium, a steady-state redox potential can be calculated for each redox active couple.[12] Steady-state redox potentials were calculated with the Nernst equation, Eh = Eo + (RT/nF) ln ([disulfide]/[thiol]2), where Eo is the standard potential for the redox couple, R is the gas constant, T is the absolute temperature, n is 2 for the number of electrons transferred and F is Faraday’s constant. The standard potential, Eo, at pH 7.4 for Cys/CySS was −250 mV and for GSH/GSSG was −264 mV [1].
3 Results
3.1 Chromatography and mass spectrometry of standards
Ion exchange chromatography was chosen based on the Loughlin et. al method for rapid measurement of GSH and GSSG [17]. A standard consisting of Cys, CySS, cysteine-glutathione disulfide (CySSG), GSH and GSSG was tested. The chromatographic separation consisted of a period at initial conditions needed for desalting, a formic acid gradient for separation, a short isocratic phase with high acid to elute more tightly bound analytes, and an equilibration period prior to the next run. The minimum time needed for each of these phases was evaluated and the total run time established. Optimum conditions were; initial conditions, 2 min; gradient, 4 min; isocratic, 2 min; equilibration, 1 min; making the final run time 9 minutes. Mobile phase conditions were systemically tested for optimum separation with 25%, 30%, 40% and 50% acetonitrile and 0.5%, 0.75%, 1% and 2% maximum formic acid. 50% acetonitrile and 1% formic acid provided the best results. Optimum flow rate was defined as the highest flow rate that maintained separation, and this was found to be 0.35 ml/min.
Optimal ionization was accomplished using a steady injection of the standard mixture directly into the electrospray source and simultaneously tuning on the most chemically different species. Cys and GSSG were used as the extremes in size, and GSSG and CySS were used as the extremes in polarity. Spray voltage, sheath gas, capillary temperature, capillary voltage and tube lens voltage were systematically altered to acquire the conditions that gave optimum ionization and detection. A single microscan was unable to effectively detect both GSSG and Cys, so a wide range scan, which is a composite of 2 microscans using 2 different scan ranges, was used. The number of ions collected for each microscan was systematically tested to optimize signal without causing loss of mass accuracy due to ion-ion distortion and found to be 3 million ions. LC-FTMS parameters were stored in a software file on the controlling computer, and a column switching valve was employed to allow for easy daily set-up and multiple instrument uses. A representative ion chromatogram of a standard mixture is shown in Figure 1. This LC-FTMS method was saved to the system’s controlling computer and subsequent analyses used the pre-programmed method with comparable results. Standard curves were linear with concentrations of 10 nmol/l to 150 µmol/l, with GSH and GSSG showing much better ionization efficiency than Cys and CySS.
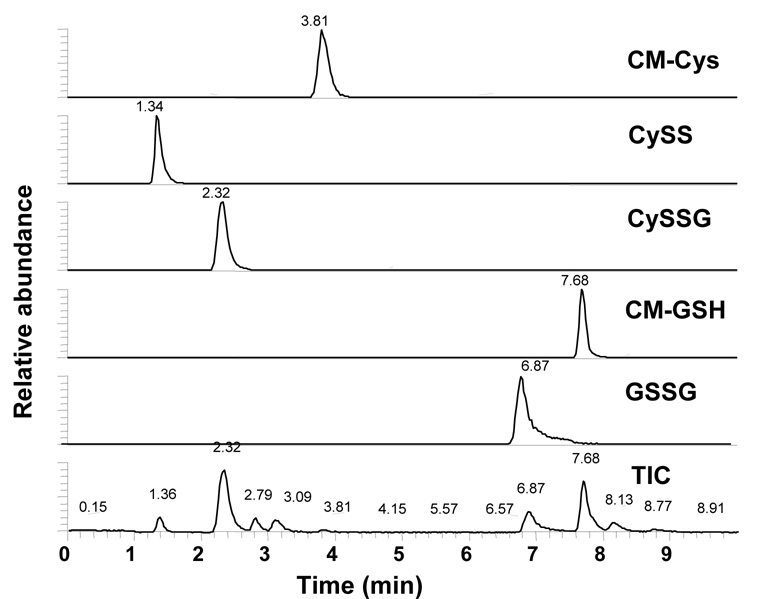
Figure 1. Ion Chromatogram of Cys, CySS, CySSG, GSH, And GSSG Standard Mixture.
A standard containing S-carboxymethyl-cysteine (CM-Cys), cystine (CySS), cysteine-glutathione disulfide (CySSG), S-carboxymethyl-glutathione (CM-GSH) and glutathione disulfide (GSSG) was used to test chromatographic and mass spectrometric conditions, and the resulting ion chromatogram was viewed with m/z windows of 180.0307–180.0343 for CM-Cys, 241.0287–241.0335 for CySS, 427.0920–427.0960 for CySSG, 366.0929–366.1002 for CM-GSH, 613.1530-613.165 + 307.0801-307.0863 for singly and doubly charged GSSG, and the total ion chromatogram (TIC).
3.2 Protein precipitation and sample preparation
Plasma samples were thawed to room temperature. Protein removal was evaluated using acetone (1:1), methanol (2:1), acetonitrile (1:1 and 2:1), acidifying with HCl (0.1 mol/l) with acetonitrile (1:1 and 2:1) and methanol (2:1), and acidifying with formic acid (1%) with acetonitrile (2:1) followed by centrifugation for 2 minutes to remove the protein. Both acetonitrile (2:1) and methanol (2:1) extracted proteins well, removing 98% and 96% respectively, but acetonitrile provided the best recovery of all five endogenous chemicals by LC-FTMS (Fig. 2a).
Figure 2. Ion chromatogram of Cys, CySS, CySSG, GSH and GSSG and quantification of Cys and CySS in human plasma.
A) the ion chromatogram was viewed with a m/z window of 180.0307–180.0343 for CM-Cys, 241.0287–241.0335 for CySS, 427.0920–427.0960 for CySSG, 366.0929–366.1002 for CM-GSH, 613.1530-613.165 + 307.0801–307.0863 for singly and doubly charged GSSG, and the total ion chromatogram (85–850 m/z). B) Quantification of plasma Cys and CySS was obtained relative to internal standards. Stable isotopic standards, 10 µmol/l [13C2]-CM-Cys and 125 µmol/l [3,3’-13C2]-CySS were added prior to removal of plasma protein. The ratio of peak areas for m/z 180.0307–180.0343 and 182.0374–182.041 was used to calculate Cys concentration and the ratio of peak areas for m/z 241.0287–241.0335 and 243.0354–243.0402 was used to calculate CySS concentration. Each sample was run in quadruplicate. A representative ion chromatogram is shown. S-carboxymethyl-cysteine, CM-Cys; cystine, CySS; cysteine-glutathione disulfide, CySSG; S-carboxymethyl-glutathione, CM-GSH; glutathione disulfide, GSSG; [1’,2’ 13C]-S-carboxymethyl-cysteine, [13C2]-CM-Cys; [3,3’ 13C]-cystine, [13C2]-CySS.
To ensure correct identification, MS/MS was performed in the ion trap with detection of daughter ions in the FTMS. The standard mixture containing 10 µmol/l Cys, CySS, CySSG, GSH and GSSG was used as reference spectra. The MS/MS ion spectra showed the same 180 → 163 for CM-Cys, 241 → 152 for CySS, 427 → 298 for CySSG, 366 → 237 and 219 for CM-GSH and 613 → 355 and 298 for GSSG in both standard and plasma samples.
Ion chromatograms were viewed with varying m/z resolution to determine the resolving power required to correctly distinguish each chemical from others with the same nominal mass present in plasma. Correct identification of GSH and GSSG required a resolving power of 50 ppm, Cys and CySS required 25 ppm and CySSG required 10 ppm.
3.3 Quantification of Cys, CySS, GSH and GSSG
Stable isotopic standards were synthesized for Cys, GSH and GSSG (as described in Materials and Methods) and [13C2]-CySS was purchased. A stable isotopic standard was not made for CySSG, so no quantification was done. A stock solution of isotopic standards was spiked into plasma prior to protein precipitation with the final concentrations of isotopic standards adjusted to be similar to known concentrations; 10 µmol/l Cys, 125 µmol/l CySS, 2.5 µmol/l CM-GSH and 100 nmol/l GSSG [1]. An artifactual reduction of CySS to Cys due to ammonium iodide formed in the preservation solution necessitated recrystallization of the IAA prior to use. Recrystallization largely eliminated this artifact, but an internal validation procedure based upon the amount of [13C]-CM-Cys, the product of [13C2]-CySS reduction, was introduced as a correction factor to assure accurate analysis. Quantification was carried out by Quan Browse™ software (Thermo Fisher Scientific) using the area under the curve relative to the respective isotopic standard (Fig. 2b). Integration parameters were set to allow for peak tailing and integration was stable with consecutive integrations varying by <1%.
3.4 Comparison of results with previously validated method using dansyl chloride and fluorescence detection
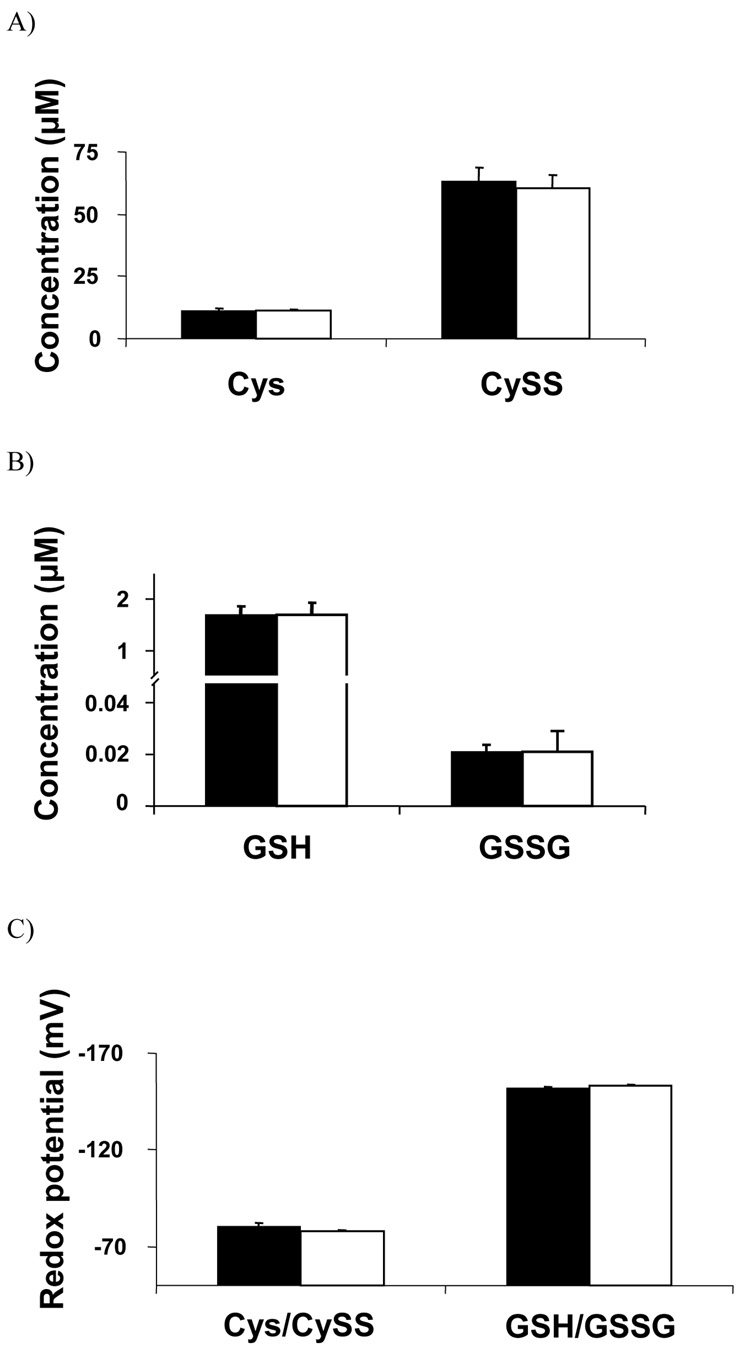
The LC-FTMS method was compared to a previously developed and validated HPLC method [11] to if see this new method, which required less processing and had a shorter run time, provided comparable concentrations of Cys, CySS and Cys/CySS steady-state redox potential. Results showed that the methods were comparable in terms of absolute concentrations for Cys and CySS (Fig. 3a). Similarly, the steady-state redox potential values were comparable with values for the LC-FTMS being −78 ± 1 mV compared to the value of −77 ± 2 mV using fluorescence detection (Fig. 3c).
Figure 3. Comparison of LC-FTMS method to previously validated HPLC with fluorescent detection method for analysis of Cys, CySS, GSH and GSSG concentration and steady-state redox potential values.
A) The concentrations of Cys (11.2 ± 0.7, 11.0 ± 0.4, p=0.73) and CySS (63.4 ± 5.5, 60.5 ± 5.5, p=0.95) were not significantly different between the two methods. B) The concentrations of GSH (0.026 ± 0.2, 1.7 ± 0.2, p=0.94) and GSSG (0.024 ± 0.002, 0.026 ± 0.01, p=0.72) were not significantly different between the methods. C) The steady-state redox potential for the Cys/CySS couple (−76.6 ± 1.5, −77.8 ± 0.8, p=0.16) GSH/GSSG couple (−152.1 ± 0.4, −152.9 ± 0.7, p=0.36) was not significantly different between the 2 methods. All samples were run in quadruplicate. All values are mean ± standard error. S-carboxymethyl-cysteine, CM-Cys; cystine, CySS; S-carboxymethyl-glutathione, CM-GSH; glutathione disulfide, GSSG; [1’,2’ 13C]-S-carboxymethyl-cysteine, [13C2]-CM-Cys; [3,3’ 13C]-cystine, [13C2]-CySS.
GSH, GSSG and GSH/GSSG steady-state redox potential was also analyzed by both LC-FTMS and HPLC with fluorescence detection. Results showed that the methods were comparable in terms of absolute concentration for GSH and GSSG (Fig. 3b). The steady-state redox potential values were also comparable between the two methods with the LC-FTMS being −152.9 ± 0.7 mV compared to the value of −152.1 ± 0.4 mV for the HPLC assay (Fig. 3c).
3.5 Reproducibility, stability and recovery of Cys and CySS by LC-FTMS method
To evaluate reproducibility of the method and stability of samples, plasma was collected as described in the methods and stored at −80° C. On the day after collection (day 1), an aliquot was processed with 5 replicate LC-FTMS runs. Within day coefficient of variation for these runs was determined to be 2.1% for Cys and 3.6% for CySS. Five replicate LC-FTMS runs were also performed on days 8 and 15, and the week-to-week coefficient of variation was 8.0% for Cys and 7.5 % for CySS. An aliquot was processed with 6 replicate LC-FTMS runs on each of 2 columns, and column to column variability was 3.2% for Cys and 4.3% for CySS. Aliquots were processed at 6 months and 1 y with 5 replicate LC-FTMS runs, and sample stability was assessed by calculating the percentage of day 1 Cys and CySS that was present. The measured values expressed as a percentage of the initial measurement, Cys (6 months, 99 ± 2%; 1 y 101 ± 3%) or CySS (6 months, 97 ± 4%; 1 y, 104 ± 4%) showed that there was no significant loss with the storage conditions. Recovery of Cys and CySS from plasma was evaluated by spiking 0, 10, 20, 30, 40 and 50 µmol/l of each compound prior to protein precipitation. Recovery of Cys was 103 ± 4% and of CySS was 97 ± 2%.
3.6 Evaluation of plasma thiol/disulfide oxidative stress with age
Previous studies have shown characteristic changes in plasma thiol levels and steady-state redox potential with age, becoming highly significant between young (<30) and older (>60) adults, irrespective of other demographic factors [1,19–22]. To evaluate clinical utility of the FTMS method, 5 young (mean age = 25.7 ± 3.3 y) and 5 older (mean age = 67.8 ± 6.3 y) adults were recruited to determine the ability of the LC-FTMS method to detect and quantify plasma thiol levels and steady-state redox potential changes with age. Although Cys, CySS and Cys/CySS steady-state redox potential were the primary outcome, GSH, GSSG and GSH/GSSG steady-state redox potential were also measured. Cys concentration was not significantly different (p=0.10) between young (11.3 ± 2.9 µmol/l) and older (7.3 ± 2.2 µmol/l) adults, but CySS concentration was significantly (p<0.01) lower in young (51.72 ± 4.2 µmol/l) than in older (104.8 ± 4.8 µmol/l) adults (Fig 4a). GSH concentrations were significantly (p<0.01) higher in the young (2.8 ± 0.2 µmol/l) than in the older (0.9 ± 0.1 µmol/l) adults, but GSSG levels were not significantly different (p=0.29) in young (0.035 ± 0.01 µmol/l) than in older (0.05 ± 0.01 µmol/l) adults (Fig. 4b). Cys/CySS steady-state redox potential was significantly (p<0.01) more reduced in young (−84.6 ± 2.9 mV) than in older (−61.0 ± 2.2 mV) adults due mainly to the increased CySS in older adults. GSH/GSSG steady-state redox potential was also significantly (p<0.01) more reduced in young (−155.1 ± 4.2 mV) than in older (−121.2 ± 4.4 mV) adults (Fig 4c) due mainly to decreased GSH concentrations in older adults. These data are consistent with previous findings and confirm the utility of the new method for evaluation of relevant steady-state redox potential differences for use as a clinical measurement of oxidative stress in humans [1, 23–25].
Figure 4. Use of LC-FTMS method to determine Cys, CySS, GSH, GSSG and steady-state redox potential values in plasma from young adults (20–30 y) and older adults (>60 y).
A) Young (black) and older (white) adults did not have significantly different Cys levels (11.3 ± 2.9, 7.3 ± 2.2, p=0.10) but had significantly different CySS levels (51.72 ± 4.2, 104.8 ± 4.8, p<0.01). B) Younger adults had significantly more GSH than older adults (2.8 ± 0.2, 0.9 ± 0.1, p<0.01) but GSSG was not significantly different (0.035 ± 0.01, 0.05 ± 0.01, p=0.29). C) Plasma redox was significantly more oxidized in older adults compared with young adults in both the Cys/CySS couple (−84.6 ± 2.9, −61.0 ± 2.2, p<0.01) and the GSH/GSSG couple (−155.1 ± 4.2, −121.2 ± 4.4, p<0.01). All values are mean ± standard error. S-carboxymethyl-cysteine, CM-Cys; cystine, CySS; S-carboxymethyl-glutathione, CM-GSH; glutathione disulfide, GSSG; [1’,2’ 13C]-S-carboxymethyl-cysteine, [13C2]-CM-Cys; [3,3’ 13C]-cystine, [13C2]-CySS.
* p<0.01
4. Discussion
Methods for measuring and quantifying low molecular weight thiols and disulfides in plasma are done using HPLC separation with fluorescence, ultraviolet, colorimetric or electrochemical detection. Many of these procedures require derivatization prior to analysis and long HPLC separation times to avoid co-elution. Electrochemical methods eliminate the necessity of derivatization but sample stability presents a problem for routine clinical use. Recently, HPLC separation has also been linked to mass spectrometry detection, which eliminates the need for derivatization procedures and allows shorter HPLC time without compromising sample stability, but these methods do not detect cystine (Table 1).
Table 1.
Common HPLC methods used to measure and quantify Cys and CySS in human plasma
| Method | Prep timea | HPLC run time | Cys detection | CySS detection | Stability |
|---|---|---|---|---|---|
| Fluorescent: mBrB [23, 26] |
1 hr | 35 min per fractionb |
Cys-mBrB | Indirectc | > 14 days @ −80°, − 20° and 4° |
| Ultraviolet: CMQT [27] |
40 min | 10 min per fractionb |
Cys-CMQT | Indirectc | Not determined |
| Colorimetric: DTMB [28] |
4 hr | 2 hr per fractionb |
Cys-DTMB | Indirectc | Not determined |
| Amino acid analyzer: ninhydrin [9, 29] |
none | 80 min per fractionb |
Cys- ninhydrin |
Indirectc | Stable over time of analysis |
| Electrochemical: dual electrode [30] |
30 min | 15 min | Direct | Direct | 1 wk @ −20°, 5 min @ RT |
| Colorimetric: DNFB [31] |
5+ hr | 40 min | Cys-CM or CYs-NEM |
Direct | 2 wk @ 4° |
| Fluorescent: Dansyl [11, 12] |
16+ hr | 1 hr | Cys-CM | Direct | 3 mo @ −80° |
| MS/MS: [15, 32] |
2 min | 15 min | Cys-DTMB | Not detected | 1 day @ RT, 1 mo @ −80° |
|
Current method: FTMS |
2 min | 9 min | Cys-CM | Direct | 1 yr @ −80+° |
Preparation time needed prior to HPLC – includes time for protein precipitation
At least 2 fractions (normal and reduced) needed for analysis
Detected by subtracting the amount in the normal fraction from the amount in the reduced fraction (Total plasma cysteine – Cys = CySS)
The current LC-FTMS method provides a new method to rapidly quantify Cys/CySS steady-state redox potential based exclusively on the chemicals themselves and not derivatization products or daughter ions. In a practical test, the method confirmed previous studies showing increased oxidative stress associated with age [1,23–25].
A potential problem with the method due to artifactual reduction of CySS to Cys by ammonium iodide formed in the preservation solution was initially addressed by substituting maleimides for IAA. These derivatives were not reliably ionized by ESI (data not shown). Consequently, a procedure was introduced to correct for any reduction based upon the amount of [3-13C]-CM-Cys detected. This approach provided accurate quantification of endogenous Cys and CySS. While the current method can simultaneously measure Cys, CySS, GSH and GSSG, the dynamic range with real samples did not allow quantification of GSSG in every analysis. With a sample prep time of 2 min and run time of 9 min, the method is suitable for relatively high throughput analysis of clinical samples and can analyze almost ten times the number currently used methods (Table 1) for human plasma. In principle, this run time could be decreased to 4 min if only Cys and CySS were analyzed. While this method was developed and validated using a FTMS, it likely could be adapted to a triple quad mass spectrometer with the use of multiple reaction monitoring. However, due to the m/z resolving power required, currently available single quad mass spectrometers are not adequate.
In summary, the present study uses LC-FTMS for rapid separation with accurate mass detection and quantification of Cys and CySS in human plasma based on stable isotopic dilution. This method can be performed on a multiple use instrument by simple column switching and, in principle, can simultaneously provide quantification of a large number of other metabolites for which isotopic standards are available. For the latter, validation studies with MS/MS would be needed. Results show that the method is comparable to a current HPLC method in quantification, and significantly decreases preparation and analysis times. The method was validated in terms of recovery and application to oxidative stress associated with aging. Thus, this LC-FTMS method has characteristics suitable for routine analysis of Cys/CySS steady-state redox potential as a clinical biomarker of oxidative stress.
Acknowledgements
This research was supported by NIH Grant ES009047 and the Emory – Georgia Tech Predictive Health Institute. The authors would like to acknowledge Siobhan Moriarty-Craige for blood collection.
List of Abbreviations
- CySS
cystine
- Cys
cysteine
- CM-Cys
S-carboxymethyl cysteine
- CySSG
cysteine glutathione disulfide
- GSH
glutathione, reduced
- CM-GSH
S-carboxymethyl glutathione
- GSSG
glutathione disulfide
- LC-FTMS
liquid chromatography - Fourier transform mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 2.Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17:48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer LR, Glass JD. Axonal degeneration in motor neuron disease. Neurodegener Dis. 2007;4:431–442. doi: 10.1159/000107704. [DOI] [PubMed] [Google Scholar]
- 4.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 5.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 6.Moriarty SE, Shah JH, Lynn M, et al. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Medved I, Brown MJ, Bjorksten AR, Leppik JA, Sostaric S, McKenna MJ. N-acetylcysteine infusion alters blood redox status but not time to fatigue during intense exercise in humans. J Appl Physiol. 2003;94:1572–1582. doi: 10.1152/japplphysiol.00884.2002. [DOI] [PubMed] [Google Scholar]
- 8.Jonas CR, Puckett AB, Jones DP, et al. Plasma antioxidant status after high-dose chemotherapy: a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am J Clin Nutr. 2000;72:181–189. doi: 10.1093/ajcn/72.1.181. [DOI] [PubMed] [Google Scholar]
- 9.Brigham MP, Stein WH, Moore S. The Concentrations Of Cysteine And Cystine In Human Blood Plasma. J Clin Invest. 1960;39:1633–1638. doi: 10.1172/JCI104186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katrusiak AE, Paterson PG, Kamencic H, Shoker A, Lyon AW. Pre-column derivatization high-performance liquid chromatographic method for determination of cysteine, cysteinyl-glycine, homocysteine and glutathione in plasma and cell extracts. J Chromatogr B Biomed Sci Appl. 2001;758:207–212. doi: 10.1016/s0378-4347(01)00182-7. [DOI] [PubMed] [Google Scholar]
- 11.Jones DP, Carlson JL, Samiec PS, et al. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 12.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 13.Petrlova J, Mikelova R, Stejskal K, et al. Simultaneous determination of eight biologically active thiol compounds using gradient elution-liquid chromatography with Coul-Array detection. J Sep Sci. 2006;29:1166–1173. doi: 10.1002/jssc.200500425. [DOI] [PubMed] [Google Scholar]
- 14.Nolin TD, McMenamin ME, Himmelfarb J. Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: application to studies of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:554–561. doi: 10.1016/j.jchromb.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaving G, Rocks BF, Iversen SA, Titheradge MA. Simultaneous quantitation of homocysteine, cysteine and methionine in plasma and urine by liquid chromatography-tandem mass spectrometry. Ann Clin Biochem. 2006;43:474–480. doi: 10.1258/000456306778904605. [DOI] [PubMed] [Google Scholar]
- 16.Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM, Jr, Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. Faseb J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 17.Loughlin AF, Skiles GL, Alberts DW, Schaefer WH. An ion exchange liquid chromatography/mass spectrometry method for the determination of reduced and oxidized glutathione and glutathione conjugates in hepatocytes. J Pharm Biomed Anal. 2001;26:131–142. doi: 10.1016/s0731-7085(01)00402-2. [DOI] [PubMed] [Google Scholar]
- 18.Jennifer M, Johnson FHS, Dean P Jones. LC-FTMS for High-Throughput Analysis of Oxidative Stress Biomarkers in Human Plasma, in 54th ASMS Conference on Mass Spectrometry; ASMS; Seattle, WA. 2006. [Google Scholar]
- 19.Chan YC, Suzuki M, Yamamoto S. A comparison of anthropometry, biochemical variables and plasma amino acids among centenarians, elderly and young subjects. J Am Coll Nutr. 1999;18:358–365. doi: 10.1080/07315724.1999.10718876. [DOI] [PubMed] [Google Scholar]
- 20.Blanco RA, Ziegler TR, Carlson BA, et al. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86:1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 21.Droge W. The plasma redox state and ageing. Ageing Res Rev. 2002;1:257–278. doi: 10.1016/s1568-1637(01)00008-3. [DOI] [PubMed] [Google Scholar]
- 22.Di Giuseppe D, Frosali S, Priora R, et al. The effects of age and hyperhomocysteinemia on the redox forms of plasma thiols. J Lab Clin Med. 2004;144:235–245. doi: 10.1016/j.lab.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Giustarini D, Dalle-Donne I, Lorenzini S, Milzani A, Rossi R. Age-related influence on thiol, disulfide, and protein-mixed disulfide levels in human plasma. J Gerontol A Biol Sci Med Sci. 2006;61:1030–1038. doi: 10.1093/gerona/61.10.1030. [DOI] [PubMed] [Google Scholar]
- 24.Andriollo-Sanchez M, Hininger-Favier I, Meunier N, et al. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: the ZENITH study. Eur J Clin Nutr. 2005;59 Suppl 2:S58–S62. doi: 10.1038/sj.ejcn.1602300. [DOI] [PubMed] [Google Scholar]
- 25.Droge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exp Gerontol. 2002;37:1333–1345. doi: 10.1016/s0531-5565(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 26.Mansoor MA, Svardal AM, Ueland PM. Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma. Anal Biochem. 1992;200:218–229. doi: 10.1016/0003-2697(92)90456-h. [DOI] [PubMed] [Google Scholar]
- 27.Bald E, Chwatko G, Glowacki R, Kusmierek K. Analysis of plasma thiols by high-performance liquid chromatography with ultraviolet detection. J Chromatogr A. 2004;1032:109–115. doi: 10.1016/j.chroma.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Ohmori S, Ikeda M, Kasahara E, Hyodoh H, Hirota K. A colorimetric determination of total glutathione based on its C-terminal glycine residue and its application to blood, liver, and yeast. Chem Pharm Bull (Tokyo) 1981;29:1355–1360. doi: 10.1248/cpb.29.1355. [DOI] [PubMed] [Google Scholar]
- 29.Hack V, Breitkreutz R, Kinscherf R, et al. The redox state as a correlate of senescence and wasting and as a target for therapeutic intervention. Blood. 1998;92:59–67. [PubMed] [Google Scholar]
- 30.Kleinman WA, Richie JP., Jr Status of glutathione and other thiols and disulfides in human plasma. Biochem Pharmacol. 2000;60:19–29. doi: 10.1016/s0006-2952(00)00293-8. [DOI] [PubMed] [Google Scholar]
- 31.Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 32.Guan X, Hoffman B, Dwivedi C, Matthees DP. A simultaneous liquid chromatography/mass spectrometric assay of glutathione, cysteine, homocysteine and their disulfides in biological samples. J Pharm Biomed Anal. 2003;31:251–261. doi: 10.1016/s0731-7085(02)00594-0. [DOI] [PubMed] [Google Scholar]