Abstract
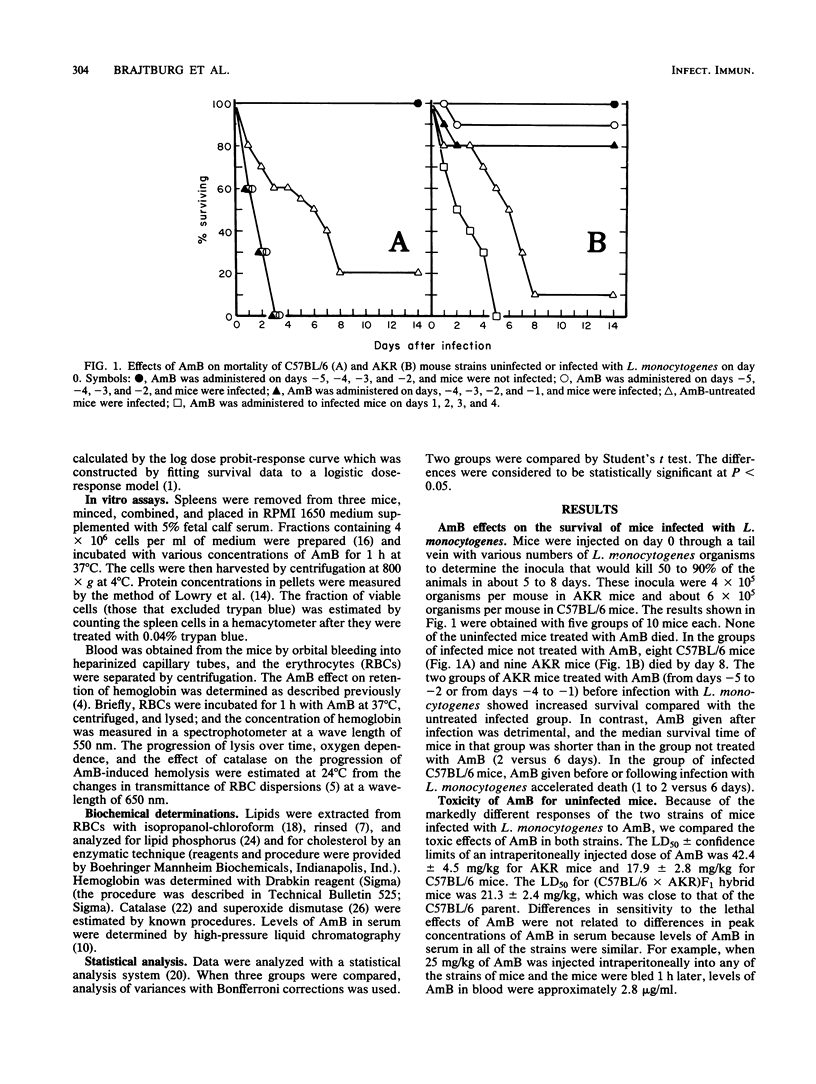
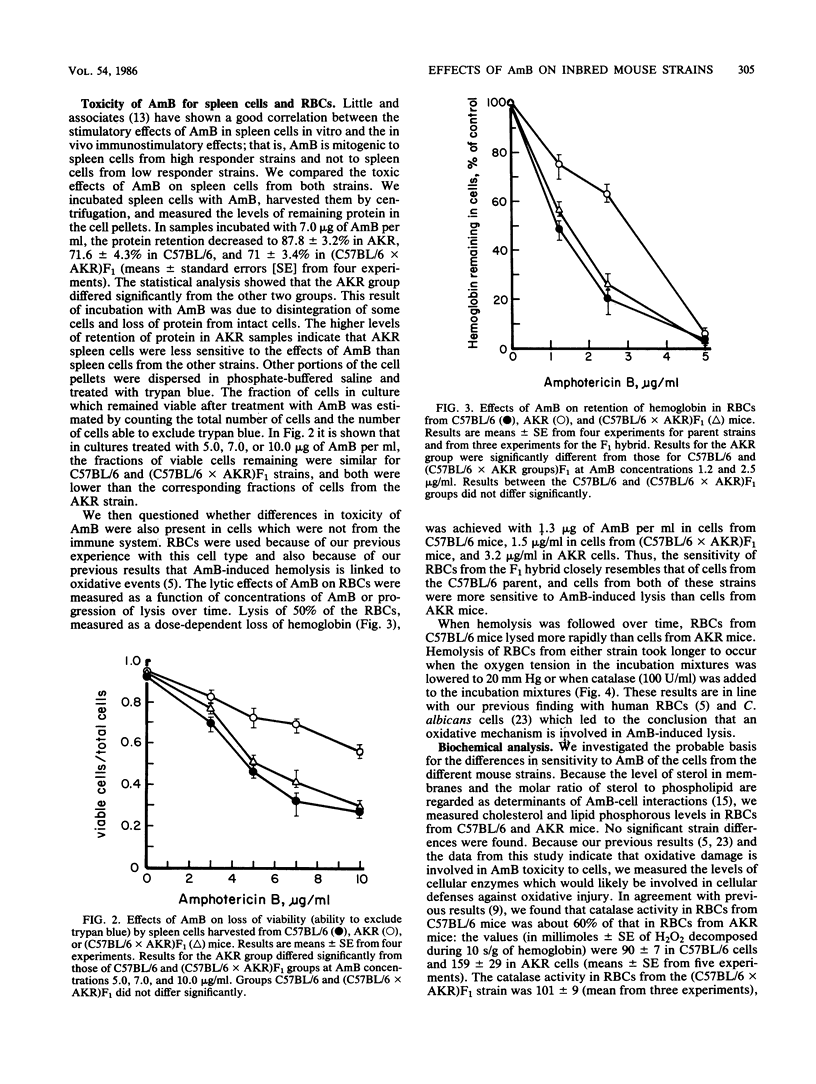
Amphotericin B (AmB) treatment before infection with the bacterium Listeria monocytogenes prolonged survival of AKR mice but shortened survival of C57BL/6 mice compared with survival of untreated infected controls. C57BL/6 mice were also more sensitive to the acute toxic effects of AmB than AKR mice, as were (C57BL/6 X AKR)F1 hybrid mice. Spleen cells and erythrocytes (RBCs) from the C57BL/6 and the F1 hybrid mice were both more sensitive to the lytic and lethal effects of AmB than corresponding cells from AKR mice. Biochemical analysis indicated that catalase levels in RBCs from C57BL/6 and F1 hybrid mice were about 60% of those found in RBCs from AKR mice. The lysis by AmB of RBCs from all these strains of mice was inhibited by catalase or incubation in a low-oxygen environment. These findings suggest that (i) the low catalase levels in C57BL/6 and F1 hybrid mice may limit the protection of cells from the oxidant damage involved in AmB action, and (ii) the toxicity which occurs at low concentrations of AmB in the mouse strains with low intracellular catalase levels may interfere with or ablate the AmB-induced increases in mouse resistance to L. monocytogenes infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bistoni F., Vecchiarelli A., Mazzolla R., Puccetti P., Marconi P., Garaci E. Immunoadjuvant activity of amphotericin B as displayed in mice infected with Candida albicans. Antimicrob Agents Chemother. 1985 Apr;27(4):625–631. doi: 10.1128/aac.27.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke T. J., Little J. R., Shirley S. F., Lynch R. G. Augmentation of murine immune responses by amphotericin B. Cell Immunol. 1977 Sep;33(1):180–190. doi: 10.1016/0008-8749(77)90145-9. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Kobayashi G. S., Medoff G. Effects of serum lipoproteins on damage to erythrocytes and Candida albicans cells by polyene antibiotics. J Infect Dis. 1986 Mar;153(3):623–626. doi: 10.1093/infdis/153.3.623. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Schwartz D. R., Vertut-Croquin A., Schlessinger D., Kobayashi G. S., Medoff G. Involvement of oxidative damage in erythrocyte lysis induced by amphotericin B. Antimicrob Agents Chemother. 1985 Feb;27(2):172–176. doi: 10.1128/aac.27.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Canono B. P., Henson P. M., Campbell P. A. Genetically determined resistance to listeriosis is associated with increased accumulation of inflammatory neutrophils and macrophages which have enhanced listericidal activity. Immunology. 1985 Jul;55(3):511–518. [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Godfrey R. W., Wilder M. S. Relationships between oxidative metabolism, macrophage activation, and antilisterial activity. J Leukoc Biol. 1984 Oct;36(4):533–543. doi: 10.1002/jlb.36.4.533. [DOI] [PubMed] [Google Scholar]
- Hoffman H. A., Rechcigl M., Jr Erythrocyte catalase in inbred mice. Enzyme. 1971;12(2):219–225. doi: 10.1159/000459534. [DOI] [PubMed] [Google Scholar]
- Kim H., Lin C. High-pressure liquid chromatographic method for determination of Sch 28191 in biological fluids. Antimicrob Agents Chemother. 1984 Jan;25(1):45–48. doi: 10.1128/aac.25.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Little J. R., Abegg A., Plut E. The relationship between adjuvant and mitogenic effects of amphotericin methyl ester. Cell Immunol. 1983 Jun;78(2):224–235. doi: 10.1016/0008-8749(83)90277-0. [DOI] [PubMed] [Google Scholar]
- Medoff G., Brajtburg J., Kobayashi G. S., Bolard J. Antifungal agents useful in therapy of systemic fungal infections. Annu Rev Pharmacol Toxicol. 1983;23:303–330. doi: 10.1146/annurev.pa.23.040183.001511. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Stewart S. J., Ellner J. J. Amphotericin B-induced resistance to Schistosoma mansoni. J Immunol. 1981 May;126(5):1667–1670. [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Sadarangani C., Skamene E., Kongshavn P. A. Cellular basis for genetically determined enhanced resistance of certain mouse strains to listeriosis. Infect Immun. 1980 May;28(2):381–386. doi: 10.1128/iai.28.2.381-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine K. I. Myocardial ischemia: ionic events in ischemia and anoxia. Am J Pathol. 1981 Feb;102(2):256–261. [PMC free article] [PubMed] [Google Scholar]
- Shirley S. F., Little J. R. Immunopotentiating effects of amphotericin B. I. Enhanced contact sensitivity in mice. J Immunol. 1979 Dec;123(6):2878–2882. [PubMed] [Google Scholar]
- Sinha A. K. Colorimetric assay of catalase. Anal Biochem. 1972 Jun;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Sokol-Anderson M. L., Brajtburg J., Medoff G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J Infect Dis. 1986 Jul;154(1):76–83. doi: 10.1093/infdis/154.1.76. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Rothblat G. H. Sterol to phospholipid molar ratios of L cells with qualitative and quantitative variations of cellular sterol. Proc Soc Exp Biol Med. 1974 Sep;146(4):1166–1172. doi: 10.3181/00379727-146-38267. [DOI] [PubMed] [Google Scholar]
- Thomas M. Z., Medoff G., Kobayashi G. S. Changes in murine resistance to Listeria monocytogenes infection induced by amphotericin B. J Infect Dis. 1973 Apr;127(4):373–377. doi: 10.1093/infdis/127.4.373. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975 Feb;85(2):337–341. [PubMed] [Google Scholar]


