Abstract
Bone marrow mesenchymal stem cells (MSCs) participate in myocardial repair following myocardial infarction. However, their in vivo reparative capability is limited due to lack of their survival in the infarcted myocardium. To overcome this limitation, we genetically engineered male rat MSCs overexpressing CXCR4 in order to maximize the effect of stromal cell-derived factor-1α (SDF-1α) for cell migration and regeneration. MSCs were isolated from adult male rats and cultured. Adenoviral transduction was carried out to over-express either CXCR4/green fluorescent protein (Ad-CXCR4/GFP) or Ad-null/GFP alone (control). Flow cytometry was used to identify and isolate GFP/CXCR4 over-expressing MSCs for transplantation. Female rats were assigned to one of four groups (n = 8 each) to receive GFP-transduced male MSCs (2 × 106) via tail vein injection 3 days after ligation of the left anterior descending (LAD) coronary artery: GFP-transduced MSCs (Ad-null/GFP-MSCs, group 1) or MSCs over-expressing CXCR4/GFP (Ad-CXCR4/GFP-MSCs, group 2), or Ad-CXCR4/GFP-MSCs plus SDF-1α (50 ng/μl) (Ad-CXCR4/GFP-MSCs/SDF-1α, group 3), or Ad-miRNA targeting CXCR4 plus SDF-1α (Ad-miRNA/GFP-MSCs + SDF-1α treatment, group 4). Cardiodynamic data were obtained 4 weeks after induction of regional myocardial infarction (MI) using echocardiography after which hearts were harvested for immunohistochemical studies. The migration of GFP and Y-chromosome positive cells increased significantly in the peri- and infarct areas of groups 2 and 3 compared to control group (p<0.05), or miRNA-CXCR4 group (p<0.01). The number of CXCR4 positive cells in groups 2, 3 was intimately associated with angiogenesis and myogenesis. MSCs engraftment was blocked by pretreatment with miRNA (group 4). Cardiac function was significantly improved in rats receiving MSCs over-expressing CXCR4 alone or with SDF-1α. The up-regulation of matrix metalloproteinases (MMPs) by CXCR4 overexpressing MSCs perhaps facilitated their engraftment in the collagenous tissue of the infarcted area. CXCR4 over-expression led to enhance in vivo mobilization and engraftment of MSCs into ischemic area where these cells promoted neomyoangiogenesis and alleviated early signs of left ventricular remodeling.
Keywords: CXCR4 over-expression, Engraftment, Stem cells, Myocardium infarction, Matrix metalloproteinases
1. Introduction
Mesenchymal stem cells (MSCs) have a potential to regenerate cardiac myocytes following acute myocardial infarction (MI) [1]. However, mobilized cells into infarcted tissue are subject to cell death due to insufficient oxygen [2]. SDF-1α and its unique receptor, CXCR4 play an important role in stem cell homing, chemotaxis, expression of adhesion molecules, engraftment [3], proliferation, and survival [4]. CXCR4 expression is endogenously regulated by tissue environmental factors such as cytokines, chemokines, stromal cells, adhesion molecules, myocardial ischemia and proteolytic enzymes [5]. SDF-1α, secreted by cells within ischemic myocardium, is a potent chemo-attractant for cells expressing CXCR4. In a recent report, Zhang et al. have shown that transplantation of SDF-1α over-expressing MSCs could enhance the cardioprotective effects of MSCs [6]. Although mobilization of endothelial progenitor cells (EPCs) in the setting of acute myocardial infarction has been observed [3], little is known about the effect of MSCs over-expressing CXCR4 on their mobilization and engraftment within infarcted myocardium. We determined the potential role of the SDF-1α/CXCR4 axis in mobilization of MSCs and their survival in infarcted myocardium. Overexpression of CXCR4 appears to be an effective strategy to accelerate mobilization of these cells toward ischemic area. Due to low native levels of CXCR4 expression in MSCs, they migrate sluggishly toward a SDF-1α gradient following MI [7]. We hypothesized that over-expression of CXCR4 in MSCs will enhance their engraftment and transdifferentiation in ischemic myocardium.
2. Materials and methods
2.1. Laboratory animals
All protocols conformed to the Guidelines for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication No. 85-23, revised 1996) and were approved by the University of Cincinnati Animal Care and Use Committee. MSCs were obtained from Sprague–Dawley (SD) male rats. Female SD rats were grouped to receive tail vein injections with green fluorescent protein (GFP)-transduced male MSCs (2 × 106). All injections in female rats were performed 3 days after LAD occlusion. These animals were randomly divided into four groups (n = 8 each group) to receive various treatments, as follows: Group 1, received adenoviral vector without CXCR4 gene expression (Ad-Null/GFP-MSCs); Group 2, received MSCs with CXCR4 overexpression (AdCXCR4/GFP-MSCs); Group 3, the same as group 2 plus SDF-1α (50 ng/μl) injected intramyocardially in the infarct area (Ad-CXCR4/GFP-MSCs/SDF-1α); Group 4, received adenovirus-based miRNA targeting CXCR4 plus intramyocardial SDF-1 (Ad-miRNA/GFP-MSCs/SDF-1). Rats in groups 1 and 2 received saline injections of equal volumes and in identical intramyocardial regions as those in groups 3 and 4 that received SDF-1α injections.
After measuring cardiodynamic variables at 4 weeks, hearts were harvested and analyzed for various studies.
2.2. Generation of recombinant adenovirus vector
The AdEasy TM Vector System (Qbiogene, Inc.) was used for regenerating recombinant adenovirus according to manufacturer's instructions. In brief, rat CXCR4 cDNA (MGC-36266) was purchased from ATCC (American Type Culture Collection), and sub-cloned in the BglII and HindIII sites of plasmid pEGFP-C1 (Clonetech) by polymerase chain reaction (PCR) technology. The primers for PCR containing BglII (5′) and HindIII (3′) linkers (in bold) were synthesized as follows: CXCR4 forward primer: 5′-CAGA AGATCT GTT GCC ATG GAA CCG ATC-3′; reverse primer: 5′-CAGA AAGCTT GGG TTA GCT GGA GTG-3′. The identity of the gene was confirmed by sequencing, and was subsequently cloned into the same restriction sites on the shuttle vector pAdTrack-CMV which contains the enhanced green fluorescence protein (EGFP) expression cassette. The resulting shuttle vector plasmid was linearized with PmeI, mixed with supercoiled pAdEasy-1, and used to electroporate BJ5183 cells. The recombinants were identified by restriction endonuclease mapping (PacI). Once recombination was confirmed, supercoiled plasmid DNA was transformed into DH10B cells for large-scale amplification. The recombinant construct was linearized with PacI and used to transfect HEK-293 cells. Recombinant adenovirus expressing both green fluorescent protein (GFP) and CXCR4 [each under a separate cytomegalovirus (CMV) promoter, Fig. 1A] was harvested after 7 days, purified by CsCl gradient centrifugation, and viral titers were determined.
Fig. 1.
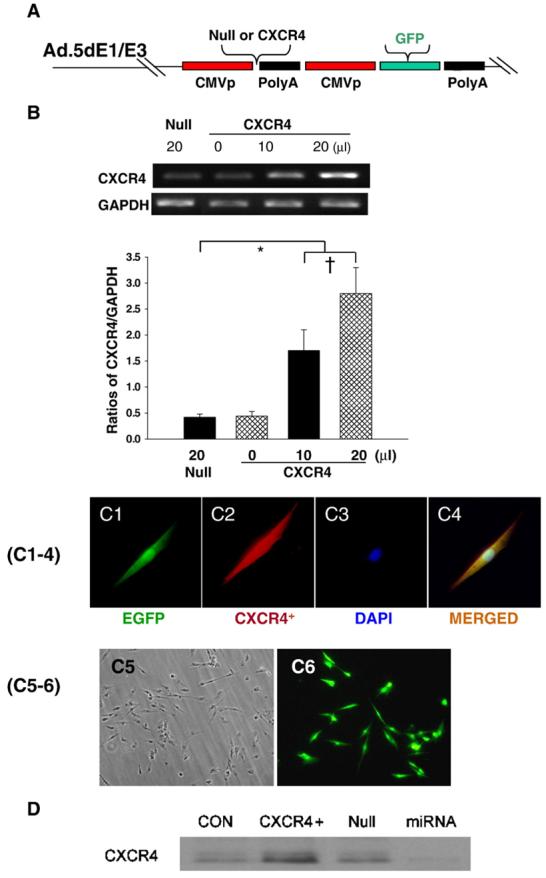
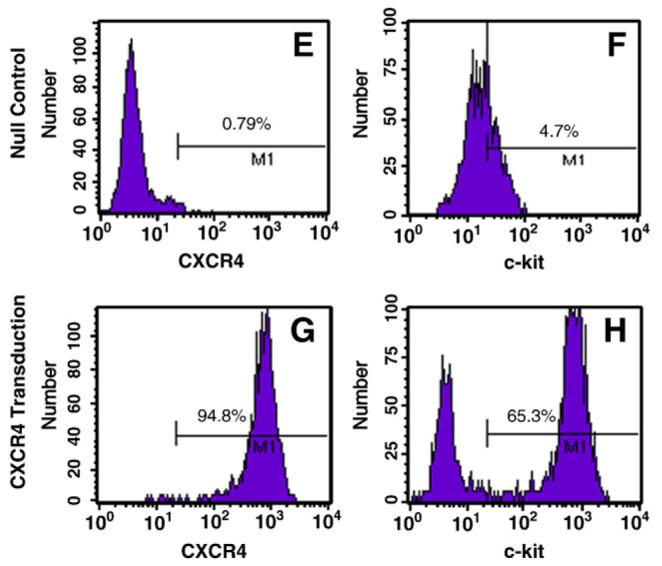
A: Generation of recombinant adenovirus vectors. B: RT-PCR for CXCR4 overexpression in MSCs. Panel B shows dose dependent CXCR4 overexpression. Null indicates Ad-null-GFP group. *p<0.05 vs. null group; †p<0.05 vs. CXCR4 (0 μl) group. Values mean±S.E.M., n=6 for each group. C(1–4): Immunofluorescence staining for EGFP and CXCR4. Panel C1 shows EGFP positive MSC, C2: Same MSC as “C1” except CXCR4 antibody staining (red color); C3: Same MSC as “C1” except nuclear staining by DAPI (blue color); C4, Same MSC as “C3” except DAPI co-localized with CXCR4 antibody staining and EGFP. Color codes for antibody staining are indicated. Panels C5–6 show the uniform transfection of MSCs with GFP and CXCR4 genes. C5, Non-transfected MSCs; C6, Adenovirus mediated EGFP and CXCR4 MSCs. Most of MSCs are uniformly transfected with EGFP and CXCR4 genes. D: CXCR4 expression on the membrane was determined by Western blotting using MSCs membrane extraction. CON indicates control group; CXCR4+, Ad-CXCR4/GFP group; Null, Ad-null-GFP group; miRNA, AdmiRNA targeting CXCR4 group. E–H: Flow cytometry of MSCs. Ad-null characterized cell fraction expressing CXCR4 (E) and c-kit (F); Ad-CXCR4 (G) and (H). Horizontal bar of each label in panel represents subpopulation of MSCs.
2.3. Preparation of mesenchymal stem cells
MSCs primary culture
Bone marrow cells (BMCs) were extracted from SD 8-week-old male rats. The animals were killed by cervical dislocation and BMCs were flushed out of tibias and femurs. After washing, centrifuged cells were resuspended in normal culture medium to a final concentration of 5 × 105 viable cells per milliliter in a T75 flask. Normal culture medium (NM) consisted of Dulbecco's modified Eagle's medium (DMEM, Gibco NY, USA) containing 10% (v/v) fetal bovine serum (FBS,) and antibiotics. The T75 flask was kept in a humidified 5% CO2 incubator at 37 °C for 72 h. Culture medium was changed every 3–4 days, non-adherent cells were removed by changing the medium.
MSCs subculture
Confluent primary cultures were washed once with PBS (Gibco). A 0.25% trypsin solution containing 0.01% EDTA (Sigma) was laid onto the monolayer and incubated for 10 min at 37 °C. After thorough detachment, cells were resuspended in NM and seeded in three T75 flasks until they were 80–90% confluent. Subsequent passages were performed in similar fashion. Passage 2–4 MSCs were used in the study.
2.4. CXCR4 labeling of MSCs
The adenoviral vector (Ad) without CXCR4 gene (Null) or with CXCR4 gene (Ad-CXCR4) was used. MSCs cultures were initiated in six-well plates in NM. MSCs were transduced overnight using dilutions of concentrated virus equivalent to 1× 107 infectious units in non-FBS medium. MSCs transduced with Ad-null-GFP served as control. Flow cytometry was used for isolating MSCs overexpressing GFP/CXCR4 for transplantation.
2.5. Transfection efficiency of micro-RNA (miRNA) in MSCs
We chose adenovirus-based miRNA constructs to express miRNA, which can knock-down CXCR4. Adenoviral system mediated transduction allows a stable cell line with CXCR4 knockdown by blasticidin selection. The CXCR4 top strand sequence: TGCTGTATAT ACTCACACTG ATCGGTGTTT TGGCCACTGA CTGACACCGA TCAGTGAGTATATA; the bottom strand sequence: CCTGTATATA CTCACTGATCG GTGTCAGTCAG TGGCCAAAACA CCGATCAGTGT GAGTATATAC. To evaluate transfection efficiency, we used the BLOCK-iT™ Adenoviral RNAi Expression System with EmGFP to deliver the specific double-stranded DNA oligonucleotides (ds oligo) that encoded the target CXCR4 pre-miRNA. The adenovirus without ds oligo served as a control. Briefly, 12 h before transduction, MSCs were maintained in Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum (FBS), supplemented with glutamine and penicillin/streptomycin in 6-well plates. Adenoviral stock (100 μl; 2 × 105 TU/ml) was added, the cells were incubated for 8 h, the adenoviral media was removed, and the cells were cultured in DMEM with 10% FBS until used.
2.6. Western blotting for membrane CXCR4 expression
Membrane proteins were obtained by membrane proteins extraction kit according to manufacturer's instructions (Calbiochem). In brief, the cells were transferred into centrifuge tube(s) and centrifuged at 300×g for 10 min at 4 °C. The pellet was washed with 2 ml ice cold PBS. The supernatant was removed without disturbing the pellet and discarded. 10 μl Protease Inhibitor Cocktail was added to the wall of the tube and immediately 2 ml ice-cold Extraction Buffer I was added to the cell pellet. The cell pellet was mixed, resuspended and incubated for 10 min at 4 °C under gentle agitation. Insoluble material was sedimented at 16,000×g and 4 °C for 15 min. The supernatant was discarded and the pellet kept on ice. Protease Inhibitor Cocktail 5 μl was added to the wall of the tube followed immediately ice-cold Extraction Buffer II by added 1 ml to the cell pellet. The cell pellet was then mixed, resuspended and maintained for 30 min at 4 °C under gentle agitation. Insoluble material was sedimented by centrifuge at 16,000×g for 15 min at 4 °C. The supernatant (membrane fraction) was stored at −20 °C until used for western blotting analysis. Membrane protein extract (20 μg) was separated by SDS-PAGE and transferred to PVDF membrane. The membrane was blocked with 5% milk in Tris-buffer saline solution (pH 7.6) containing 0.05% Tween-20 (TBS/T), and incubated with primary antibodies for CXCR4 (AnaSpec, Inc.) overnight at 4 °C. The membrane was then incubated for 1 h with HRPconjugated secondary antibody at room temperature, washed and developed with the ECL plus kit (GE Healthcare, USA).
2.7. RT-PCR analysis for matrix metalloproteinases
For measuring matrix metalloproteinase-9 (MMP-9) and membrane type 1 MMP (MT1-MMP), MSCs were subjected to hypoxic conditions, 1% O2, 5% CO2, and 94% N2 at 37 °C in an airtight Plexiglas chamber (Billups-Rothenberg, Del Mar, CA) for 6 h followed by 2 h reoxygenation. Control cells were cultured under normoxic conditions with 5% CO2 at 37 °C. Cells were reoxygenated by replacing ischemic buffer with culture medium at 5% CO2 and 37 °C for another 2 h. Total RNA isolated from MSCs was used for cDNA synthesis with SuperScript III RNase H-reverse transcriptase (Invitrogen). The cDNA as template was amplified with Taq DNA polymerase (Invitrogen). The sequences of primers: Rat CXCR4 sense primer sequence: 5′-gctgaggagcatgacagaca-3′ and antisense primer sequence: 5′-gatgaaggccaggatgagaa-3′; Rat MMP-9 sense primer sequence: 5′-ttcgacgctgacaagaagtg-3′and antisense primer sequence: 5′-aggggagtcctcgtggtagt-3′; Rat MT1-MMP sense primer sequence: 5′-gtaccccaagtcagctctgc-3′ and antisense primer sequence: 5′-cagtgaacgctggcagtaaa-3′; Rat GAPDH sense primer sequence: 5′-ctcatgaccacagtccatgc-3′ and antisense primer sequence: 5′-ttcagctctgggatgacctt-3′.The PCR products were size-fractionated by 1.2% agarose gel electrophoresis.
2.8. Surgical procedures
Myocardial infarction (MI) model was developed in SD female rats (200–250 g), as previously described [8]. Briefly, Isoflurane anesthesia was induced by spontaneous inhalation. The inhalation gas was a mixture of air and oxygen (total oxygen: 40%) and 2.4% isoflurane. A midline cervical skin incision was performed for intubation. The animals were mechanically ventilated with room air supplemented with oxygen (1.5 L/min) using a rodent ventilator (Model 683, Harvard Apparatus, South Natick, MA). Body temperature was carefully monitored with a probe (Cole-Parmer Instrument, Vernon Hill, IL) and was maintained at 37 °C throughout the surgical procedure. The heart was exposed by left side limited thoracotomy and the left anterior descending artery (LAD) was ligated with a 6-0 polyester suture 1 mm from tip of the normally positioned left auricle. Immediately after ligation of the LAD, SDF-1α (50 ng/μl) was injected into the sites in the ischemic area. Controls animals underwent LAD ligation and only saline was injected. GFP-transduced male MSCs (2 × 106) were injected into female rats via tail vein 3 days after LAD ligation. Approximately 10% of rats succumbed during surgical procedures.
2.9. Migration assay
To investigate migration of MSCs overexpressing CXCR4, a chemotaxis assay was performed using a chemotaxis chamber. We performed a chemotaxis assay in vitro using transwells (5-μm pore) (Corning, Corning, NY) as described [9]. Ad-null-MSCs or Ad-CXCR4-MSCs (1 × 105), or MSCs transduced with adenovirus-mediated miRNA (Ad-miRNA) in 100 μl medium were added to the upper chamber, and 600 μl medium with or without SDF-1α (0, 10, 100 ng/ml) was placed in the lower chamber. After incubation for 4 h at 37 °C, the cells migrating (bottom chamber) were counted randomly under fluorescence microscope. All groups were studied at least in triplicate.
2.10. Immunohistochemistry and FISH analysis
The immunohistochemical studies were performed as described previously [10,11]. Cells were labeled with a reliable cell marker, green fluorescent protein (GFP). Primary antibodies, CXCR4, sarcomeric α-actinin (Sigma), fluorescence labeled secondary antibodies (Jackson ImmunoResearch Laboratories or Molecular Probes) were used for immuostaining. To determine the expression and distribution of CXCR4 in transplanted heart, CXCR4 was detected by anti-CXCR4 (Cat: 28147, AnaSpec, Inc.). Endothelial cells were identified using CD31 antibody (PECAM-1, Santa Cruz Biotechnology). Vascular smooth muscles were identified immunohistochemically using antibodies against α-smooth muscle actin (M0951, DAKO). Myocytes were identified with α-sarcomeric actin antibody (Sigma) and 4′, 6-diamino-2-phenylindole (DAPI, Sigma) was used to stain nuclei [12]. Fluorescent imaging was performed with an Olympus BX41 microscope (Olympus America Inc., Melville, NY, USA) equipped with epiflouresence attachment and images were recorded using a digital camera with MagnaFire™ 2.1 software. FISH analysis of heart tissue samples was carried out using STAR* FISH Rat 12/Y Paints Protocol (CA-1631), as described previously [13]. Confocal images were obtained with a Leitz DMRBE fluorescence microscope equipped with a TCS 4D confocal scanning attachments (Leica, Inc.).
2.11. Echocardiography
Left ventricular (LV) function variables were assessed by transthoracic echocardiography, which was performed at 4 weeks after MI using iE33 Ultrasound System (Phillips) with a 15-MHz probe. After the induction of light general anesthesia, hearts were imaged two-dimensionally in long-axis view at the level of the greatest LV diameter. This view was used to position the M-mode cursor perpendicular to the LV anterior and posterior walls. The LV end-diastolic diameters and LV end-systolic diameters were measured from M-mode recordings according to the leading-edge method. LV ejection fraction (LVEF) was calculated as: LVEF (%) = [left ventricular end-diastolic dimension (LVDd)3 − left ventricular end-systolic dimension (LVDs)3]/(LVDd)3 × 100. All echocardiographic measurements were averaged from at least 3 separate cardiac cycles.
2.12. Measurement of fibrosis
Fixed hearts were embedded in paraffin and LV cross sections from apex, mid-LV, and base were stained with Trichrome-Masson. Image of LVarea of each slide was taken by Olympus BX41 microscope equipped with CCD (Magna-Fire™, Olympus) camera. Fibrosis area and total LV area of each image were measured using the Image-Pro Plus (Media Cybernetics Inc., Carlsbad, CA, USA), and the percentage of the fibrosis was calculated as (fibrosis area/total LV area) × 100.
2.13. Statistical analysis
Results were statistically analyzed with the use of the StatView 5.O software package (Abacus Concepts Inc., Berkeley, CA). All values were expressed as mean ±S.E.M. and were analyzed by one-way analysis of variance (ANOVA) for repeated measures, followed by Bonferroni/Dunn test or unpaired t-test. P-value 0.05 was considered statistically significant.
3. Results
3.1. Adenovirus mediated CXCR4 overexpression
CXCR4 overexpression in MSCs was verified by RT-PCR (Fig. 1B). Quantitative RT-PCR showed that CXCR4 expression was significantly higher in Ad-CXCR4 overexpressing MSCs in the presence of CXCR4 (10 and 20 μl) as compared with Ad-null group (Null), and this increase was concentration-dependent. MSCs also strongly expressed EGFP and CXCR4 by immunofluorescence staining (Figs. 1C1-6). The overexpression of CXCR4 was confirmed by Western blotting using membrane extraction (Fig. 1D). Flow cytometry showed the fraction of MSCs expressing CXCR4 and it was more than 100-fold greater (94.8%) in Ad-CXCR4 group (Fig. 1G) than in the Ad-null group for CXCR4 (0.74%, Fig. 1E). Similarly, the fraction of MSCs with c-kit positive expression in Ad-CXCR4 group (65.3%. Fig. 1H) was more than 12-fold greater than in MSCs of the Ad-null group (4.7%, Fig. 1F). CXCR4 expression was blocked by miRNA targeting CXCR4 (Figs. 1D and 2A, B).
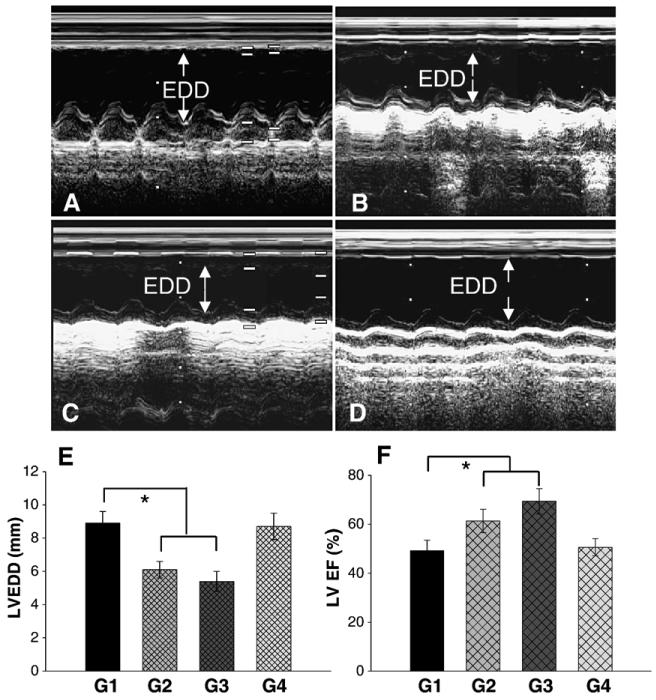
Fig. 6.
Assessment of cardiac function. M-mode echocardiograms are shown at 4 weeks in group 1 (A), group 2 (B), group 3 (C) and group 4 (D). Left ventricular enddiastolic diameters (LVDD, E) and ejection fraction (EF) (F) in hearts treated with CXCR4 overexpressing MSCs (G2), or SDF-1α plus CXCR4 overexpression (G3) was significantly better (F) than in control group (G1), or miRNA-CXCR4 group (G4). All values were expressed as mean±S.E.M. *p<0.05 vs. control group (G1). n=6 for each group.
Fig. 2.
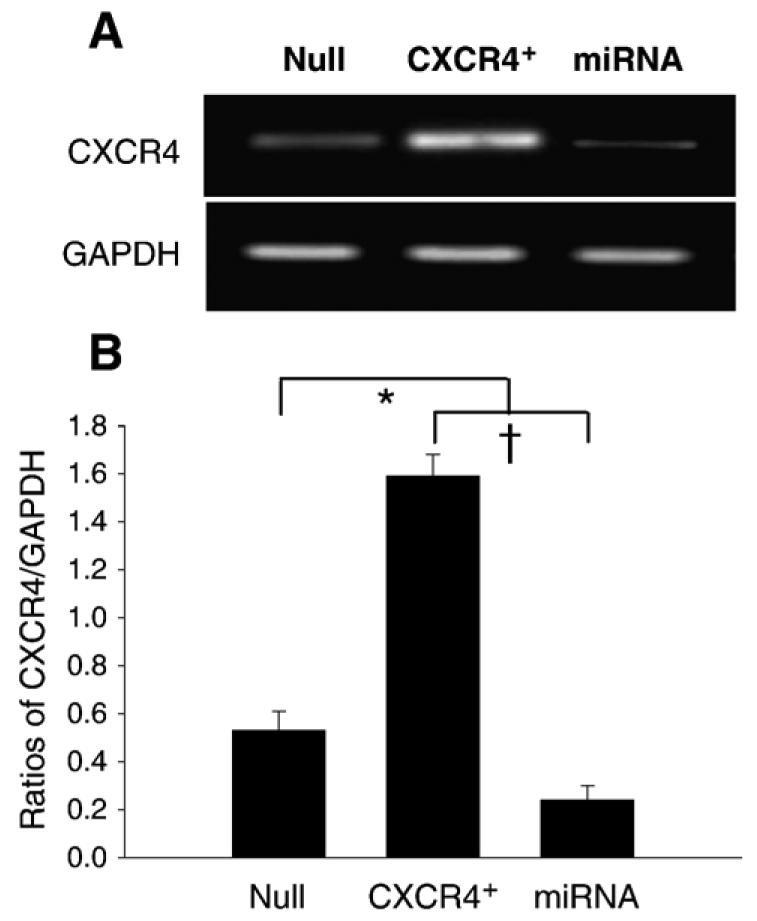
Effects of micro-RNA (miRNA) on MSCs. RT-PCR shows the transfection efficiency of CXCR4-miRNA on MSCs (A). CXCR4 expression was knocked down by miRNA targeting CXCR4 gene (B). Values are mean±S.E.M., n=6 for each group. *p<0.05 vs. null group; †p<0.01 vs. miRNA group.
3.2. Migration assay
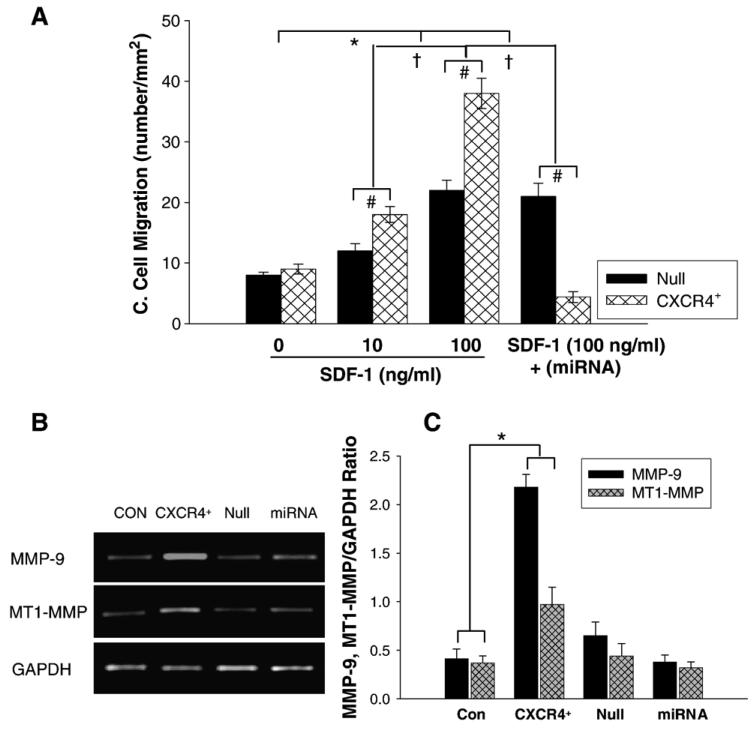
To determine whether SDF-1α/CXCR4 interaction is involved in stem cell migration and proliferation, we subjected MSCs transduced with either Ad-CXCR4/GFP or Ad-miRNA to different concentrations of SDF-1α for chemotaxis assay. We observed that exposure of Ad-CXCR4-MSCs to SDF-1α for 4 h caused a robust cell migration in a concentration dependent manner (Fig. 3A). The cell migration was significantly higher in Ad-CXCR4 group in the presence of SDF-1α (10 and 100 ng/ml) than in Ad-null group. However, in the presence of miRNA targeting CXCR4, cell migration in response to SDF-1α was blocked (Fig. 3A), suggesting that overexpression of CXCR4 is functional and responded to SDF-1α.
Fig. 3.
Cell migration and MMPs expression. A: Chemotaxis experiments were carried out by using transwells (5-μm pore). Cultured MSCs transduced with AdCXCR4/GFP (1 × 105) or Ad-miRNA targeting CXCR4 in 100 μl medium were added to the upper chamber, and 600 μl medium with or without SDF-1α (0, 10, 100 ng/ml) was placed in the bottom chamber. After 4 h incubation at 37 °C, migrating (bottom chamber) cells were counted. Exposure of Ad-CXCR4-MSCs to SDF-1α for 4 h resulted in strong cell migration, in a concentration dependent manner. The cell migration was significantly higher in Ad-CXCR4 group in the presence of SDF-1α (10 and 100 ng/ml) than in Ad-null group, p<0.05). The treatment of miRNA targeting CXCR4 prevented cell migration in response to SDF-1α, suggesting that CXCR4+-MSCs was functional and responded to SDF-1α. All values were expressed as mean±S.E.M. n=6 for each group. *p<0.05 vs. SDF-1α (0 ng/ml) group; †p<0.01 vs. other groups; #p<0.05 vs. null group. B: RT-PCR shows MMP-9 and MT1-MMP mRNA expression in CXCR4 overexpressing MSCs. CON indicates control MSCs group (lane 1); CXCR4+, CXCR4 overexpressing MSC group (lane 2); Null, adenoviral null treated MSCs (lane 3); miRNA, miRNA targeting CXCR4 (lane 4). GAPDH was used as the mRNA internal control to ensure equivalent loading. C: Quantitative assessment of CXCR4+-MSCs mRNA expression. All values were expressed as mean±S.E.M. n=6 for each group. *p<0.05 vs. control group.
3.3. MMPs expression in CXCR4 overexpressing MSCs (CXCR4+-MSCs)
MMP-9 and MT1-MMP expression was evaluated by RTPCR (Fig. 3B). MMP-9 and MT1-MMP were upregulated significantly only in CXCR4+-MSCs exposed to 6 h hypoxia followed 2 h reoxygenation. The hypoxic effect was not observed in other groups (Figs. 3B and C).
3.4. Myocardial regeneration
New myocytes were identified by EGFP (green) which was unambiguous evidence for the origin of MSCs forming new myocytes (Fig. 4A). The majority of EGFP cells were positive for sarcomeric α-actin suggesting that allogeneic MSCs migrated into the MI scar and periinfarcted area (group 3, Figs. 4A–C). As compared with group 1 (Fig. 4I), homing of MSCs to damaged cardiac area was higher in group 2. The migration of MSCs was the highest in group 3 (Fig. 4I). However, MSCs homing was blocked by miRNA against CXCR4 in group 4 (Fig. 4I). The presence of Y-chromosome positive nuclei (arrow) as determined by FISH was clearly shown in differentiated cardiomyocytes in group 1, 2, 3, and 4. As compared with group 1 (Fig. 4E), more Y-chromosome positive nuclei were located either in differentiated cardiomyocytes or between myofibers in group 2 (Fig. 4F). The differentiated cardiomyocytes with Y-chromosome positive nuclei in the peri-infarct area was the highest in group 3 (Fig. 4G). However, Y-chromosome positive nuclei were significantly reduced in group 4, which was treated with miRNA (Figs. 4H and J). FISH analysis confirmed that migrated cells were observed either in the peri- or infarcted region but not in the remote viable myocardium.
Fig. 4.
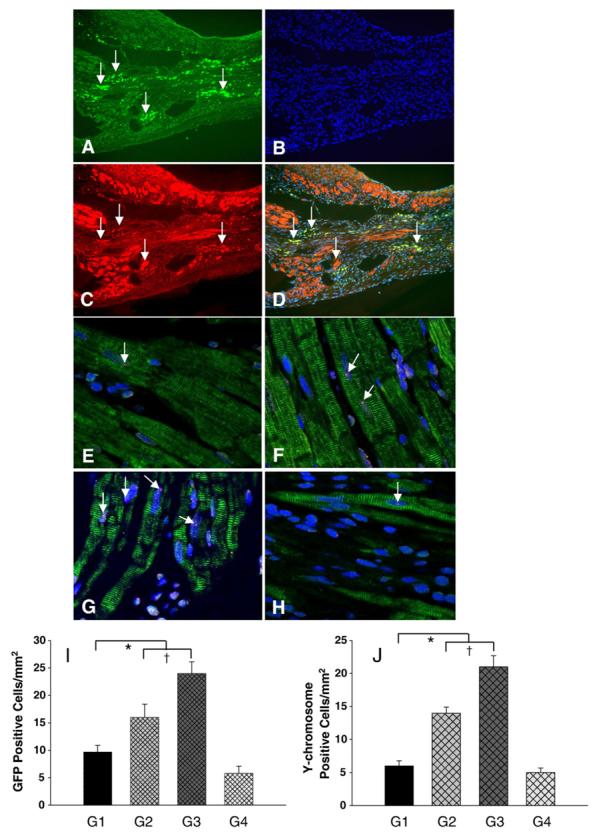
Migration and differentiation of MSCs in the infarcted myocardium. Panels A–D shows immunofluorescent staining in group 3 (data in other groups not shown). A: Immunostaining for EGFP-positive MSCs which migrated to the border zone of infarcted myocardium (green fluorescence, arrow). B: Same section as “A” except all nuclei (arrow) were identified by DAPI; C: Same section as “A and B” except α-sarcomeric actin staining for cardiac myocytes (red fluorescence); D: Same section as “A, B and C” except for colocalization of α-sarcomeric actin, EGFP and DAPI. Magnification × 200. Panels E–H shows FISH analysis for Y-chromosome. The presence of Y-chromosome positive nuclei (arrow) in the differentiated cardiomyocytes in group 1 (E), group 2 (F), group 3 (G) and group 4 (H). Myocytes were identified by sarcomeric α-actin (green). All nuclei were identified by DAPI staining (blue) (original magnification × 630, confocal imaging). I shows average number of transplanted GFP positive cells per unit area (mm2) in the peri-infarcted area after various treatments. J shows average number of transplanted Y-chromosome positive cells per unit area (mm2) in the peri-infarcted area after various treatments. All values were expressed as mean±S.E.M. n=6 for each group. *p<0.05 vs. control group; †p<0.05 vs. other groups. G1 indicates group 1; G2, group 2; G3, group 3; G4, group 4.
3.5. Immunohistochemical evidence for angiogenesis and CXCR4 expression
PECAM-1(CD31) was used as a marker for endothelial cells. Semiquantitative analysis demonstrated that the number of capillary density was significantly increased in group 2 and group 3 as compared with group 1 or group 4 (Figs. 5A and E). The number of endothelial cells per randomly chosen field did not differ between group 2 (48.6±5.6) and group 3 (52.4±4.1), but they were increased two-fold in both group 2 and group 3 compared with group 1 (23.8±3.6, p<0.01) or group 4 (25.9±4.3, p<0.05). Similarly the presence of CXCR4 antibody positive cells was prominent in groups 2 (Fig. 5C) and 3 compared to groups 1 and 4 (Fig. 5F). The CXCR4 expression was predominantly observed in the vascular endothelial cells in the damaged myocardium, being the highest in group 2 (16.3±2.4) and group 3 (18.5±2.1) as compared with group 1 (6.7±1.4, p<0.01) while CXCR4 expression in group 4 (3.8±0.8, p<0.01) was minimal due to inhibition of CXCR4 by miRNA (Fig. 5F). Smooth muscle α-actin was used as a marker for vascular smooth muscle cells. Similarly, vascular density was significantly increased in group 2 and group 3 as compared with group 1 or group 4 (Fig. 5G). The number of α-actin positive vessels located in the infarcted area was increased in group 3 (Fig. 5B). At 4 weeks after MSCs injection, Ychromosome positivity was observed in the nuclei of blood vessels in group 3 (Fig. 5D) (data not shown for other groups). The presence of Y-chromosome positive nuclei (arrow) was determined by FISH.
Fig. 5.
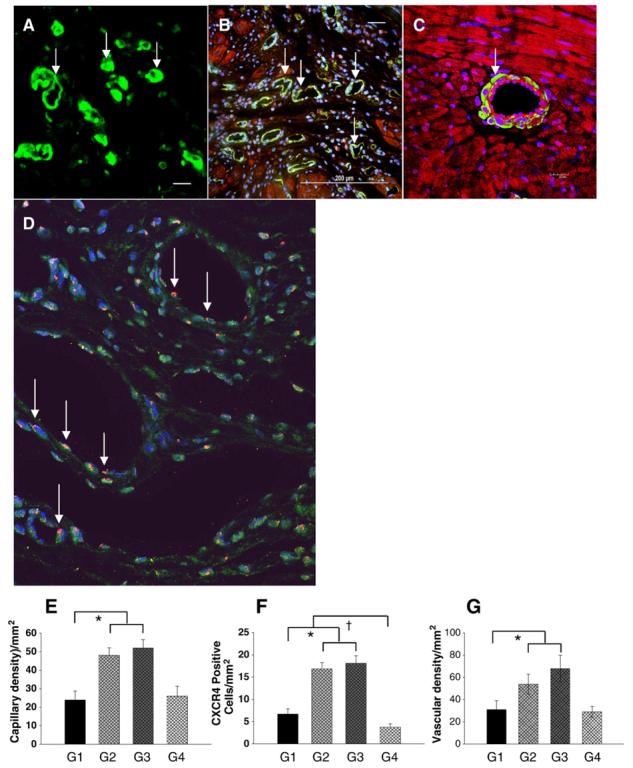
Assessment of blood vessel density. Blood vessels were stained with CD31 (A), α-smooth muscle actin (SMA) (B) and Y-chromosome positive nuclei (D). Capillary density was identified by CD31 antibody in group 3 (A, arrows) and quantified (E). Immunocytochemistry of the heart tissues with polyclonal antibody revealed an intense staining of CXCR4 predominantly in the endothelial cells in the damaged myocardium in group 2 (C, arrow). D shows the presence of Ychromosome positive nuclei (arrow) in the differentiated blood vessels in group 3 (data in other groups not shown). DAPI-stained nuclei (blue) (original magnification × 630, confocal imaging). F shows CXCR4 positive vessels per unit area (mm2) in the infarcted area of various treatment groups. The number of capillary vessels located in the infarcted area is shown in group 3 (E, green color). F shows quantitative analysis of vascular density (anti α-smooth muscle actin antibody staining) was determined by various treatments. All values were expressed as mean±S.E.M. *p<0.05 vs. control group; †p<0.05 vs. other groups. n=6 for each group. (Original magnification × 200 except confocal imaging.) G1 indicates group 1; G2, group 2; G3, group 3; G4, group 4.
3.6. Parameters for cardiac function and fibrosis
Cardiac function analyzed by echocardiography showed a significant improvement in left ventricular end-systolic and end-diastolic diameters in rats which received CXCR4 over-expressing MSCs (group 2, Fig. 6B), or received CXCR4 overexpressing MSCs and SDF-1α (group 3, Fig. 6C) than in group 1 which was treated with ordinary MSCs (Fig. 6A). Left ventricle end-diastolic diameters (LVEDD) were significantly reduced (p<0.05) in groups 2 and 3 compared with the other two groups (Fig. 6E). Consistent with function restoration, LV ejection fraction was higher in group 2 and group 3 than in other groups at 4 weeks after MI (p<0.05; Fig. 6F and Table 1). Table 1 shows a comparison of echocardiography findings among the different treatment groups. LVDs showed a tendency similar to that for LVDd. LVDs decreased in the groups 2 and 3 compared to groups 1 and 4, with no significant difference between groups 2 and 3. However, addition of SDF-1α with CXCR4-overexpressing MSCs did not further improve LVEDD, LVDs and ejection fraction in group 3. The groups receiving ordinary MSCs (group 1) or miRNA (group 4) treatment were no different from each other (Table 1).
Table 1.
Assessment of the cardiodynamic variables by echocardiography in various treatment at 4 weeks after MI
| Group | Normal | G1 | G2 | G3 | G4 |
|---|---|---|---|---|---|
| LVDd (mm) | 5.2±0.2 | 8.8±0.5 | 5.9±0.4* | 5.3±0.7* | 8.6±0.3 |
| LVDs (mm) | 3.3±0.4 | 7.0±0.8 | 4.3±0.5* | 3.6±0.9* | 6.8±0.8 |
| EF (%) | 78±4.7 | 49.2±2.3 | 61.3±5.1* | 69.3±3.9* | 50.6±3.4 |
LVDd indicates left ventricular end-diastolic dimension; LVDs, left ventricular diameter at end-systole. EF, ejection fraction; AMD, AMD3100.
p<0.05 vs. control n=6 in each group. Data represent the mean±S.E.M. G1 indicates group 1; G2, group 2; G3, group 3; G4, group 4.
Fig. 7 indicates the heart sections after Masson-Trichome staining (A) and percentage of fibrosis (B). Four weeks after MI, the percentage of fibrosis in the left ventricle wall in group 2 and group 3 was significantly reduced as compared with group 1, or group 4 and was the lowest in group 3 as compared with other groups (Figs. 7A and B).
Fig. 7.
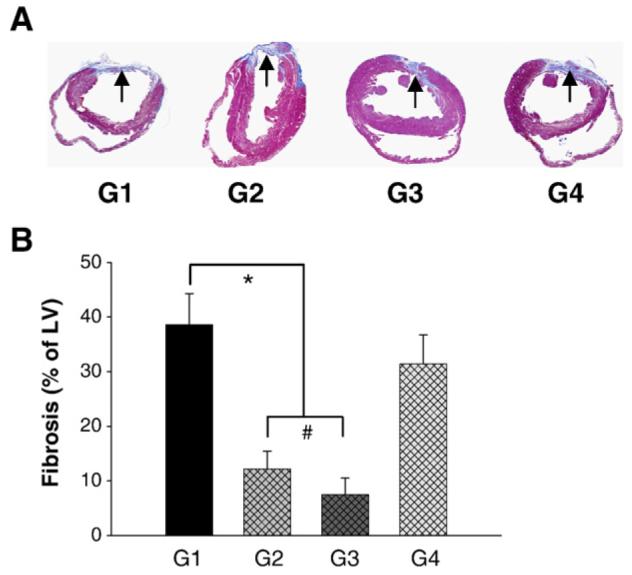
Measurement of fibrosis. Panel A shows sections for Masson-Trichome staining in various treatments. Hearts receiving CXCR4 overexpressing MSCs in group 2 (G2) or combination of CXCR4 overexpressing MSCs with SDF-1α in group 3 (G3) exhibited reduction in the fibrosis (arrow) as compared to group 1 (G1) or group 4 (G4) (A, G1–G4). Panel B shows percentage of fibrosis in hearts after permanent LAD occlusion and various treatments. Fibrosis in G2 and G3 was significantly reduced to 12.2±2.3%, 7.5±2.8%, respectively as compared with G1 (38.6±5.7%) or G4 (31.4±5.3%), B: All values were expressed as mean±S.E.M. *p<0.05 vs. control group; #p<0.05 vs. group 2 (n=6 in each group). G1 indicates group 1; G2, group 2; G3, group 3; G4, group 4.
4. Discussion
The major conclusion of this study is that engraftment of CXCR4 overexpressing MSCs in the infarcted myocardium is effective for successful angiomyogenesis in the infarcted myocardium. Bone marrow-derived MSCs are multipotent progenitor cells, which are increased in the peripheral circulation [14] and in the myocardium following myocardial infarction [15]. Although MSCs have been shown to participate in myocardial repair, their homing to infarcted area and their survival post transplantation are limited [16]. The cell viability may ultimately determine the final outcome of cardiac repair [17]. CXCR4 is receptor for SDF-1α and is very important in the stem cell migration, homing, and survival [18]. However, the mechanisms of MSCs recruitment to the damaged myocardium by CXCR4/SDF-1α interaction remain unclear. In this study, we showed that SDF-1α/CXCR4 interaction played an important role in recruitment of MSCs to the infarcted myocardium for its repair.
CXCR4 is critical for homing, transendothelial migration, and engraftment of hematopoietic stem and progenitor cells [19]. The importance of the SDF-1α/CXCR4 axis in hematopoiesis is supported by genetic studies in mice [20]. The SDF-1α/CXCR4 axis is an essential component of cell differentiation in numerous tissues as shown by the use of SDF-1α and CXCR4 knockout mice. SDF-1α/CXCR4 interaction also has a physiological role during embryogenesis in vascular development, cardiogenesis, and cerebral development [21-23]. The current study demonstrated that CXCR4 overexpressing MSCs increase the expression of c-kit. This is of potential importance because c-kit positive cells play a significant contributing role in cardiac repair and restoration of heart function after myocardial infarction [24]. The significance of the increased number of c-kit positive cells further underscores the factor that c-kit deficient cardiac progenitor cells in W/Wv mice prevent cell differentiation during aging or after injury [24] and that c-kit positive cells are also associated with enhanced angiogenesis [25].
CXCR4 expression is dynamic and is regulated by various factors including cytokines, chemokines, stromal cells, adhesion molecules, and proteolytic enzymes [26]. CXCR4 is up-regulated in human stem and progenitor cells by short-term treatment with cytokines under in vitro culture condition [27,28]. This subsequently enhances their in vitro migration toward an SDF-1α gradient [27,29] and is also in agreement with our study. Low concentrations of SDF-1α together with other cytokines enhance the survival and proliferation of both human CD34+ cells and murine stem cells [30,31]. On the other hand the SDF-1α/CXCR4 interaction is also involved in the retention of stem and progenitor cells in the bone marrow [20,32,33]. SDF-1α is up-regulated in the heart immediately after MI [14] causing migration of CXCR4 expressing MSCs towards the infarcted myocardium. Besides their migratory role, these CXCR4 positive MSCs participated in neovascularization and differentiated into new myocytes. In addition, MSCs secrete or express SDF-1α [34,35] and its interaction with CXCR4 increases survival of progenitor cells [6]. SDF-1α could serve as an adhesive molecule by binding CXCR4-expressing cells to the vessel wall [36], which is consistent with our study that a high degree of CXCR4 expression is observed in the vascular endothelial cells. Our results demonstrated that MSCs overexpressing CXCR4 enhanced vascularization in the damaged myocardium. SDF-1α up-regulation in the myocardium after MI, as shown in this study is important in stem cell recruitment [13]. However, it was also reported that SDF-1α expression alone did not increase vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) which may explain the ineffectiveness of MSCs in the repair of damaged heart [37]. Another recent study showed lack of myocytes regeneration by overexpressing SDF-1α in the infarcted area despite improvement of cardiac function [6]. This protection could occur by recruiting the anti-apoptotic kinases ERK and Akt [36]. Our results further suggest that the combined effects of MSCs overexpressing CXCR4 in the infarcted area with secretion of SDF-1α by myocytes are essential for successful cardiac regeneration. This conclusion is supported by our data that inhibition of CXCR4 by miRNA-CXCR4 significantly reduced homing of transplanted MSCs to the ischemic myocardium resulting in limited myocardial regeneration and angiogenesis. Our data support recent reports that the SDF-1α/CXCR4 interaction is important for MSCs migration to the infarcted myocardium and angiogenesis [6,38]. However, the approach in this study used the delivery of CXCR4 over-expressing MSCs rather than delivery of SDF-1α expressing MSCs.
The effect of MSCs overexpressing CXCR4 might be different as these cells up-regulate MMP-9 and MT1-MMP under hypoxic conditions which may enhance the ability of CXCR4+-MSCs to cross the reconstituted basement membrane to generate new myocytes (Fig. 3B). MSCs in culture are known to upregulate MMP-2, MMP-9, and membrane type-1 (MT1)-MMP, leading to significantly increase vessel network formation [39]. The finding that CXCR4+-MSCs overexpressing MMPs could be exciting. It is likely that these MMPs may soften the collagenous area and facilitate their homing and engraftment in the infarcted area. The combined effect of accelerated engraftment of these cells in the infarcted or border zones and increase in number of newly differentiated myocytes and endothelial cells resulted in improved regional LV function and reduction in scar size.
The potential difficulties in assessing the success of cell transplantation involve the tracking and the fate of the donor cells in vivo. This problem was facilitated by the use of adenovirus encoding both CXCR4 and green fluorescent protein, which is easy to identify MSCs by immunofluorescent microscopy. The other potential difficulty is how to quantify the homed MSCs in the damaged myocardium. Using this model, however, we were able to identify homed cells by their green color and the presence of the Y-chromosome as unique and reliable markers of mobilized cells not only for myogenesis but also for angiogenesis. Sex mismatched cell transplantation using male GFP+-MSCs in female rats helped to identify the Y chromosome positive donor cells in the recipient heart thus confirming the source of stem cells.
4.1. Conclusion
Overexpression of CXCR4 in MSCs was extremely effective in their engraftment in the infarcted myocardium for neomyoangiogenesis. The strategy of exploiting homing of CXCR4 overexpressing MSCs to the infarcted myocardium would be of significant importance in the cell based therapy of infarcted myocardium.
Acknowledgments
This work was funded by the National Institutes of Health grants HL-081859-01 (Y. Wang); HL-74272, HL-080686 (M. Ashraf): HL083236 (M. Xu); HL87861-01 (GC. Fan). The authors wish to thank Professor R.W. Millard, PhD for helpful discussion.
References
- 1.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–61. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 3.Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110(20):3213–20. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 4.Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–16. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–9. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1α expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 7.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Ahmad N, Wang B, Ashraf M. Chronic preconditioning: a novel approach for cardiac protection. Am J Physiol Heart Circ Physiol. 2007;292:H2300–5. doi: 10.1152/ajpheart.01163.2006. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Ahmad N, Wani MA, Ashraf M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol. 2004;37:1041–52. doi: 10.1016/j.yjmcc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol. 2006;40:736–45. doi: 10.1016/j.yjmcc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res Dec. 2007 doi: 10.1093/cvr/cvm025. Electronic publication ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Haider HKH, Ahmad N, Zhang D, Ashraf M. Evidence for ischemia induced host-derived bone marrow cell mobilization into cardiac allografts. J Mol Cell Cardiol. 2006;41:478–87. doi: 10.1016/j.yjmcc.2006.06.074. [DOI] [PubMed] [Google Scholar]
- 14.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–8. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–23. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 17.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–67. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–30. [PubMed] [Google Scholar]
- 20.Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324–32. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 21.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 22.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 23.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 24.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:2304–9. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CH, Anderson N, Li SH, Szmitko PE, Cherng WJ, Fedak PW, et al. Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circ Res. 2006;99:617–25. doi: 10.1161/01.RES.0000243210.79654.fd. [DOI] [PubMed] [Google Scholar]
- 26.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 27.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 28.Forster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, et al. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160:1522–31. [PubMed] [Google Scholar]
- 29.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–96. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grafte-Faure S, Levesque C, Ketata E, Jean P, Vasse SC, Vannier JM. Recruitment of primitive peripheral blood cells: synergism of interleukin 12 with interleukin 6 and stromal cell-derived factor-1. Cytokine. 2000;12:1–7. doi: 10.1006/cyto.1999.0520. [DOI] [PubMed] [Google Scholar]
- 31.Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, et al. The SDF-1–CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells. 2001;19:453–66. doi: 10.1634/stemcells.19-5-453. [DOI] [PubMed] [Google Scholar]
- 32.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–71. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 33.Kawabata K, Ujikawa M, Egawa T, Kawamoto H, Tachibana K, Iizasa H, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci U S A. 1999;96:5663–7. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- 35.Van Overstraeten-Schlögel N, Beguin Y, Gothot A. Role of stromal-derived factor-1 in the hematopoietic-supporting activity of human mesenchymal stem cells. Eur J Haematol. 2006;76:488–93. doi: 10.1111/j.1600-0609.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–63. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 38.Elmadbouh I, Haider HKH, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–9. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]