Abstract
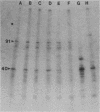
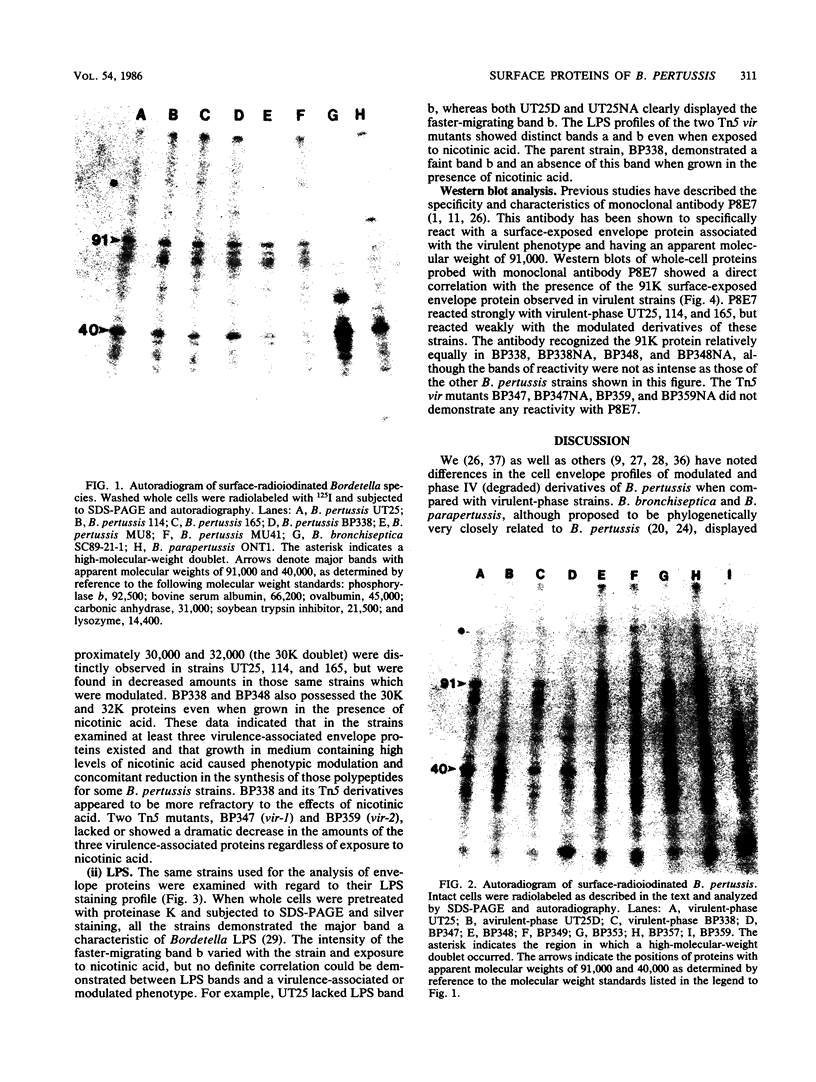
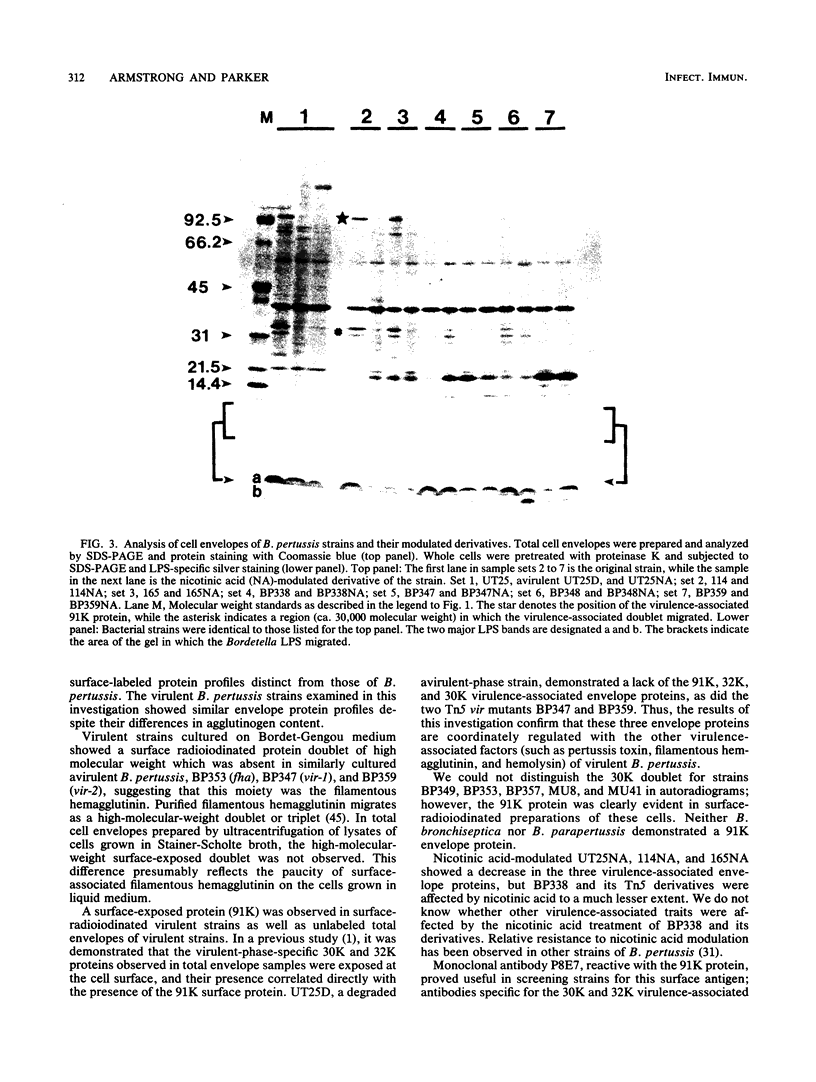
The surface proteins of several Bordetella strains and their modulated derivatives were examined by surface radioiodination, cell fractionation, and Western blotting. A surface protein with a high Mr, missing in a mutant lacking the filamentous hemagglutinin, was identified in virulent Bordetella pertussis and Bordetella parapertussis cells and was absent in avirulent B. pertussis strains. The electrophoretic profiles of lipopolysaccharide and the 40,000-Mr anion-selective porin were not determinants which correlated with phase variation or phenotypic modulation. At least three envelope proteins (91,000, 32,000, and 30,000 molecular weight) were found only in virulent B. pertussis strains and were absent or diminished in the avirulent phase and most phenotypically modulated strains. Two transposon-induced mutants unable to produce hemolysin, dermonecrotic toxin, pertussis toxin, and filamentous hemagglutinin also lacked these three envelope proteins, confirming that virulence-associated envelope proteins were genetically regulated with other virulence-associated traits.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong S. K., Parker C. D. Heat-modifiable envelope proteins of Bordetella pertussis. Infect Immun. 1986 Oct;54(1):109–117. doi: 10.1128/iai.54.1.109-117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S. K., Parr T. R., Jr, Parker C. D., Hancock R. E. Bordetella pertussis major outer membrane porin protein forms small, anion-selective channels in lipid bilayer membranes. J Bacteriol. 1986 Apr;166(1):212–216. doi: 10.1128/jb.166.1.212-216.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie R. M., Coote J. G., Parton R. Adenylate cyclase activity during phenotypic variation of Bordetella pertussis. J Gen Microbiol. 1985 Jan;131(1):27–38. doi: 10.1099/00221287-131-1-27. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Hewlett E. L., Manclark C. R. Intracellular localization of the dermonecrotic toxin of Bordetella pertussis. Infect Immun. 1979 Sep;25(3):896–901. doi: 10.1128/iai.25.3.896-901.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzell J. W., Dobrogosz W. J., Kloos W. E., Manclark C. R. Phase-shift markers in Bordetella: alterations in envelope proteins. J Infect Dis. 1981 Apr;143(4):562–569. doi: 10.1093/infdis/143.4.562. [DOI] [PubMed] [Google Scholar]
- Ezzell J. W., Dobrogosz W. J., Kloos W. E., Manclark C. R. Phase-shift markers in the genus Bordetella: loss of cytochrome d-629 in phase IV variants. Microbios. 1981;31(125-126):171–181. [PubMed] [Google Scholar]
- Frank D. W., Parker C. D. Interaction of monoclonal antibodies with pertussis toxin and its subunits. Infect Immun. 1984 Oct;46(1):195–201. doi: 10.1128/iai.46.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. W., Parker C. D. Isolation and characterization of monoclonal antibodies to Bordetella pertussis. J Biol Stand. 1984 Oct;12(4):353–365. doi: 10.1016/s0092-1157(84)80060-8. [DOI] [PubMed] [Google Scholar]
- Goldman S., Hanski E., Fish F. Spontaneous phase variation in Bordetella pertussis is a multistep non-random process. EMBO J. 1984 Jun;3(6):1353–1356. doi: 10.1002/j.1460-2075.1984.tb01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idigbe E. O., Parton R., Wardlaw A. C. Rapidity of antigenic modulation of Bordetella pertussis in modified Hornibrook medium. J Med Microbiol. 1981 Nov;14(4):409–418. doi: 10.1099/00222615-14-4-409. [DOI] [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilus pertussis. I. Antigen structure of H. pertussis and its phases. Kitasato Arch Exp Med. 1953 Nov;26(2-3):121–133. [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilus pertussis. V. Relation between the phase of bacilli and the progress of the whooping-cough. Kitasato Arch Exp Med. 1954 Sep;27(3):57–62. [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACEY B. W. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960 Mar;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheat W. L., Wardlaw A. C., Novotny P. Modulation of Bordetella pertussis by nicotinic acid. Infect Immun. 1983 Aug;41(2):516–522. doi: 10.1128/iai.41.2.516-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Hewlett E. L., Peppler M. S., Selander R. K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986 Apr;166(1):230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. D., Armstrong S. K., Frank D. W. Surface antigens of Bordetella pertussis. Adv Exp Med Biol. 1985;185:117–127. doi: 10.1007/978-1-4684-7974-4_7. [DOI] [PubMed] [Google Scholar]
- Parker C. Role of the genetics and physiology of Bordetella pertussis in the production of vaccine and the study of host-parasite relationships in pertussis. Adv Appl Microbiol. 1976;20:27–42. doi: 10.1016/s0065-2164(08)70107-2. [DOI] [PubMed] [Google Scholar]
- Parton R., Wardlaw A. C. Cell-envelope proteins of Bordetella pertussis. J Med Microbiol. 1975 Feb;8(1):47–57. doi: 10.1099/00222615-8-1-47. [DOI] [PubMed] [Google Scholar]
- Peppler M. S. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982 Mar;35(3):840–851. doi: 10.1128/iai.35.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S., Schrumpf M. E. Isolation and characterization of Bordetella pertussis phenotype variants capable of growing on nutrient agar: comparison with phases III and IV. Infect Immun. 1984 Jan;43(1):217–223. doi: 10.1128/iai.43.1.217-223.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J., Lowe F. Enrichment medium for the isolation of Bordetella. J Clin Microbiol. 1977 Sep;6(3):303–309. doi: 10.1128/jcm.6.3.303-309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K., Parker C. D. Identification and characterization of Vibrio cholerae surface proteins by radioiodination. Infect Immun. 1985 Apr;48(1):87–93. doi: 10.1128/iai.48.1.87-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Hawkins D. C. Structure and biological properties of solubilized envelope proteins of Bordetella pertussis. Infect Immun. 1983 Feb;39(2):590–598. doi: 10.1128/iai.39.2.590-598.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. R., Parker C. D. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect Immun. 1982 Nov;38(2):548–553. doi: 10.1128/iai.38.2.548-553.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw A. C., Parton R. Bordetella pertussis toxins. Pharmacol Ther. 1982;19(1):1–53. doi: 10.1016/0163-7258(82)90041-9. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984 Aug;150(2):219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. M., Cowell J. L., Steven A. C., Carter P. H., McGrath P. P., Manclark C. R. Purification and characterization of fimbriae isolated from Bordetella pertussis. Infect Immun. 1985 May;48(2):422–427. doi: 10.1128/iai.48.2.422-427.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]