Abstract
Arousal-related processes associated with heightened heart rate (HR) predict memory enhancement, especially for emotionally arousing stimuli. In addition, phasic HR deceleration reflects “orienting” and sensory receptivity during perception of stimuli. We hypothesized that both tonic elevations in HR as well as phasic HR deceleration during viewing of pictures would be associated with deeper encoding and better subsequent memory for stimuli. Emotional pictures are more memorable and cause greater HR deceleration than neutral pictures. Thus, we predicted that the relations between cardiac activity and memory enhancement would be most pronounced for emotionally-laden compared to neutral pictures. We measured HR in 53 males during viewing of unpleasant, neutral, and pleasant pictures, and tested memory for the pictures two days later. Phasic HR deceleration during viewing of individual pictures was greater for subsequently remembered than forgotten pictures across all three emotion categories. Elevated mean HR across the entire encoding epoch also predicted better memory performance, but only for emotionally arousing pictures. Elevated mean HR and phasic HR deceleration were associated, such that individuals with greater tonic HR also showed greater HR decelerations during picture viewing, but only for emotionally arousing pictures. Results suggest that tonic elevations in HR are associated both with greater orienting and heightened memory for emotionally arousing stimuli.
Keywords: orienting, memory, heart rate, emotion, cardiac, cognitive
It is well established that emotional arousal at the time of encoding facilitates subsequent memory (Cahill & McGaugh, 1998). When measured prior to or during memorization, elevated heart rate (as one index of arousal) has been associated with better subsequent memory performance (Jennings & Hall, 1980), particularly in subjects with a history of PTSD (Nagamine, Matsuoka, Mori, Fujimori, Imoto, Kim, & Uchitomi, 2007). Pharmacological agents that alter arousal and heart rate (e.g., beta-blockers or epinephrine) show commensurate memory alterations, such that heightened heart rate (HR) is associated with memory enhancement and reduced HR with memory impairment, especially for emotionally arousing information (Cahill & Alkire, 2003; O’Carroll, Drysdale, Cahill, Shajahan, & Ebmeier, 1999). In these studies, HR may act as a proxy measure for peripheral (and possibly central) adrenergic and/or noradrenergic activation. Human and animal research suggests that central noradrenergic activation, especially within the basolateral nucleus of the amygdala, is necessary for enhancement of memory for emotionally arousing material (Cahill & McGaugh, 1998; van Stegeren, Everaerd, Cahill, McGaugh, & Gooren, 1998). Together, these studies show that adrenergic and/or noradrenergic activation and associated increases in HR predict heightened subsequent memory, especially for emotionally arousing information.
Phasic HR deceleration within seconds of apprehending a stimulus also predicts subsequent memory enhancement (Buchanan, Etzel, Adolphs, & Tranel, 2006). HR deceleration upon confrontation with a stimulus is one component of an orienting response (OR), which is a constellation of physiological responses that reflects attentional and cognitive processing of stimuli of low to moderate intensity (Cook & Turpin, 1997). Attention to the external environment and heightened sensory receptivity (“stimulus intake”) is associated with cardiac deceleration (Lacey, 1959, 1967). Early theorists argued that the OR is necessary for learning, hypothesizing that it occurs when a match for a perceived stimulus is not found in memory (e.g., Öhman, 1979). Indeed, orienting at the time of encoding (measured using a variety of physiological indicators of the OR, including HR deceleration) predicts subsequent memory performance (e.g., Buchanan et al., 2006; Palomba, Angrilli, & Mini, 1997; Siddle, Packer, Donchin, & Fabiani, 1991).
It is currently unclear how heightened HR over an extended period of time (on the order of minutes to hours) is associated with phasic decreases in HR during orienting (which occur for only a few seconds). Theoretically, both tonic HR elevation and phasic HR deceleration during picture viewing should predict memory enhancement. The current study included a 25-to-28 minute encoding period, in which subjects viewed pleasant, unpleasant, and neutral pictures. We tested relations among the following: phasic HR deceleration during individual picture viewing, mean HR during the entire encoding epoch, and subsequent recall for pictures.
Furthermore, we examined differences in the association of cardiac activity and memory for neutral vs. emotionally arousing pictures. Previous research has shown that passively viewed emotional pictures are both more memorable and result in greater orienting as measured by HR deceleration than neutral pictures (Bradley, Greenwald, Petry, & Lang, 1992; Bradley & Lang, 2000). In addition, arousal during encoding and tonic HR elevation is most predictive of memory for emotionally arousing as opposed to neutral information (Cahill, Prins, Weber, & McGaugh, 1994; O’Carroll et al., 1999). Thus, we hypothesized that both greater tonic HR elevation and greater orienting, as measured by HR deceleration, would predict memory enhancement specifically for emotionally arousing pictures.
Method
Participants
Sixty-one male undergraduate students at the University of Wisconsin were recruited for this study. Participants were excluded if they were female, younger than 18 years old, had previous experience with the slides used in this study, had a medical illness, history of head injury, self-reported mental or substance use disorder, daily tobacco use, night shift work, inability or unwillingness to complete the protocol, treatment with psychotropic medications, narcotics, beta-blockers, steroids, or any other medication that affects nervous or endocrine systems.
Participants were subjected to a laboratory-based manipulation of endogenous cortisol levels after encoding of stimuli (a 15-minute speech); including females was infeasible for the following reasons: effects of oral contraceptive use and menstrual cycle on cortisol; sex differences in the effects of cortisol on memory (Wolf, Schommer, Hellhammer, McEwen, & Kirschbaum, 2001); and inadequate funds for doubling of the sample size. Thus, findings presented here may not be generalizable to women.
Eight participants were excluded from analyses because seven failed to return to the second laboratory visit for memory tasks and one was excluded due to experimenter error (i.e., presentation of a subset of the stimuli twice). Thus, the final sample consisted of a total of 53 participants.
Design and Procedure
Participants arrived at the laboratory at either 1:30pm or 4:30pm. This timing was chosen because a subset of the participants (who began at 4:30) were used to test hypotheses regarding cortisol, negative affect, and memory, which required data collection at the same time of day for all participants (see Abercrombie, Speck, & Monticelli, 2006). All procedures were approved by University of Wisconsin Health Sciences Human Subjects Committee.
Participants viewed 21 pleasant, 21 neutral, and 21 unpleasant pictures (International Affective Picture System; Lang, Bradley, & Cuthbert, 2001). Participants were randomly assigned to view one of two affectively equivalent sets of pictures.1 Mean (SD) normative ratings for pleasantness were as follows: Set 1, pleasant 7.0 (0.41), neutral 4.89 (0.36), unpleasant 2.37 (0.43); Set 2, pleasant 7.0 (0.63), neutral 4.93 (0.35), unpleasant 2.37 (0.38). Mean (SD) normative ratings for arousal were as follows: Set 1, pleasant 5.95 (0.56), neutral 2.56 (0.32), unpleasant 5.94 (0.59); Set 2, pleasant 5.93 (0.66), neutral 2.49 (0.31), unpleasant 5.91 (0.57).
Pictures were presented for six seconds followed by an Inter Trial Interval (ITI) of seventeen seconds. Participants wore headphones that presented a 50ms blast of 95DB white noise (startle probe) at 4.5 seconds during picture presentation for 18 pictures (6 of each valence) or at either 7 or 8 seconds during the ITI for 36 pictures. Nine trials did not include startle probes. Previous research has shown that presence of a startle probe on a trial does not affect HR change in response to stimuli (Bradley, Codispoti, Cuthbert, & Lang, 2001). Analyses of our data using Repeated Measures ANOVA with the Huynh-Feldt correction with time, emotion category, and the presence of a probe as predictors did not reveal an effect for the auditory startle probe on HR deceleration (F[22, 52] = 1.68, n.s.); therefore, HR data were collapsed across probe condition. Pictures were interspersed randomly and presented in 3 separate blocks, each of which was slightly longer than 8 minutes with brief breaks in between blocks. Participants were not told that memory for these stimuli would be tested.
After picture viewing, participants completed a social stress task to manipulate endogenous cortisol levels. Participants were told to spend five minutes preparing a speech in which they described their emotional reactions to the pictures they had just viewed in front of a two-person evaluative audience for 15 minutes.
Memory testing
Participants returned to the laboratory two days later in the evening for a free recall test, in which they were given ten minutes to write down descriptions of as many of the pictures as they could remember. Two independent raters showed reliable scoring of free recall responses (IC = .99).
Physiological Data Collection and Processing
Electrodes for measurement of HR were affixed to the distal end of the right collarbone and the lower left rib cage. Interbeat interval (IBI) series were derived from the ECG series and were hand corrected for artifacts. Mean HR for each of the 3 picture viewing blocks was calculated and averaged to create an overall mean HR score during encoding (hereafter referred to as mean HR).
In order to measure HR changes during orienting, HR during presentation of each picture was estimated for every ½ second using the procedures detailed by Graham (1978). The last ½ second of the ITI prior to picture presentation was used as the baseline and change scores for each ½ second of the total six seconds were calculated by subtracting the baseline from each ½ second estimate. The maximum HR deceleration from baseline in the first 3 seconds of picture viewing was determined for each trial for each participant, using the methods of Bradley et al. (2001). Thus, “HR deceleration” refers to the maximum HR deceleration during the 1st 3 seconds of picture viewing for each trial.
Salivary cortisol levels were obtained throughout the encoding session by having participants chew briefly on cotton swabs using the Salivette sampling device (Sarstedt Inc., Newton, NC). Cortisol samples were processed using Salimetrics (State College, PA) cortisol enzyme immunoassay kits as described in Abercrombie et al. (2006). Assay results were considered acceptable only if the coefficient of variation for the duplicate measurement of a sample was less than or equal to 20%. The mean inter-assay CV% was 7.4%, and the mean intra-assay CV% was 3.8%. The detection limit for this assay was 0.007 μg/dl. Raw cortisol data were log transformed.
Data Analysis
First, we conducted a Two-Way Repeated Measures ANOVA with the Huynh-Feldt correction with time and emotion category as predictors to test whether HR deceleration during picture viewing varied in response to unpleasant, pleasant, and neutral pictures. This was to ensure that the HR response to emotional stimuli conformed to previous research (Bradley et al., 2001; i.e., with the greatest deceleration observed for emotionally arousing pictures).
Next, we used two approaches to analyze the data, both of which examined the associations among memory performance, cardiac activity, and emotionally arousing content of pictures. First, we separated remembered vs. forgotten trials, and determined whether pictures associated with greater HR deceleration were more often remembered than forgotten. Second, we examined overall memory performance; i.e., we examined the relations among cardiac variables and total number of pictures recalled, number of neutral pictures recalled, and number of emotionally arousing pictures recalled. For HR deceleration, we used a mixed model analysis predicting memory performance with both emotion category (unpleasant, pleasant, and neutral) and maximum HR deceleration entered into the model. We also examined the association between phasic HR deceleration and tonic mean HR during encoding, and we examined whether the individuals with greatest tonic mean HR during encoding showed better memory performance.
Because subjects completed a laboratory-based stressor after encoding, we examined the relations among HR during encoding, HR during the speech stressor, and memory performance. We were also interested in whether variation in cortisol levels altered the relation between cardiac activity during encoding and memory. Previous research has shown that cortisol elevations affect memory and interact with arousal-related processes in the prediction of memory (e.g., Abercrombie et al., 2006; Roozendaal, Okuda, de Quervain, & McGaugh, 2006). Thus, we tested the relations among cardiac activity, memory, and cortisol output during encoding and the speech (using area under the curve, both with respect to ground and increase; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003).
Results
Across all participants, HR during encoding ranged from 52.2 to 88.9 beats per minute (BPM). The average (SD) HR during encoding, 70.8 (9.1) BPM, was within the range of typical resting HR for men (average resting HR typically ranges between 60–80 BPM; Brownley, Hurwitz, & Schneiderman, 2000). Average (SD) HR during the speech stressor was 87.1 (13.5) BPM, and ranged from 55.1 to 117.7 BPM.
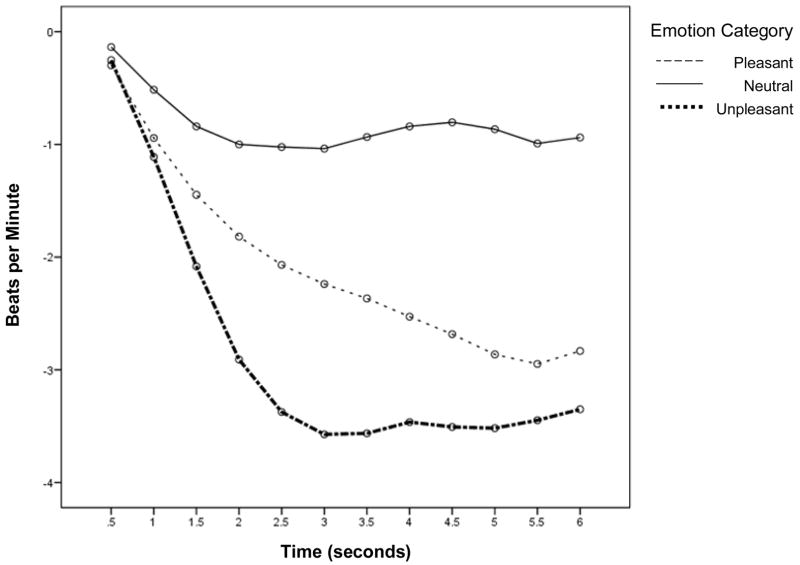
Consistent with prior research, emotion category predicted free recall scores (F[2, 52] = 139.89, p < .001; see Table 1). Post hoc Tukey analyses revealed more unpleasant pictures were recalled than pleasant pictures (see Table 1; mean difference = 5.68, p < .001) and neutral pictures (mean difference = 6.96, p < .001) and more pleasant pictures were recalled than neutral pictures (mean difference = 1.28, p < .01). Also consistent with prior research is our finding that HR deceleration during the first three seconds of picture viewing varied by emotion category, F(2, 52) = 26.84, p < .001 (Figure 1 & Table 1). Least significant difference pairwise comparisons revealed HR deceleration was greatest for unpleasant pictures, followed by pleasant and then neutral pictures (all p’s < .01).
Table 1.
Average (SD) HR Deceleration & Memory Performance
| Average maximum HR deceleration (Δ BPM) | Free recall performance (number of pictures recalled) | |
|---|---|---|
| Neutral Pictures | −3.60 (1.67) | 4.21 (1.97) |
| Pleasant Pictures | −4.71 (2.18) | 5.49 (2.04) |
| Unpleasant Pictures | −5.52 (2.66) | 11.17 (2.76) |
Note. HR values (beats per minute; BPM) were obtained by using the maximum HR deceleration from baseline in the first 3 seconds of picture viewing, modeled on methods of Bradley et al. (2001).
Figure 1.
HR deceleration in response to emotional pictures. Baseline HR (beats per minute) was subtracted from each subsequent half second HR estimate.
HR Deceleration and Memory
Remembered vs. forgotten trials
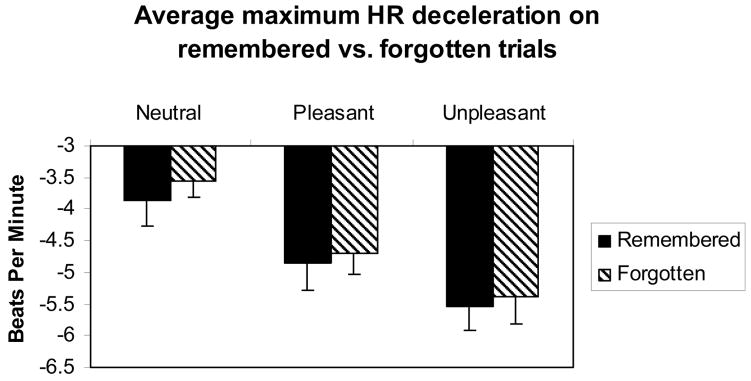
Across emotion category, HR deceleration was greater for trials that were subsequently remembered vs. those that were forgotten, F(1, 52) = 7.99, p < .005 (Figure 2). No interaction was found, such that the difference in HR deceleration for remembered vs. forgotten stimuli did not differ for neutral, pleasant, and unpleasant pictures F(2, 52) = 0.10, n.s. (Figure 2).
Figure 2.
Bar graph of average maximum HR deceleration (in beats per minute) for remembered vs. forgotten trials for neutral, pleasant, and unpleasant pictures. Across emotion category, HR deceleration was greater for trials that were subsequently remembered vs. those that were forgotten, F(1, 52) = 7.99, p < .005. Also apparent in this graph is the enhancement of HR deceleration for emotionally-laden compared to neutral pictures (also shown in Table 1 and Figure 1). Error bars represent standard error of the mean.
Prediction of total memory scores
When predicting total memory scores, average HR deceleration predicted recall of pictures (F[1, 52] = 20.43, p < .001), such that greater HR deceleration predicted better memory performance (i.e., the more deceleration on average, the more pictures remembered). As stated above, emotion category predicted recall scores, such that emotionally arousing pictures were better remembered than neutral pictures. However, the emotion category by HR deceleration interaction was not significant. These results for total memory scores are consistent with the results based on analysis of remembered vs. forgotten stimuli, which suggest that greater phasic HR deceleration predicts better memory performance regardless of emotion category.
Mean Heart Rate
HR deceleration and mean heart rate
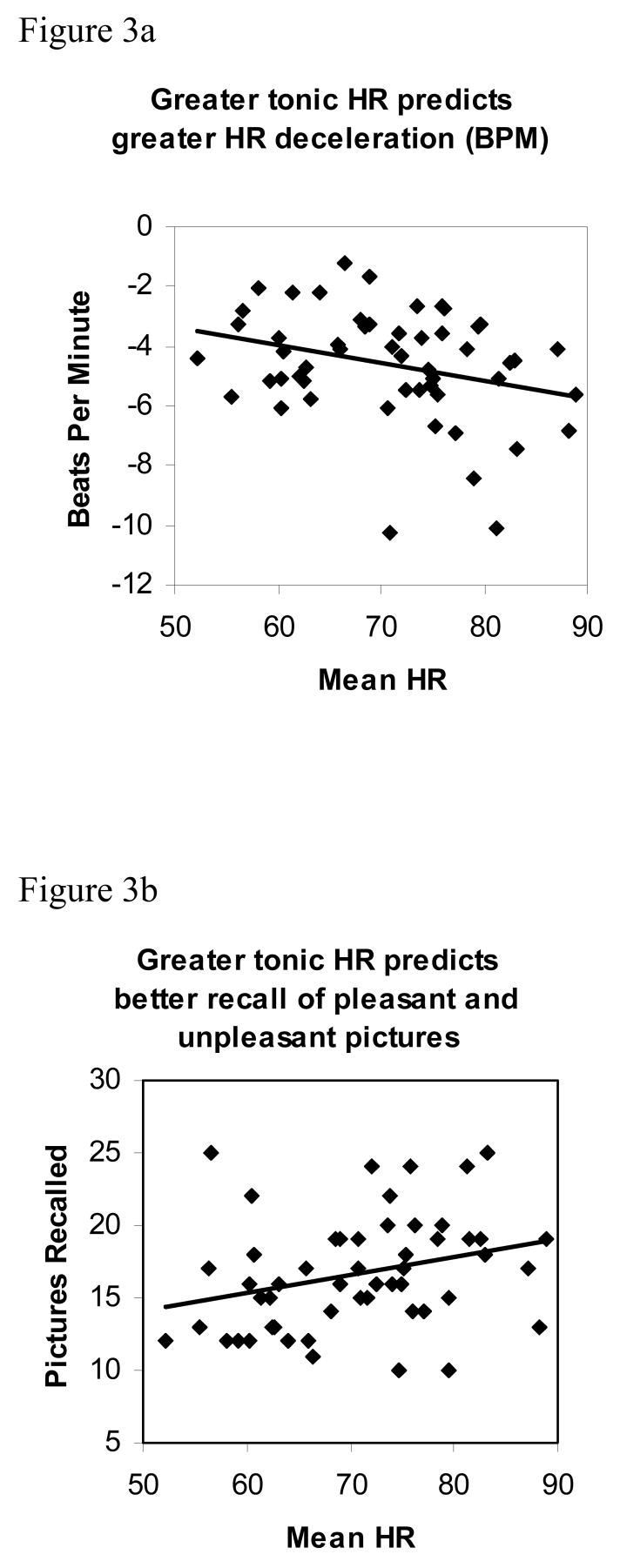
Mean HR during encoding was associated with phasic HR deceleration, r(52) = −.30, p < .05, such that individuals with the highest mean HR across the entire encoding epoch (approximately 27 minutes) showed the greatest average HR deceleration in response to viewing pictures (Figure 3a). However, the relation between mean HR and phasic HR deceleration was not apparent across all three emotion categories. Those individuals with higher mean HR showed greater HR deceleration in response to unpleasant, r(52) = −.35, p < .01, and pleasant pictures, r(52) = −.30, p < .05, but not neutral pictures, r(52) = −.06, n.s. These data suggest that individuals with higher tonic HR showed a stronger orienting response to emotionally arousing pictures, but not neutral pictures.
Figure 3.
a. Scatter plot of the relation between mean HR during encoding (Tonic HR) and maximum phasic HR deceleration during viewing of emotionally-laden and neutral pictures (in beats per minute; r[52] = −.30, p < .05). Individuals with the highest mean HR show the greatest average HR deceleration in response to viewing pictures.
b. Scatter plot of the relation between mean HR during encoding (Tonic HR) and number of pleasant and unpleasant pictures recalled (r[52] = .29, p < .05).
Mean HR and memory performance
As predicted, individuals with higher mean HR showed better memory performance for emotionally arousing pictures (unpleasant and pleasant combined), r(52) = .29, p < .05 (Figure 3b). However, there was no association between mean HR and memory scores for neutral pictures, r(52) = .03, n.s. Regression analysis (examining whether HR deceleration in response to arousing pictures accounts for additional variance in memory scores over and above variance accounted for by mean HR) showed that HR deceleration does not account for significant variance over mean HR, F(2, 52) = 1.79, n.s. When HR deceleration is placed in the model first, mean HR does not account for significant variance over HR deceleration, F(2, 52) = 2.4, n.s. These findings are consistent with the positive association between mean HR and phasic HR deceleration reported above, and suggest that mean HR and HR deceleration account for overlapping portions of variance in memory scores. In sum, individuals with higher tonic HR orient more to emotionally arousing pictures and subsequently remember more emotionally arousing pictures than individuals with lower tonic HR.
HR Response to Stress
HR during the speech (which took place approximately 10 minutes after the encoding period) was significantly positively related to mean HR during encoding, r = .67, p < .01. HR response to the speech stressor (HR during speech minus HR during encoding) was highly related to HR during the speech, r = .74, p < .001, but was not related to HR during encoding, r = −.01, n.s. Unlike HR during encoding, HR during the speech stressor and HR response to stress were unrelated to subsequent memory performance (all p’s > .15). In addition, HR during the speech and HR response to stress were not significantly related to phasic HR deceleration during picture encoding (p’s > .11 for HR deceleration during all pictures combined across emotion category). However, there was a trend for greater HR during the speech predicting greater HR deceleration during encoding of pleasant pictures, (r = −.25, p = .08). This trend was not apparent when examining the relation between HR deceleration to pleasant pictures and HR response to speech (i.e., HR during speech minus HR during encoding), r = −.05, n.s.
Cortisol, Cardiac Variables, and Memory
In the current sample, cortisol levels during encoding and cortisol output during the speech stressor (which occurred after encoding) were related neither to memory performance nor to cardiac variables (all p’s > .27). Furthermore, when entered into regression equations examining the relations between memory performance and either mean HR or HR deceleration, cortisol output neither added significant variance nor abolished the relation between cardiac variables and memory (all p’s < .46). Thus, neither cortisol levels during encoding nor cortisol response to the speech stressor alter or account for the observed relations between cardiac variables and memory performance.
Discussion
This study replicated prior research showing that greater phasic HR deceleration during picture viewing was greater for subsequently remembered than forgotten stimuli (Buchanan et al., 2006). These data suggest that a larger orienting response (as reflected in greater HR deceleration in response to a picture) is related to deeper encoding and better subsequent memory for the stimulus. We also found that individuals with higher tonic HR across the entire picture encoding epoch showed better subsequent memory for emotionally arousing pictures. Furthermore, we found that higher tonic HR predicted greater phasic HR deceleration during viewing of emotionally arousing (but not neutral) pictures. This finding suggests that individuals who were more autonomically aroused (as measured by mean HR) showed stronger orienting responses to emotionally arousing pictures (as measured by HR deceleration). In sum, those individuals with elevated HR and greater orienting responses subsequently remembered more emotionally arousing pictures than individuals with lower mean HR. Elevated tonic HR may reflect a state of physiological and emotional arousal, which is associated with increased attentional processing of emotionally salient material, reflected in enhanced phasic slowing of HR and deeper encoding of emotionally salient information.
In our data, deceleration was greatest for unpleasant pictures and least for neutral pictures. However, the difference in HR deceleration for remembered vs. forgotten pictures is stable across emotion category. These data imply that greater orienting increases the likelihood that a stimulus will be later remembered, regardless of whether the stimulus is emotionally arousing. On the other hand, the relations among tonic HR elevation, phasic HR deceleration, and subsequent memory performance were observed only for emotionally arousing pictures. Thus, autonomic arousal appears to predict the degree of orienting and subsequent memory only for emotionally arousing events. Other factors (e.g., novelty) have been shown to determine the degree of orienting to neutral stimuli (Cook & Turpin, 1997).
At first glance, it may seem paradoxical that individuals with elevated tonic HR are more likely to show greater HR deceleration during processing of emotional stimuli. However, neurogenic control of HR is complex. The two-component model of attention proposed by Porges (1992) can aid in the understanding of these relationships. This model partitions the physiological response to a stimulus into two components, reactive and sustained. The reactive portion involves a short-latency vagally-mediated heart rate deceleration occurring within one second of stimulus perception. This is followed by a longer latency period that lasts four to five seconds where heart rate response varies as a function of the stimulus. The coupling of vagal withdrawal and sympathetic activation causes this longer latency heart rate acceleration in response to important or intense stimuli whereas the response to mild stimuli involves vagal activation to cause more prolonged heart rate deceleration. The model also describes a longer duration parasympathetically-mediated response. When sustained attention is required, heart rate stabilizes and heart rate variability diminishes, which facilitates information processing. Further, the relationship between the sympathetic and parasympathetic branches of the autonomic nervous system in terms of controlling heart rate is one of “accentuated antagonism” such that sympathetic activity is partly a function of background vagal activity as recently demonstrated non-invasively in humans by Uijtdehaage and Thayer (2000).
Furthermore, autonomic activation is associated with priming of attention to stimuli promoting avoidance (Lang, Bradley, & Cuthbert, 1990). Phasic bradycardia is the typical HR response to passively viewed emotionally arousing (particularly aversive) stimuli, and represents attentional orienting to a stimulus (Bradley et al., 2001; Bradley & Lang, 2000). Thus, greater tonic HR may represent a priming of attentional systems, and therefore may promote greater heart rate deceleration to emotionally arousing stimuli.
Porges’ research has examined individual differences in vagal tone and information processing (reviewed in Porges, 1992). Their research suggests that individuals with higher baseline vagal tone show greater HR reactivity to stimulation as well as better sustained attention. While our study did not measure resting vagal tone, our data are consistent with Porges and colleagues’ data in that the individuals with the more extreme task-related HR responses (i.e., greater phasic slowing and greater tonic HR during stimulus viewing) were the individuals with the deepest encoding and best subsequent memory performance.
Past research implicates additional centrally mediated relations among arousal, orienting, and memory. For instance, the locus coeruleus-norepinephrine (LC-NE) system maintains behavioral and neuronal states that determine the level of arousal of the organism (spanning from sleep to quiet waking to arousal; Berridge & Waterhouse, 2003). The LC-NE system also plays an important role in modulating the orienting response to salient stimuli. In addition to HR deceleration, the P300 component of the human event-related potential (ERP) serves as an index of an OR (Palomba et al., 1997). P300-like components are also observed in monkeys. Pharmacological manipulation of LC firing as well as bilateral LC lesions alter the magnitude of these P300-like components in monkeys (Pineda, Foote, & Neville, 1987). Thus, the LC-NE system appears to modulate these P300-like components, and may be an important modulator of the orienting response. The LC-NE system therefore appears to modulate states of arousal as well as processing of salient sensory information, which suggests that the level of arousal (even within states of attentive waking) should predict degree of orienting. Our data showing a positive relation between heightened tonic HR (reflecting a heightened state of arousal) and greater phasic HR deceleration (reflecting greater orienting) are consistent with this view.
Noradrenergic activation of the basolateral nucleus of the amygdala may partly determine positive relations among arousal, orienting to emotional stimuli, and emotional enhancement of memory. Amygdala activation during memory formation underlies the superiority of memory for emotional information (Cahill, Haier, Fallon, Alkire, Tang, Keator, Wu, & McGaugh, 1996; Canli, Zhao, Brewer, Gabrielli, & Cahill, 2000). In particular, noradrenergic activation in the basolateral nucleus of the amygdala is necessary for memory enhancement associated with emotionally provocative events and stimuli (Liang, Juler, & McGaugh, 1986; McGaugh, 2000). In sum, norepinephrine plays a crucial role in the emotional enhancement of memory (McGaugh, 2000), and contributes to long-term alterations in synaptic strength, gene transcription, and other processes necessary for learning (Berridge & Waterhouse, 2003).
Furthermore, the amygdala plays a role in central regulation of HR, as projections from the central nucleus of the amygdala innervate the nucleus tractus solitarius (Brownley, Hurwitz, & Schneiderman, 2000). Noradrenergic activation of the amygdala may be partly responsible both for alterations in HR and heightened memorability of emotional information. Future research examining central nervous system activation is needed to further test whether amygdala activation mediates the relations between HR and memory.
Most likely, HR alterations that accompany emotionally laden events do not play a major causal role in the greater memorability (Öhman, Hamm, & Hugdahl, 2000). HR and other peripheral alterations likely reflect (rather than cause) central nervous system processes involved in the strength of encoding stimuli and events. Indeed, manipulation of central but not peripheral adrenergic systems is necessary for enhancement of memory for emotional material (van Stegeren et al., 1998).
However, various data suggest that central mechanisms may not be sufficient, and that autonomic arousal is necessary for emotional enhancement of memory. Cardiovascular responses may play a causal role in cortical sensitivity to stimuli via afferent connections from the heart to the brain (Lacey, 1967; see Öhman et al., 2000 for review). For instance, an intact nucleus tractus solitarius (NTS), which can be viewed as an integration center for afferent autonomic signals, is necessary for arousal-related memory improvement (Miyashita & Williams, 2003, 2004; Williams & McGaugh, 1993). Stimulation of afferents to the NTS during memory formation improves retention (Clark, Naritoku, Smith, Browning, & Jensen, 1999; Clark, Smith, Hassert, Browning, Naritoku, & Jensen, 1998). Thus, it appears that peripheral activation plays an important contributory role in emotional enhancement of memory, and is not merely a proxy measure for central mechanisms (see also Anderson, Yamaguchi, Grabski, & Lacka, 2006). Pharmacological blockade of parasympathetic, sympathetic, and dual blockade of parasympathetic and sympathetic influences on cardiac variation (e.g., Berntson, Cacioppo, & Quigley, 1994) could be used to test whether blocking stimulus-related variation in HR alters the differential memorability of emotionally laden vs. neutral stimuli.
Research has shown that alterations in arousal and emotion-related information processing are associated with the symptomatology of depression and anxiety. For instance, Shalev, Sahar, Freedman, Peri, Glick, Brandes, Orr, and Pitman (1998) showed that trauma victims with higher heart rates in the hospital emergency department following a traumatic incident are more likely to develop PTSD than trauma survivors with lower emergency room heart rates. Shalev and colleagues (1998) also showed that higher emergency department heart rates predicted a higher rate of intrusive memories of the traumatic incident at 4 months following the event. Furthermore, alterations in emotion-related information processing and heightened memory for negative information is often observed in depression (Mineka, Rafaeli, & Yovel, 2003; Shestyuk, Deldin, Brand, & Deveney, 2005). Measuring HR deceleration and other electrophysiological indices of information processing (e.g., ERPs) during processing of negative vs. neutral stimuli may serve as an important physiological index of alterations in emotion-related information processing in future research investigating PTSD, depression, and other forms of psychopathology. In addition, electrophysiological measures during cognitive processing of emotional material may serve as important indicators of treatment progress in cognitive behavioral therapy. Future research on clinical samples should further investigate both central and peripheral responses to emotionally evocative material and their relationships to memory and symptomatology.
Future research must also examine potential sex differences in the association between HR and emotional memory. Because the current study was conducted on men only, the results presented here may not be generalizable to women. Women often show greater enhancement of memory for emotional information than men (Canli, Desmond, Zhao, & Gabrieli, 2002). In addition, women generally show greater resting HRs than men (Taneja, Windhagen Mahnert, Passman, Goldberger, & Kadish, 2001). It should be determined whether women’s higher resting HRs are related to priming of attentional systems, and enhanced orienting and/or emotional memory.
In the sample presented here, HR and cortisol responses to the stressor were not related to memory performance and do not appear to alter the relations between HR during encoding and subsequent memory. However, because all subjects participated in the stressor immediately following encoding the results presented herein may depend on exposure to emotional arousal and stress immediately following encoding. Future research, in which exposure to a stressor is experimentally manipulated, is needed in order to determine whether an emotionally arousing stressor immediately following encoding alters relations between cardiac activity during encoding and subsequent memory.
Summary
Because emotional pictures are more memorable and cause greater HR deceleration than neutral pictures, we predicted that the relations between cardiac activity and memory enhancement would be most pronounced for emotionally-laden compared to neutral pictures. We found that elevated mean HR across the entire encoding epoch predicted better memory performance for emotionally arousing pictures. Phasic HR deceleration during viewing of individual pictures was greater for subsequently remembered than forgotten pictures across all three emotion categories. Tonic HR and phasic HR deceleration were associated, but only for emotionally arousing pictures, such that those individuals with greater tonic HR also showed greater HR decelerations during viewing of emotionally arousing pictures. Results suggest that tonic elevations in HR are associated both with greater orienting and heightened memory for emotionally arousing stimuli. These data imply that greater orienting predicts the memorability of an event, and that autonomic arousal may be associated with enhancement of the orienting response and depth of encoding during an emotionally arousing event. Future research is needed to examine the central nervous system mediators of these effects as well as their ramifications for treatment of emotion-related information processing alterations in psychopathology.
Acknowledgments
The authors would like to thank Brenda Smage for her assistance with cardiac data processing, and Nicole Speck, Anand Lakshmanan, Natasha Shallow, Ashley Lienhardt, and Marie Kay for their assistance with data collection. We also thank Priscilla Rasche, Christopher Chambreau, Richard Davidson, and Ned Kalin for advice and support. This research was funded by institutional start-up funds to Heather Abercrombie, for which we are grateful to the University of Wisconsin-Madison.
Footnotes
Set A included pictures 1450, 1650, 2495, 2840, 2850, 2870, 3000, 3030, 3053, 3064, 3168, 3180, 3230, 3400, 5390, 5470, 5480, 5510, 5600, 5628, 5740, 6260, 6312, 6570_1, 6838, 7004, 7025, 7035, 7050, 7175, 7233, 7234, 7350, 7380, 7490, 7491, 7502, 7600, 7700, 7705, 7950, 8030, 8040, 8060, 8090, 8120, 8161, 8170, 8180, 8185, 8250, 8340, 8502, 8531, 9040, 9050, 9210, 9400, 9410, 9570, 9810, 9920, 9921. Set B included pictures 1640, 2190, 2320, 2340, 2580, 2880, 3010, 3015, 3062, 3063, 3071, 3150, 3160, 3170, 3220, 3261, 3350, 5450, 5534, 5621, 5629, 5700, 5731, 5890, 5910, 6250, 6540, 7000, 7002, 7006, 7009, 7020, 7030, 7031, 7040, 7080, 7090, 7140, 7150, 7217, 7224, 7235, 7289, 7501, 8080, 8160, 8190, 8232, 8260, 8280, 8300, 8380, 8470, 8490, 8503, 9180, 9250, 9300, 9520, 9560, 9630, 9800, 9910.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31:187–196. doi: 10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Yamaguchi Y, Grabski W, Lacka D. Emotional memories are not all created equal: Evidence for selective memory enhancement. Learning & Memory. 2006;13:711–718. doi: 10.1101/lm.388906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic cardiac control. I. Estimation and validation from pharmacological blockades. Psychophysiology. 1994;31:572–585. doi: 10.1111/j.1469-8986.1994.tb02350.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: Pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling and physiology. In: Lane R, Nadel L, Ahern GL, Allen JJB, Kaszniak AW, Rapcsak SZ, Schwartz GE, editors. Cognitive neuroscience of emotion. New York: Oxford University; 2000. pp. 242–276. [Google Scholar]
- Brownley KA, Hurwitz BE, Schneiderman N. Cardiovascular psychophysiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2. New York: Cambridge University Press; 2000. pp. 224–264. [Google Scholar]
- Buchanan TW, Etzel JA, Adolphs R, Tranel D. The influence of autonomic arousal and semantic relatedness on memory for emotional words. International Journal of Psychophysiology. 2006;61:26–33. doi: 10.1016/j.ijpsycho.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiology of Learning and Memory. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire M, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neuroscience. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. β-Adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JDE. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrielli JDE, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. The Journal of Neuroscience. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nature Neuroscience. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiology of Learning and Memory. 1998;70:364–373. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- Cook EW, III, Turpin G. Differentiating orienting, startle, and defense responses: The role of affect and its implications for psychopathology. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Hillsdale NJ: Erlbaum; 1997. pp. 137–164. [Google Scholar]
- Graham FK. Constraints on measuring HR and period sequentially through real and cardiac time. Psychophysiology. 1978;15:492–495. doi: 10.1111/j.1469-8986.1978.tb01422.x. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Hall SW. Recall, recognition, and rate: Memory and the heart. Psychophysiology. 1980;17:37–46. doi: 10.1111/j.1469-8986.1980.tb02457.x. [DOI] [PubMed] [Google Scholar]
- Lacey JI. Psychophysiological approaches to the evaluation of psychotherapeutic process and outcome. In: Rubenstein EA, Parloff MB, editors. Research in psychotherapy. Vol. 1. Washington, D.C: American Psychological Association; 1959. pp. 160–208. [Google Scholar]
- Lacey JI. Somatic response patterning and stress: Some revisions of activation theory. In: Appley MH, Trumbull R, editors. Psychological stress: Issues in research. New York: Appleton-Century-Crofts; 1967. pp. 14–42. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–398. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. Technical Report A-5. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: Involvement of the amygdala noradrenergic system. Brain Research. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory – a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Mineka S, Rafaeli E, Yovel I. Cognitive biases in emotional disorders: Information processing and social-cognitive perspectives. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of affective sciences. New York: Oxford University Press; 2003. pp. 976–1009. [Google Scholar]
- Miyashita T, Williams CL. Enhancement of noradrenergic neurotransmission in the nucleus of the solitary tract modulates memory storage processes. Brain Research. 2003;987:164–175. doi: 10.1016/s0006-8993(03)03323-7. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Peripheral arousal-related hormones modulate norepinephrine release in the hippocampus via influences on brainstem nuclei. Behavioural Brain Research. 2004;153:87–95. doi: 10.1016/j.bbr.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Nagamine M, Matsuoka Y, Mori E, Fujimori M, Imoto S, Kim Y, Uchitomi Y. Relationship between heart rate and emotional memory in subjects with a past history of post-traumatic stress disorder. Psychiatry and Clinical Neurosciences. 2007;61:441–443. doi: 10.1111/j.1440-1819.2007.01677.x. [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, Drysdale E, Cahill L, Shajahan P, Ebmeier KP. Memory for emotional material: A comparison of central versus peripheral beta blockade. Journal of Psychopharmacology. 1999;13:32–39. doi: 10.1177/026988119901300104. [DOI] [PubMed] [Google Scholar]
- Öhman A. The orienting response, attention, and learning: An information-processing perspective. In: Kimmel HD, van Olst EH, Orlebeke JF, editors. The orienting reflex in humans. Hillsdale, NJ: Lawrence Erlbaum; 1979. pp. 443–471. [Google Scholar]
- Öhman A, Hamm A, Hugdahl K. Cognition and the autonomic nervous system: Orienting, anticipation and conditioning. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of psychophysiology. New York: Cambridge University Press; 2000. pp. 533–575. [Google Scholar]
- Palomba D, Angrilli A, Mini A. Visual evoked potentials, heart rate responses, and memory to emotional pictorial stimuli. International Journal of Psychophysiology. 1997;21:55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Foote SL, Neville HJ. The effects of locus coeruleus lesions on a squirrel monkey late positive component: A preliminary study. Electroencephalography and Clinical Neurophysiology. 1987;40:481–486. [PubMed] [Google Scholar]
- Porges SW. Autonomic regulation and attention. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 201–223. [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJF, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Sahar T, Freedman S, Peri T, Glick N, Brandes D, Orr SP, Pitman RK. A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Archives of General Psychiatry. 1998;55:553–559. doi: 10.1001/archpsyc.55.6.553. [DOI] [PubMed] [Google Scholar]
- Shestyuk AY, Deldin PJ, Brand JE, Deveney CM. Reduced sustained brain activity during processing of positive emotional stimuli in major depression. Biological Psychiatry. 2005;57:1089–1096. doi: 10.1016/j.biopsych.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Siddle DAT, Packer JS, Donchin E, Fabiani M. Mnemonic information processing. In: Jennings JR, Coles MGH, editors. Handbook of cognitive psychophysiology: Central and autonomic nervous system approaches. Oxford, England: John Wiley & Sons; 1991. pp. 449–510. [Google Scholar]
- Taneja T, Windhagen Mahnert B, Passman R, Goldberger J, Kadish A. Effects of sex and age on electrocardiographic and cardiac electrophysiological properties in adults. Journal of Pacing and Clinical Electrophysiology. 2001;24:16–21. doi: 10.1046/j.1460-9592.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- Uijtdehaage SHJ, Thayer JF. Accentuated antagonism in the control of human heart rate. Clinical Autonomic Research. 2000;10:107–110. doi: 10.1007/BF02278013. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Everaerd W, Cahill L, McGaugh JL, Gooren LJG. Memory for emotional events: Differential effects of centrally versus peripherally acting β-blocking agents. Psychopharmacology. 1998;138:305–310. doi: 10.1007/s002130050675. [DOI] [PubMed] [Google Scholar]
- Williams CL, McGaugh JL. Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behavioral Neuroscience. 1993;107:955–962. [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]