Abstract
Background
Chronic changes in blood flow stimulate arterial remodeling, which contributes to the maintenance of vascular homeostasis. Experimental studies suggest that remodeling represents a response to local changes in endothelial shear stress and is nitric oxide–dependent.
Methods and Results
To investigate determinants of outward arterial remodeling in humans, we measured ulnar artery flow, diameter, and flow-mediated dilation before and after removal of the adjacent radial artery in 53 patients who were undergoing coronary bypass surgery (age 60±11 years; 13% female). Removal of the radial artery increased ulnar artery blood flow by 35% (P=0.009) and increased ulnar artery diameter by 9% (P<0.001) 4 to 8 weeks after surgery. At 1 week, ulnar artery shear stress was increased by 58% (P<0.001), but it was no longer different from baseline at longer-term follow-up. The contralateral ulnar artery was unaffected, which suggests that these findings were not attributable to the systemic effects of medications or the postoperative state. Extent of outward remodeling correlated with the increase in blood flow (r=0.50, P=0.001) and with flow-mediated dilation at baseline (r=0.50, P=0.001). Remodeling correlated inversely with baseline endothelial expression of P-selectin in the radial artery (r=−0.76, P=0.004, n=14).
Conclusions
A sustained increase in blood flow in the ulnar artery induced outward arterial remodeling despite the presence of risk factors and coronary artery disease. The remodeling response was related to endothelial phenotype, as reflected by flow-mediated dilation and expression of P-selectin. These findings provide evidence that the endothelium plays an important role in the regulation of vascular structure in humans.
Keywords: remodeling, endothelium, shear stress, blood flow, P-selectin
Chronic increases in arterial blood flow over days to weeks induce compensatory changes in arterial structure that result in an enlarged arterial lumen with normal wall thickness.1,2 This chronic remodeling response contrasts with the acute vasodilator response that occurs within seconds to minutes after an increase in blood flow. Chronic arterial remodeling occurs during normal growth and development, repetitive exercise, and other clinical settings and helps maintain the appropriate balance between tissue demand and blood supply. The primary signal for flow-induced arterial remodeling and flow-mediated dilation is believed to be altered shear stress at the endothelial surface. Shear stress is the frictional force produced by flowing blood, and it relates directly to blood flow and inversely to the third power of the arterial radius. A chronic increase in flow increases local shear stress and stimulates outward remodeling that continues until shear stress has been restored to baseline. Conversely, decreased blood flow leads to an endothelium-dependent decrease in the size of the arterial lumen.3 This feedback mechanism maintains shear stress in the physiological range and endothelial cells in a quiescent and atheroprotective phenotype.4
Flow-induced arterial remodeling has important links to atherosclerosis. During lesion development, arterial remodeling is an adaptive response that helps maintain lumen size (Glagov phenomenon).5 On the other hand, activation of proinflammatory mechanisms during the remodeling process may contribute to plaque vulnerability, restenosis, and cardiovascular events.6–8 Remodeling depends on the bioavailability of endothelium-derived nitric oxide9 and activation of proinflammatory signaling mechanisms and may be influenced by cardiovascular risk factors.2,10
In experimental animals, arterial remodeling is often studied by ligation of 1 carotid artery, which produces a sustained increase in blood flow in the contralateral carotid artery at a defined point in time.11 In the present study, we took advantage of a parallel situation in patients and examined the consequences of increased blood flow in the ulnar artery as it takes over sole blood flow to the hand after removal of the radial artery for use as a coronary bypass conduit. We hypothesized that a chronic increase in ulnar artery flow would induce outward arterial remodeling, and we sought to investigate clinical predictors of this response.
Methods
Study Subjects
We enrolled consecutive patients with coronary artery disease undergoing nonemergent coronary artery bypass surgery using the radial artery as a bypass conduit at Boston Medical Center. The radial artery harvest technique has been described previously and is illustrated in Figure 1.12,13 The present study focused on the structural and functional consequences of radial artery harvest on conduit ulnar artery structure and blood flow. All subjects provided written informed consent, and the protocol was approved by the Boston Medical Center Institutional Review Board.
Figure 1.
Forearm angiogram illustrating the relationship of ulnar and radial arteries in the forearm before radial artery harvest (top) and a photograph of the left arm of a patient after radial artery harvest illustrating the location the surgical scar at the wrist and the site of ultrasound imaging (lower panel). The lower panel was reproduced with permission from work by Shapira and colleagues (Wiley-Blackwell Publishing).13
Preoperative Assessment
Study personnel reviewed medical records and interviewed subjects to determine age, gender, ethnicity, medications, body mass index, cigarette smoking, and clinical history of diabetes mellitus, hypertension, hypercholesterolemia, and unstable angina. We recorded the preoperative complete blood count, serum creatinine, and lipid values from the medical record and measured blood pressure and heart rate (average of 3 recordings) using an automatic physiological recorder (Dinamap, General Electric Healthcare, Waukesha, Wis).
We measured diameter, resting blood flow, peak reactive hyperemia, and flow-mediated dilation in both ulnar arteries by ultrasound as described previously.14–16 Briefly, 2D ultrasound images were recorded before and 1 minute after induction of reactive hyperemia by 5-minute inflation of a blood pressure cuff on the upper arm to the greater of 200 mm Hg or 50 mm Hg above systolic pressure. In a subset of 5 patients, we measured ulnar artery diameter 3 minutes after sublingual nitroglycerin 0.4 mg. Doppler flow signals were recorded at baseline and for 15 seconds after cuff release to identify peak reactive hyperemia. Digitized images were analyzed with customized software (Medical Imaging Applications, LLC, Coralville, Iowa) in a blinded manner.
Baseline and hyperemic flows were expressed as flow volume (mL/min) calculated from flow velocity and vessel cross-sectional area. Flow-mediated dilation was expressed as percentage change from baseline and as the ratio of flow-mediated dilation to hyperemic flow. Ulnar artery shear stress was calculated as 8 μV/diameter, where μis blood viscosity (assumed to be 0.035 dyne·s−1 ·cm−2) and V is ulnar velocity at baseline or at peak hyperemia.
We tested subjects in a postabsorptive state and, when relevant, asked them to refrain from smoking overnight before the study. Vasoactive medications were continued without interruption before the study, because we previously demonstrated no effect of such medications on vascular function in comparable patients.17 Reproducibility for measurement of arterial flow in our laboratory has been reported previously.16 We measured ulnar artery diameter 3 times in 46 patients to evaluate reproducibility. The correlation coefficients for paired measurements averaged 0.99, with an absolute difference between measurements of 0.06 mm (2%) and a coefficient of variation of 2%.
Follow-Up Assessment
We repeated measurement of resting ulnar diameters and flows when the subject had achieved a stable condition 3 to 7 days after surgery. We repeated measurement of ulnar artery diameter, flow, peak reactive hyperemia, flow-mediated dilation, and postnitroglycerin diameter at the time of a postoperative outpatient follow-up visit (4 to 8 weeks after surgery). In some cases, we were unable to make follow-up measurements of diameter or flow because of surgical dressing, intravenous infusion catheters, or other clinical circumstances.
Histological Analysis
Radial artery tissue was available from a subset of 14 subjects, and expression of endothelial markers of inflammation in those subjects was included in a previous report.12 In the present study, we compared adhesion molecule expression in the radial artery to the ulnar artery remodeling response. As described in detail previously,12 segments of radial artery were fixed in glutaraldehyde, frozen, sectioned, and stained with anti-human P-selectin (BD Pharmingen, San Diego, Calif), vascular cell adhesion molecule-1 (Dako, Carpinteria, Calif), or intercellular adhesion molecule-1 (Santa Cruz Biotechnology, Santa Cruz, Calif) antibodies that had been diluted 1:200, 1:200, and 1:50 with BioGenex diluent (BioGenex, San Ramon, Calif), respectively. Secondary antibody staining was performed with the StrAviGen multilink biotinylated kit (BioGenex). The intensity of staining was assessed by 3 blinded observers using a semiquantitative scale (0, none; 1, weak; 2, moderate; and 3, strong staining), which has good agreement among observers (weighted κ=0.61).
Statistical Analysis
The primary end point of the study was the change in ulnar artery diameter induced by removal of the radial artery (arterial remodeling). We divided study subjects into 2 groups using the median change in ulnar artery diameter at the final visit as a cut point and compared the clinical characteristics for the 2 groups using the χ2 test for categorical variables and the Student’s t test or Mann–Whitney test for continuous variables that had a normal or skewed distribution, respectively. Measurements of vascular structure and function were made at baseline and at the 2 postoperative visits, and we evaluated time-dependent changes using repeated-measures ANOVA.
Although we were interested in the local response to increased ulnar artery blood flow, we recognized that coronary bypass surgery would have systemic effects that could influence ulnar artery structure and function. To distinguish local from systemic effects, we completed an analysis that examined each variable expressed as the ratio of that variable measured in the surgical and nonsurgical arms.
Finally, we explored the clinical predictors of the arterial remodeling response expressed as the percent change in ulnar artery diameter. In this analysis, we identified clinical and vascular variables measured at baseline that correlated with change in ulnar artery diameter. We selected variables that demonstrated a correlation with the extent of ulnar artery remodeling with a P value <0.10 and included them in multivariable models into which age and sex were forced (history of smoking was the only clinical variable that met this criterion). Analysis was completed with SPSS for Windows version 12.0.1 (SPSS Inc, Chicago, Ill). Data are presented as mean and SD, and P<0.05 was considered statistically significant.
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Study Subjects
Fifty-three patients enrolled in the study; their clinical characteristics stratified by outward remodeling response are displayed in Table 1. Most had the radial artery removed from the left arm as illustrated in Figure 1. The enrolled subjects were predominantly male and overweight to obese, with a high prevalence of risk factors, as expected in patients undergoing coronary bypass surgery. Subjects with a remodeling response below the median were more likely to have a history of cigarette smoking. The other clinical characteristics were similar for the 2 groups. Notably, we observed no relation between remodeling response and prescribed medications, including vasoactive drugs and statins.
Table 1.
Clinical Characteristics
| Ulnar Remodeling Response
|
|||
|---|---|---|---|
| Clinical Characteristic | Below Median (n=26) | Above Median (n=27) | P |
| Age, y | 58±11 | 62±10 | 0.32 |
| Female, % | 8 | 19 | 0.25 |
| Black race, % | 12 | 4 | 0.26 |
| Surgical arm, right/left | 1/25 | 4/23 | 0.19 |
| Body mass index, kg/m2 | 30.2±3.6 | 29.6±6.2 | 0.66 |
| Systolic blood pressure, mm Hg | 136±22 | 129±23 | 0.26 |
| Diastolic blood pressure, mm Hg | 73±11 | 70±8 | 0.35 |
| Glucose, mg/dL | 137±57 | 128±57 | 0.27 |
| Total cholesterol, mg/dL | 187±47 | 169±41 | 0.17 |
| HDL cholesterol, mg/dL | 43±12 | 42±12 | 0.91 |
| Triglycerides, mg/dL | 178±128 | 126±75 | 0.11 |
| LDL cholesterol, mg/dL | 109±30 | 105±34 | 0.66 |
| Creatinine, mg/dL | 0.78±0.20 | 0.84±0.21 | 0.29 |
| White blood count (1000/μL) | 9.1±3.3 | 8.6±2.8 | 0.56 |
| Hemoglobin, g/dL | 12.9±2.3 | 13.2±2.2 | 0.68 |
| Platelet count (1000/μL) | 223±70 | 196±60 | 0.16 |
| Diabetes mellitus, % | 50 | 44 | 0.69 |
| Hypertension, % | 77 | 82 | 0.68 |
| Hypercholesterolemia, % | 92 | 89 | 0.67 |
| Any history of cigarette smoking, % | 85 | 59 | 0.04 |
| Statin treatment, % | 96 | 96 | 0.98 |
| ACE inhibitor treatment, % | 46 | 48 | 0.88 |
| Nitrate treatment, % | 50 | 33 | 0.22 |
| Calcium blocker treatment, % | 12 | 19 | 0.48 |
| Insulin treatment, % | 8 | 15 | 0.41 |
| Aspirin treatment, % | 100 | 100 | 0.99 |
| Unstable presentation, % | 46 | 41 | 0.69 |
ACE indicates angiotensin-converting enzyme.
Effect of Radial Artery Removal on Ulnar Artery Blood Flow and Structure
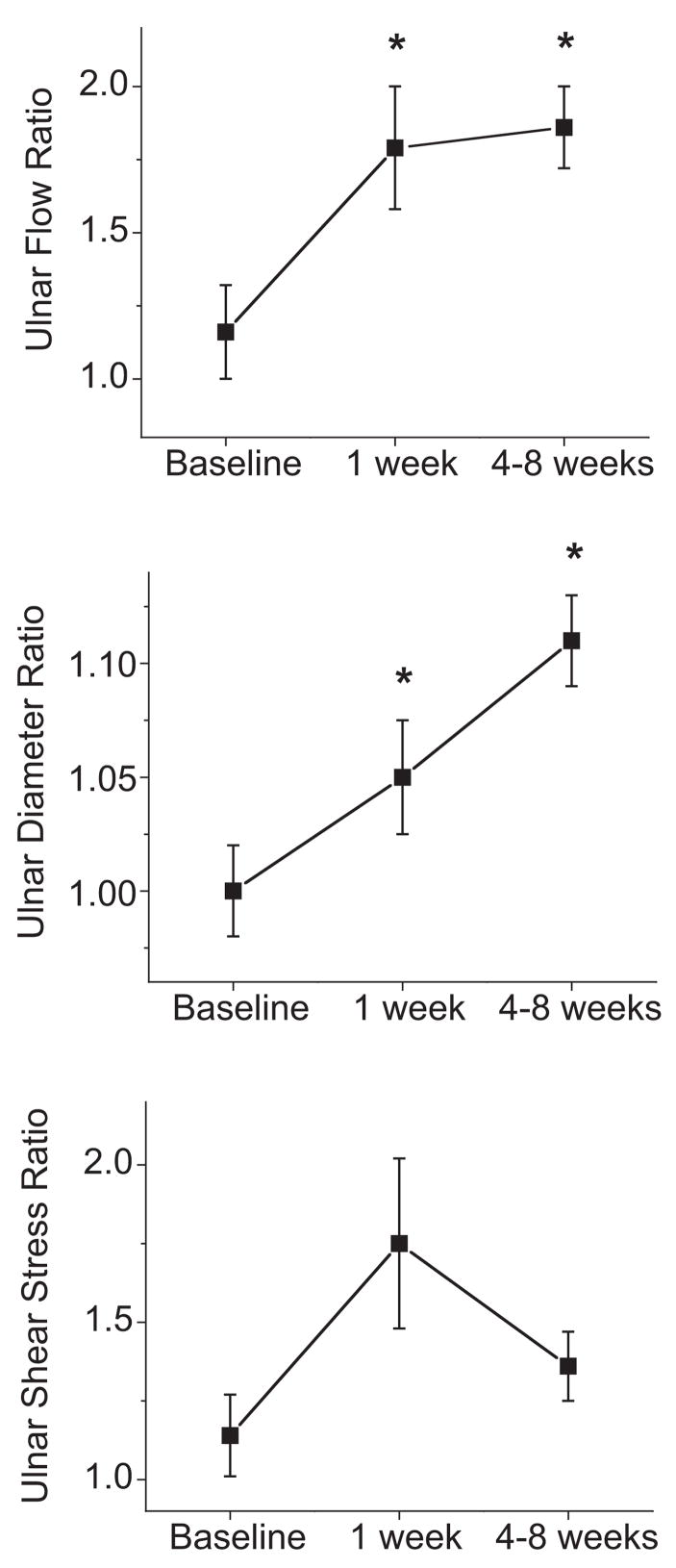
The effects of radial artery removal on the ulnar artery are displayed in Table 2. As shown, ulnar artery blood flow increased markedly at 1 week and remained significantly elevated at longer-term follow-up. Ulnar artery diameter increased at 1 week and continued to increase at 4 to 8 weeks. Arterial shear stress was increased at 1 week but was no longer increased compared with baseline at the long-term follow-up visit. In contrast, there were no significant changes compared with baseline in flow, diameter, or shear stress in the nonsurgical arm, in which the radial artery remained intact.
Table 2.
Ulnar Artery Characteristics After Removal of Radial Artery
| Ulnar Artery Characteristic | Baseline | 1 Week | 4 to 8 Weeks | P |
|---|---|---|---|---|
| Surgical arm | ||||
| Flow, mL/min (n=42) | 80±61 | 146±98* | 108±77* | <0.001 |
| Diameter, mm (n=44) | 2.69±0.43 | 2.88±0.51* | 2.92±0.48* | <0.001 |
| Hyperemic diameter, mm (n=20) | 2.84±0.51 | 3.00±0.57 | 3.15±0.47* | 0.001 |
| Shear stress, dyne/cm2 (n=42) | 23±13 | 37±22* | 27±19 | 0.001 |
| Hyperemic flow, mL/min (n=19) | 270±138 | 473±200* | 424±221† | 0.001 |
| Hyperemic flow, % increase (n=18) | 433±280 | 357±201 | 537±370 | 0.13 |
| Flow-mediated dilation, % (n=20) | 6.6±3.8 | 9.2±4.9 | 10.8±7.1 | 0.05 |
| Flow-mediated dilation/hyperemic flow ratio, 100 · % ·mL−1 ·min−1 (n=17) | 2.9±2.7 | 2.6±2.0 | 3.7±4.1 | 0.57 |
| Nonsurgical arm | ||||
| Flow, mL/min (n=39) | 77±68 | 97±93 | 68±68 | 0.11 |
| Diameter, mm (n=41) | 2.75±0.50 | 2.82±0.41 | 2.66±0.42 | 0.12 |
| Hyperemic diameter, mm (n=15) | 3.03±0.46 | 2.95±0.41 | 3.01±0.40 | 0.56 |
| Shear stress, dyne/cm2 (n=39) | 21±14 | 26±21 | 21±15 | 0.18 |
| Hyperemic flow, mL/min (n=15) | 285±138 | 348±156 | 318±124 | 0.39 |
| Hyperemic flow, % increase (n=15) | 425±300 | 373±250 | 427±220 | 0.69 |
| Flow-mediated dilation, % (n=15 ) | 10.6±8.1 | 10.0±5.9 | 11.8±5.8 | 0.64 |
| Flow-mediated dilation/hyperemic flow ratio, 100 ·% ·mL−1 ·min−1 (n=12) | 5.9±5.6 | 5.8±9.0 | 4.1±2.8 | 0.57 |
P<0.01 and
P<0.05 compared to baseline by post hoc comparison.
To adjust for the possible systemic effects of the postoperative state or concurrent medications, we examined flow, diameter, and shear stress expressed as the ratio of the surgical arm to the nonsurgical arm. As shown in Figure 2, removal of the radial artery was associated with a relative increase in ulnar artery flow and diameter at 1 week. After 4 to 8 weeks, the ulnar artery displayed further outward remodeling and a persistent increase in arterial flow. There was a trend for increased shear stress at 1 week that did not achieve statistical significance.
Figure 2.
Effect of radial artery harvest on ulnar artery flow, diameter, and shear stress expressed as the ratio of the surgical arm to the nonsurgical arm. Data are shown for the subset of subjects with data for both arms at all 3 time points (n=39, 35, and 35, respectively). As shown, removal of the radial artery led to increased ulnar artery flow (overall P=0.004 by repeated-measures ANOVA) and an associated increase in ulnar diameter (overall P<0.001) that reflected outward remodeling. *P<0.05 compared with baseline by post hoc analysis. There was a trend for increased shear stress at the 1-week time point (P=0.09).
Distinguishing Effects on Structure From Effects on Arterial Tone
The measures of resting lumen diameter used in the present study likely reflect both arterial structure and tone. To help distinguish these components, we measured ulnar diameter after administration of sublingual nitroglycerin in a subset of 5 patients who had an increase in ulnar artery flow after removal of the radial artery. Postnitroglycerin diameter increased 9.9% from 3.35±0.60 mm at baseline to 3.68±0.48 mm at the 4- to 8-week time point (P=0.004). To further address this question, we examined the diameter of the ulnar artery 1 minute after the vasodilator stimulus produced by hyperemia (hyperemic diameter). As shown in Table 2, hyperemic diameter increased progressively at the 2 follow-up visits in the surgical arm, whereas there was no change in hyperemic diameter in the nonsurgical arm. These findings suggested that the present results represent remodeling rather than vasodilation.
It is possible that early changes in ulnar diameter represent vasodilation, whereas changes at the long-term time point reflect slower changes in arterial structure. For the whole group of subjects, a large portion of the time-dependent changes in ulnar diameter were already present at the 1-week time point (Table 2), which might suggest that we were primarily observing flow-mediated dilation rather than remodeling. To better understand the time course among those subjects with a good remodeling response, we examined the subgroup with a remodeling response above the median (clinical characteristics of this subgroup are shown in Table 1). As shown in Figure 3, diameter continued to increase between the early and later follow-up visits, which may be consistent with ongoing remodeling.
Figure 3.
Time course of arterial remodeling among “responders” (patients with a remodeling response above the median, n=19). As shown, the increase in ulnar artery diameter is progressive over time after removal of the radial artery compared with the control arm (P<0.001 by repeated-measures ANOVA, *P<0.001 by post hoc analysis).
Effects of Radial Artery Removal on Vasodilator Function
As shown in Table 2, we observed a marked increase in hyperemic flow and a strong trend for increased flow-mediated dilation in the ulnar artery at the 2 follow-up visits. This increase in hyperemic flow volume likely reflects the increase in resting flow, because there was no significant change when hyperemic flow was expressed as percent change from baseline. Hyperemic flow is the stimulus for flow-mediated dilation, and the trend for improved flow-mediated dilation might reflect the increased stimulus, rather than a local improvement in endothelial function.18 To address this issue, we calculated the ratio of flow-mediated dilation to hyperemic flow at each time point, and as shown in Table 2, we observed no significant change over time. Interpretation of these findings is further complicated by the increase in baseline ulnar diameter, because larger arteries would be expected to dilate less to a given stimulus. Overall, it appears unlikely that the changes in reactive hyperemia and flow-mediated dilation were attributable to an improvement in endothelial function.
Predictors of the Outward Remodeling Response
Multivariable predictors of the outward remodeling response are displayed in Table 3. We have postulated that the primary stimulus for outward remodeling is the chronic increase in ulnar artery flow after removal of the radial artery. In support of this possibility, we observed direct correlations between the increase in flow at 1 week or 4 to 8 weeks and the degree of outward remodeling at long-term follow-up. We also hypothesized that measures of endothelial function, including flow-mediated dilation, would influence the outward remodeling response.19 As shown in Table 3, we observed a significant correlation between the extent of remodeling at long-term follow-up and baseline flow-mediated dilation and the ratio of flow-mediated dilation to hyperemic flow (Figure 4).
Table 3.
Predictors of Arterial Remodeling Response at Long-Term Follow-Up
| Correlates of Percent Change in Ulnar Artery Diameter | r* | P |
|---|---|---|
| Increase in ulnar flow at 1 week, % | 0.50 | 0.001 |
| Increase in ulnar flow at 4 to 8 weeks, % | 0.46 | 0.001 |
| Baseline flow-mediated dilation, % | 0.48 | 0.002 |
| Baseline flow-mediated dilation/hyperemic flow, 100 ·% ·mL−1 ·min−1 | 0.50 | 0.002 |
| P-selectin (semiquantitative scale) | −0.83 | 0.002 |
Correlation coefficient adjusted for age, sex, and smoking.
Figure 4.
Relationship between ulnar artery flow-mediated dilation (FMD) at baseline (adjusted for extent of hyperemic flow) and ulnar artery remodeling response at the long-term follow-up visit (unadjusted r=0.50, P=0.001, n=40).
To gain additional insight into the relation between local endothelial phenotype and remodeling, we performed immunohistochemical analysis of radial artery tissue from a subset of 14 patients. As shown in Figure 5, we observed a significant inverse correlation between endothelial expression of P-selectin and remodeling. There was no correlation between endothelial expression of intercellular adhesion molecule-1 or vascular cell adhesion molecule-1 and extent of arterial remodeling (data not shown). Interestingly, there was a significant inverse correlation between P-selectin expression and flow-mediated dilation adjusted for hyperemic flow at baseline (r=−0.65, P=0.04).
Figure 5.
P-selectin expression and outward remodeling. Radial artery segments were harvested and stained for P-selectin as described in Methods. Expression was rated by 3 blinded observers using a 0-to-3 scale. Representative images for patients with weak staining (+1) and strong staining (+3) are shown in the left panel (original magnification X20). P-selectin expression in the radial artery segment at baseline correlated inversely with the degree of outward remodeling of ulnar artery at the long-term follow-up visit (unadjusted r=−0.69, P=0.007, n=14).
Discussion
The present study characterized the ulnar artery remodeling response after surgical removal of the radial artery and used the contralateral arm with an intact radial artery as a control for the effects of background medications and the postoperative state. We observed an increase in ulnar artery flow and shear stress early after radial artery removal. On longer-term follow-up, we observed outward arterial remodeling and a restoration of shear stress toward baseline. Consistent with the premise that increased flow is the primary stimulus for outward remodeling in this setting, there was a direct correlation between the increase in flow and remodeling. We also examined clinical and local vascular predictors of remodeling. Cigarette smoking was associated with a diminished response, but other risk factors were unrelated. Interestingly, remodeling correlated directly with endothelial function as reflected by flow-mediated dilation and, in a subgroup, correlated inversely with local endothelial expression of P-selectin.
In the present study, it was not possible to fully distinguish between vasodilation and remodeling as an explanation for the increase in ulnar artery diameter. The observation that a large portion of the observed increase in arterial diameter occurred within 1 week might be consistent with vasodilation. On the other hand, the observed increase in hyperemic diameter, the subgroup results showing an increase in postnitroglycerin diameter, and the progressive increase in diameter at the later time point in the “responders” support remodeling. Animal studies demonstrate that structural remodeling begins to occur 3 to 7 days after a chronic change in flow.11,20 The present findings likely reflect a combination of vasodilation and remodeling. It is interesting that experimental studies suggest that arterial remodeling occurs when initial changes in arterial tone are “entrenched” by cross-linking of extracellular matrix proteins and that this process begins within days after a chronic change in flow.21,22
Use of the radial artery as a coronary bypass conduit is feasible because of the dual blood supply to the hand.23 Prior studies of patients undergoing radial artery harvest demonstrated the expected increase in ulnar artery flow velocity by Duplex ultrasound and preserved or only mildly decreased blood pressure and tissue perfusion in the digits early after surgery.24,25 The extent of reactive hyperemia is initially diminished but returns to normal over time.26 All of these findings are consistent with the results of the present study. The present study extends that prior work by directly imaging the conduit ulnar artery to characterize flow-induced remodeling and relating it to systemic risk factors and local arterial function.
We observed that removal of the radial artery acutely increased resting blood flow and shear stress. Over time, blood flow remained elevated, whereas shear stress returned to baseline in parallel with outward arterial remodeling. These findings fit well with our current understanding of arterial remodeling as a homeostatic response to alterations in shear stress.1,2 Although beyond the scope of the present study, it would have been interesting to obtain longer-term follow-up. Because shear stress had returned to baseline, we would predict that further remodeling would be minimal. Importantly, the observed levels of shear stress in the ulnar artery (20 to 40 dyne/cm2) are physiologically relevant and comparable to those observed in the coronary circulation.4,27
Several prior studies examined flow and shear stress in the radial artery after creation of an arterial-venous dialysis fistula in patients with chronic renal failure.28–30 As in the present study, those studies demonstrated outward remodeling of the radial and/or brachial artery that tended to restore shear stress to baseline. Notably, linking the artery directly to the low-pressure venous system produces nonphysiological flow patterns with high diastolic flow.29 The present study may be more relevant, because we examined a situation in which flow was chronically increased without disruption of the usual relationship between the conduit artery and downstream microvasculature.
To the best of our knowledge, no prior study prospectively examined the clinical predictors of outward remodeling; however, a number of cross-sectional studies have examined this issue. For example, patients with diabetes mellitus had higher shear stress in the upstream artery after creation of an arterial-venous fistula, which suggests impaired outward remodeling.10 Studies of coronary artery remodeling by intravascular ultrasound or autopsy also examined this question, although the lack of a sustained increase in blood flow and the presence of developing coronary artery lesions makes the situation different from the present study. Those cross-sectional studies linked hypertension,31,32 low HDL,32,33 insulin-dependent diabetes mellitus,34 and cigarette smoking35 with impaired outward remodeling. The latter finding is consistent with our finding that smokers had a reduced remodeling response. It is perhaps most striking, however, that we observed so little effect of systemic risk factors on remodeling. Interpretation of these negative findings should be made with caution because of the high prevalence of risk factors and background drug therapy in the present study subjects. However, they may be consistent with the idea that arterial remodeling is a fundamental response of the vasculature that is maintained in stenosis-free arteries despite the presence of risk factors. This concept is based on Glagov’s original observation that outward remodeling occurs in patients with risk factors and early atherosclerotic lesions.5
The present study demonstrated a correlation between flow-mediated dilation and the remodeling response. Because flow-mediated dilation depends on local production of nitric oxide,19 the present findings are consistent with experimental studies linking the remodeling response to the bioavailability of endothelium-derived nitric oxide.9,36,37 For example, Tronc and colleagues9 demonstrated that nitric oxide synthase inhibition prevents outward arterial remodeling and compensatory reductions in shear stress in the rabbit carotid artery after creation of an arterial-venous fistula. In the setting of chronic decreases in flow after ligation of the mouse carotid artery, deletion of the gene for endothelial nitric oxide synthase prevents inward remodeling and enhances intimal thickening.36 Thus, the present study provides new evidence in humans that flow-induced arterial remodeling relates to the functional status of the endothelium.
The mechanisms of arterial remodeling have been reviewed extensively,1,7,8,22 and it is interesting that remodeling is associated with a proinflammatory endothelial phenotype. Remodeling requires controlled and self-limited activation of nuclear factor-κB and endothelial expression of adhesion molecules that facilitate accumulation of inflammatory cells in the arterial wall. There is subsequent reorganization of intercellular matrix and proliferation and migration of vascular cells that results in an enlarged artery with normal wall architecture. In the present study, we observed that patients with greater expression of P-selectin at baseline had less outward remodeling. Mouse studies suggest that P-selectin and recruitment of leukocytes into the arterial media are important for both inward and outward arterial remodeling.11,38 Although the explanation for the present findings is unknown, they raise the interesting possibility that outward remodeling may not occur normally if endothelial cells have a proinflammatory phenotype before the increase in blood flow. Further studies will be required to confirm this possibility.
The present study has a number of limitations. First, the study included patients undergoing coronary bypass surgery. Although a study of normal subjects might have allowed us to better investigate the effects of risk factors on remodeling, radial artery harvest obviously could not be performed in that setting. Second, we studied patients in the postoperative state, in which pain, fluid shifts, and prescribed medications likely affected vascular function. We adjusted for such systemic factors by using the contralateral arm as a control. We cannot exclude the possibility that local inflammation or edema in the surgical arm might have affected our results, although such changes are unlikely to explain results at the later time point when the surgical incisions were well healed. Third, the postnitroglycerin data in a subset of patients support a change in arterial structure over time, but it was not logistically possible to administer nitroglycerin to all of the study subjects. Counterbalancing these limitations is the relatively large sample size, the prospective study design, and the unique human model.
In summary, the present study demonstrated outward arterial remodeling of the ulnar artery in response to a sustained increased in blood flow in patients undergoing coronary bypass surgery. We observed little correlation with risk factors, but there were significant correlations with the extent of flow increase and with baseline endothelial function. Although we have not measured nitric oxide directly, the present results are consistent with experimental studies indicating that the endothelium is the primary sensor for changes in blood flow and that endothelium-derived nitric oxide and proinflammatory factors influence the remodeling response. These findings provide insights into mechanisms of arterial remodeling in human subjects and are relevant to a variety of clinical situations, including growth and development, exercise, angiogenesis, collateral formation, atherosclerosis, and restenosis after arterial injury.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants HL083269, HL083801, and HL081587. Dr Shenouda is supported by a National Institutes of Health training grant (T32 HL07224), and Dr Hamburg is supported by the Boston University Medical Center Leadership Program in Vascular Medicine (K12 HL083781).
Footnotes
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Disclosures
None.
References
- 1.Silver AE, Vita JA. Shear-stress–mediated arterial remodeling in atherosclerosis: too much of a good thing? Circulation. 2006;113:2787–2789. doi: 10.1161/CIRCULATIONAHA.106.634378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 3.Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 4.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 5.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 6.Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002;105:939–943. doi: 10.1161/hc0802.104327. [DOI] [PubMed] [Google Scholar]
- 7.Ward MR, Pasterkamp G, Yeung AC, Borst C. Arterial remodeling: mechanisms and clinical implications. Circulation. 2000;102:1186–1191. doi: 10.1161/01.cir.102.10.1186. [DOI] [PubMed] [Google Scholar]
- 8.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 9.Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996;16:1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- 10.Tuka V, Slavikova M, Svobodova J, Malik J. Diabetes and distal access location are associated with higher wall shear rate in feeding artery of PTFE grafts. Nephrol Dial Transplant. 2006;21:2821–2824. doi: 10.1093/ndt/gfl290. [DOI] [PubMed] [Google Scholar]
- 11.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 12.Shapira OM, Eskenazi BR, Anter E, Joseph L, Christensen TG, Hunter CT, Lazar HL, Vita JA, Shemin RJ, Keaney JF., Jr Endoscopic versus conventional radial artery harvest for coronary artery bypass grafting: functional and histologic assessment of the conduit. J Thorac Cardiovasc Surg. 2006;131:388–394. doi: 10.1016/j.jtcvs.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Shapira OM, Eskenazi BR, Hunter CT, Anter E, Bao Y, Murphy R, Lazar HL, Shemin RJ. Endoscopic versus conventional radial artery harvest: is smaller better? J Card Surg. 2006;21:329–335. doi: 10.1111/j.1540-8191.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 14.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 15.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gokce N, Holbrook M, Hunter LM, Palmisano J, Vigalok E, Keaney JF, Jr, Vita JA. Acute effects of vasoactive drug treatment on brachial artery reactivity. J Am Coll Cardiol. 2002;40:761–765. doi: 10.1016/s0735-1097(02)02034-x. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 20.Tulis DA, Unthank JL, Prewitt RL. Flow-induced arterial remodeling in rat mesenteric vasculature. Am J Physiol Heart Circ Physiol. 1998;43:H874–H882. doi: 10.1152/ajpheart.1998.274.3.H874. [DOI] [PubMed] [Google Scholar]
- 21.Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96:119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- 22.Langille BL, Dajnowiec D. Cross-linking vasomotor tone and vascular remodeling: a novel function for tissue transglutaminase? Circ Res. 2005;96:9–11. doi: 10.1161/01.RES.0000153883.55971.81. [DOI] [PubMed] [Google Scholar]
- 23.Meharwal ZS, Trehan N. Functional status of the hand after radial artery harvesting: results in 3 977 cases. Ann Thorac Surg. 2001;72:1557–1561. doi: 10.1016/s0003-4975(01)03088-0. [DOI] [PubMed] [Google Scholar]
- 24.Lohr JM, Paget DS, Smith JM, Winkler JL, Wladis AR. Upper extremity hemodynamic changes after radial artery harvest for coronary artery bypass grafting. Ann Vasc Surg. 2000;14:56–62. doi: 10.1007/s100169910010. [DOI] [PubMed] [Google Scholar]
- 25.Pola P, Serricchio M, Flore R, Manasse E, Favuzzi A, Possati GF. Safe removal of the radial artery for myocardial revascularization: a Doppler study to prevent ischemic complications to the hand. J Thorac Cardiovasc Surg. 1996;112:737–744. doi: 10.1016/S0022-5223(96)70060-0. [DOI] [PubMed] [Google Scholar]
- 26.Royse AG, Royse CF, Maleskar A, Garg A. Harvest of the radial artery for coronary artery surgery preserves maximal blood flow of the forearm. Ann Thorac Surg. 2004;78:539–542. doi: 10.1016/j.athoracsur.2004.02.094. [DOI] [PubMed] [Google Scholar]
- 27.Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003;108:438–444. doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 28.Ene-Iordache B, Mosconi L, Antiga L, Bruno S, Anghileri A, Remuzzi G, Remuzzi A. Radial artery remodeling in response to shear stress increase within arteriovenous fistula for hemodialysis access. Endothelium. 2003;10:95–102. doi: 10.1080/10623320303365. [DOI] [PubMed] [Google Scholar]
- 29.Dammers R, Tordoir JH, Kooman JP, Welten RJ, Hameleers JM, Kitslaar PJ, Hoeks AP. The effect of flow changes on the arterial system proximal to an arteriovenous fistula for hemodialysis. Ultrasound Med Biol. 2005;31:1327–1333. doi: 10.1016/j.ultrasmedbio.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Girerd X, London G, Boutouyrie P, Mourad JJ, Safar M, Laurent S. Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension. 1996;27:799–803. doi: 10.1161/01.hyp.27.3.799. [DOI] [PubMed] [Google Scholar]
- 31.Britten MB, Zeiher AM, Schachinger V. Effects of cardiovascular risk factors on coronary artery remodeling in patients with mild atherosclerosis. Coron Artery Dis. 2003;14:415–422. doi: 10.1097/00019501-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Isoda K, Arakawa K, Kamezawa Y, Nishizawa K, Nishikawa K, Shibuya T, Ohsuzu F, Nakamura H. Effect of coronary risk factors on arterial compensatory enlargement in Japanese middle-aged patients with de novo single-vessel disease: an intravascular ultrasound study. Clin Cardiol. 2001;24:443–450. doi: 10.1002/clc.4960240605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor AJ, Burke AP, Farb A, Yousefi P, Malcom GT, Smialek J, Virmani R. Arterial remodeling in the left coronary system: the role of high-density lipoprotein cholesterol. J Am Coll Cardiol. 1999;34:760–767. doi: 10.1016/s0735-1097(99)00275-2. [DOI] [PubMed] [Google Scholar]
- 34.Kornowski R, Mintz GS, Lansky AJ, Hong MK, Kent KM, Pichard AD, Satler LF, Popma JJ, Bucher TA, Leon MB. Paradoxic decreases in atherosclerotic plaque mass in insulin-treated diabetic patients. Am J Cardiol. 1998;81:1298–1304. doi: 10.1016/s0002-9149(98)00157-x. [DOI] [PubMed] [Google Scholar]
- 35.Tauth J, Pinnow E, Sullebarger JT, Basta L, Gursoy S, Lindsay J, Jr, Matar F. Predictors of coronary arterial remodeling patterns in patients with myocardial ischemia. Am J Cardiol. 1997;80:1352–1355. doi: 10.1016/s0002-9149(97)00682-6. [DOI] [PubMed] [Google Scholar]
- 36.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawashima S, Yamashita T, Ozaki M, Ohashi Y, Azumi H, Inoue N, Hirata K, Hayashi Y, Itoh H, Yokoyama M. Endothelial NO synthase overexpression inhibits lesion formation in mouse model of vascular remodeling. Arterioscler Thromb Vasc Biol. 2001;21:201–207. doi: 10.1161/01.atv.21.2.201. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi S, Watanabe N, Nakazawa K, Suzuki J, Tsushima K, Tamatani T, Sakamoto S, Isobe M. Roles of P-selectin in inflammation, neointimal formation, and vascular remodeling in balloon-injured rat carotid arteries. Circulation. 2000;102:1710–1717. doi: 10.1161/01.cir.102.14.1710. [DOI] [PubMed] [Google Scholar]