Abstract
Vascular cognitive impairment (VCI) is the second most prevalent type of dementia in the world. The white matter damage that characterizes the common subcortical ischemic form of VCI can be modeled by ligating both common carotid arteries in the Wistar rat to induce protracted cerebral hypoperfusion. In this model, we find that repetitive intranasal administration of recombinant E-selectin to induce mucosal tolerance and to target immunomodulation to activating blood vessels potently suppresses both white matter (and possibly gray matter) damage and markers of vessel activation (tumor necrosis factor and E-selectin); it also preserves behavioral function in T-maze spontaneous alternation, T-maze spatial discrimination memory retention, and object recognition tests. Immunomodulation may be an effective novel strategy to prevent progression of VCI.
Keywords: E-selectin, immunomodulation, mucosal tolerance, vascular cognitive impairment
Introduction
As life expectancy increases worldwide, the prevalence of cognitive impairment and dementia is emerging as major public health problems in many countries of the world. Vascular cognitive impairment (VCI), the second most prevalent form of dementia, comprises a number of heterogeneous pathological conditions due to ischemic or hemorrhagic brain lesions or to protracted cerebral hypoperfusion (Roman et al, 2002). Ischemic white matter lesions, found commonly in elderly people, are the characteristic pathological changes in the subcortical ischemic form of VCI and the degree of cognitive dysfunction is related to lesion severity (Hachinski et al, 1987; Pantoni et al, 1999). White matter lesions are prominent in several types of VCI, such as Binswanger’s disease, cerebral amyloid angiopathy, and cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. These cerebrovascular white matter lesions are caused by chronic cerebral hypoperfusion, which results from stenosis of arteries or arterioles mainly in deep white matter (Roman, 2004).
Cerebrovascular white matter lesions can be experimentally induced in Wistar rat brains as a result of protracted hypoperfusion induced by permanent occlusion of both common carotid arteries (Wakita et al, 1994). In this VCI model, the impeded cerebral blood flow, which gradually increases from 33 to 81% of normal for an extended period (Otori et al, 2003), leads to delayed white matter lesions and memory impairment correlated with damage to frontal-subcortical circuits (Sarti et al, 2002; Wakita et al, 1994). Although the white matter overwhelmingly bears the brunt of the apparent histopathology after occlusion of the carotids in this model, it should be noted that bilateral carotid occlusion exposes the entire forebrain to protracted hypoperfusion. Reports of selective neuronal necrosis in similar, but not identical, models raise the possibility that gray matter damage could contribute to observed functional deficits in this model. This point will be further addressed in the Discussion.
Previous studies using this model have showed that CD4 + or CD8 + T cells infiltrate the neural parenchyma and that microglia are activated and express major histocompatibility complex (MHC) class I and II antigens briefly after ischemia in a manner that predicts the extent and the severity of demyelination and axonal damage (Wakita et al, 1994, 2002). Similar activation of microglia can also be detected in early stages of human cerebrovascular white matter lesions (Suenaga et al, 1994). These data suggest that immunological and inflammatory reactions augment white matter damage during chronic ischemia.
A novel method has been developed, in which E-selectin is used to generate regulatory T cells targeted to activating blood vessels (Takeda et al, 2002). E-selectin is a glycoprotein adhesion molecule that is specifically expressed on endothelial cells (Bevilacqua et al, 1989), but it is only expressed when endothelium becomes activated (Bevilacqua, 1993). E-selectin becomes expressed in experimental (Zhang et al, 1996) and clinical (Fassbender et al, 1999) brain ischemia. Recombinant E-selectin can, therefore, serve as an immunological tolerization antigen, which can focus immunomodulation to regions of the vascular tree in which thrombosis or hemorrhage are threatened. Intranasal instillation of recombinant human E-selectin induces mucosal tolerance to that antigen with the generation of E-selectin-specific regulatory T cells (Chen et al, 2003; Takeda et al, 2002). In spontaneously hypertensive stroke-prone rats, mucosal tolerance to E-selectin prevents spontaneous ischemic and hemorrhagic strokes (Takeda et al, 2002) and protects against ischemic brain damage after permanent middle cerebral artery occlusion (Chen et al, 2003). E-selectin-specific regulatory T cells may prevent stroke and protect against ischemic brain damage through ‘bystander suppression,’ in which immunomodulatory cytokines are released locally (Miller et al, 1991).
This study examines the capacity of mucosal tolerance to E-selectin to suppress white matter damage and memory impairment in a preclinical VCI model. The results strongly implicate inflammatory and immunological reactions in the development of white matter lesions under conditions of protracted hypoperfusion. The results also provide initial support for immunomodulation strategies to suppress development of VCI in patients at risk for that disorder.
Materials and methods
Animals and Experimental Protocols
A total of 34 male and female Wistar rats (Charles River Laboratories, Wilmington, MA, USA) aged 9 weeks were used. The National Institute of Neurological Disorders and Stroke Animal Care and Use Committee approved all experiments.
Tolerization Schedule
Intranasal application of E-selectin was carried out with the animals under brief anesthesia with 5% isoflurane in 30% O2/70% N2O.
Intranasal instillations were as follows:
Phosphate-buffered saline (PBS) (Quality biological Inc., Gaithersburg MD, USA).
Recombinant human E-selectin (Novavax, Rockville, MD, USA).
The tolerization schedule was as follows:
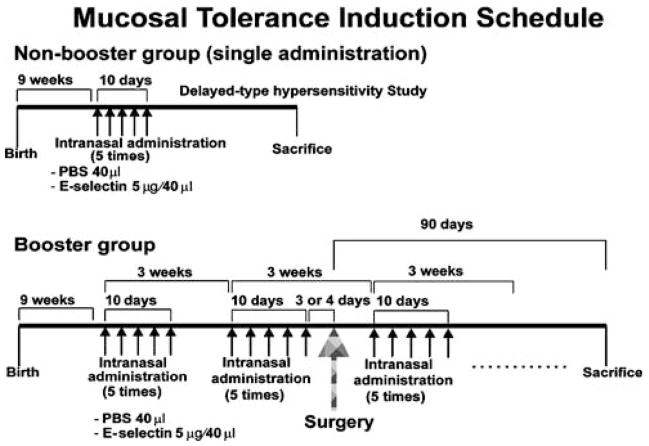
Single: PBS (20 μL) or E-selectin (2.5 μg/20 μL) was instilled into each nostril every other day for 10 days (total of five administrations) (Figure 4).
Booster: intranasal instillations of the same substance at the same volume and concentration on the same schedule, as described above, were repeated at 3-week intervals from 1 month before surgery to 3 months after surgery (Figure 1).
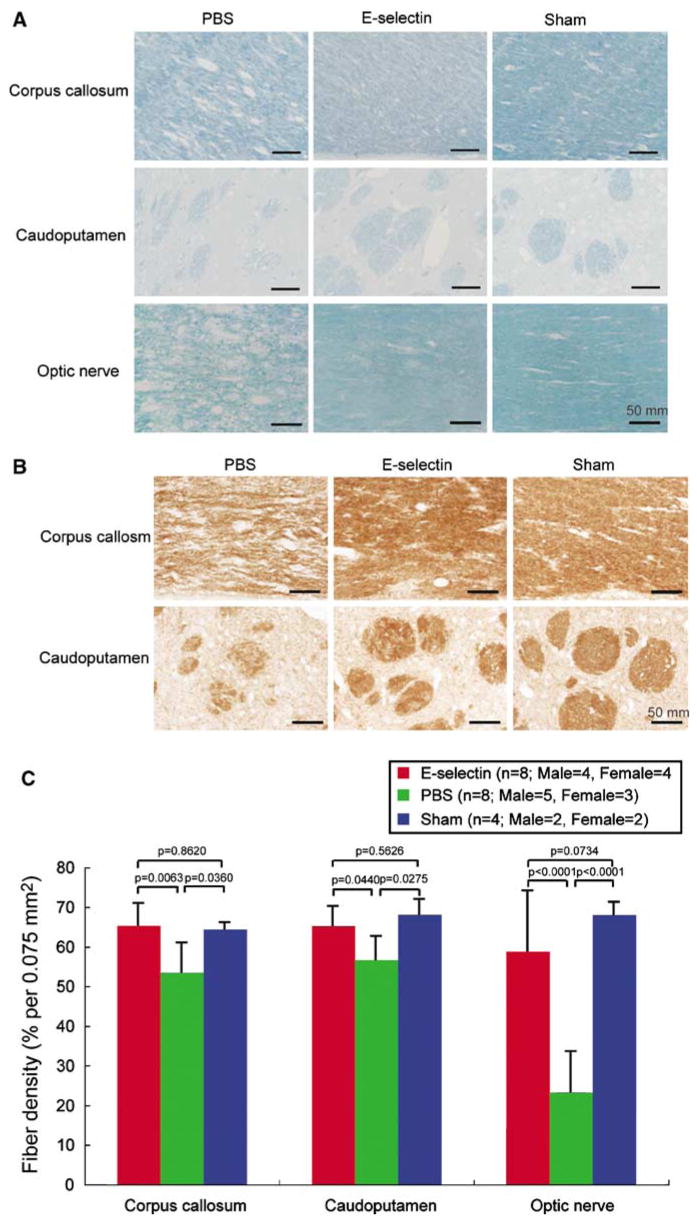
Figure 4.
Protective effect of mucosal tolerance to E-selectin against white matter damage. (A) Photomicrographs of luxol fast blue staining of the corpus callosum, caudoputamen, and optic nerve (scale bars=50 μm). (B) Photomicrographs of the immunohistochemical staining for the cocktail of monoclonal antibodies directed against nonphosphorylated neurofilaments (SMI 311) of the corpus callosum and caudoputamen (scale bars=50 μm). The extent of the white matter rarefaction was less severe in the E-selectin-treated and sham-operated rats, as compared with PBS group. (C) Histograms of the fiber densities of the corpus callosum, caudoputamen, and optic nerve in rats subjected to a sham operation or to bilateral ligation of the carotid arteries and intranasal administration of either PBS or E-selectin on a booster tolerization schedule. The fiber densities in E-selectin-treated animals were significantly higher than those in PBS-treated animals.
Figure 1.
Mucosal tolerance induction schedule. For induction of mucosal tolerance, E-selectin was instilled intranasally.
Delayed-Type Hypersensitivity Reaction
A single-course tolerization schedule with PBS or E-selectin was applied for assessing the delayed-type hypersensitivity (DTH) reaction (n = 4 per group), as described previously (Chen et al, 2003; Takeda et al, 2002).
Surgery
Booster tolerization was repeated at 3-week intervals from 1 month before surgery to 3 months after surgery. To adjust the surgery workload, half of the rats from each group were randomly selected and subjected to surgery 3 days after the last dose of the first tolerization schedule; surgery was performed 4 days after the first tolerization schedule for the remaining half of rats. Animals were anesthetized with 5% isoflurane for induction and 1.5% isoflurane for maintenance in 30% O2/70% N2O by facemask. Core body temperature was monitored and maintained at 37.0±0.5°C by heating pad and heat lamp. Through a midline cervical incision, both common carotid arteries were exposed and double-ligated with 5-0 silk sutures, as described previously (Wakita et al, 1994). After the operation, rats were kept in cages with food and water ad libitum. Four sham-operated control animals were subjected to the same surgical procedures without bilateral carotid ligation.
Behavioral Assessments
Behavioral assessments were performed without knowledge of animal group assignment. See Supplementary information for details.
Object Recognition Test
A rat is placed in a glass box, which is illuminated by a lamp suspended above the box in a darkened room. The apparatus was formed by a glass box (30×60×30 cm). The apparatus was illuminated by a 100W lamp suspended 70cm above the box in a darkened room. The day before testing, rats were habituated to the test environment by exploring the box for 6 mins without objects. On the day of the test, rats were subjected to a session of two trials. The intertrial interval was 60 mins. In the first trial, two identical objects were placed on the centerline of the long axis of the floor, 5cm from each end of the apparatus. Rats were placed into the center of the box and allowed to explore the two objects for 6 mins. The time spent exploring each object was recorded. During the second trial, one of the objects presented in the first trial was replaced by a novel object and rats were left in the box for 6 mins. The time spent for the exploration of the familiar (Tf) and the novel object (Tn) was recorded separately. Exploration was considered sniffing at the object within a distance of 2 cm from the object or touching it with the nose. A discrimination index (Tn−Tf/Tn+Tf) was calculated. For each animal, one pair of objects in the first trial was selected at random from a set of three plastic objects that differed in shape and color (red cubes, green pyramids, and blue cylinders of 6 cm height), and the role (familiar and novel object) and the position of the two objects in the second trial were randomly changed to avoid object and place preference. After each exposure, the apparatus and the objects were cleaned carefully with 70% alcohol to avoid olfactory stimuli.
T-Maze Spontaneous Alternation
Spontaneous alternation was investigated in an acrylic T-shaped runway. It consisted of a start box (20×18 cm) and start arm (60cm long), and two identical goal arms (both 50cm long). All arms were 10cm wide and 10cm high. Rats were placed in the start box of the T-maze, and a maximum time of 5 mins was allowed for them to explore the maze. Spontaneous alternation was defined as following: the rat entered with all four feet into one goal arm, came back, and then entered with all four feet into the opposite goal arm. The number of rats that alternated was recorded.
T-Maze Left/Right Discrimination Memory Retention
The dimensions of the T-maze apparatus were described above. The exit of the start box and the entrances of the goal arms could be blocked by guillotine doors. Careful consideration was given to avoid providing the animals with any spatial cues. To minimize olfactory cues, the maze was wiped carefully after each run with 70% alcohol.
Training Sessions for Left/Right Discrimination Memory Retention
The day before training, after the spontaneous alternation test, rats were habituated for 15 mins to the presence of food pellets (Bacon Softies; Bio-Serv, Frenchtown, NJ, USA) placed at the end of each arm in the T-maze. On days 1 to 3, the rats were deprived of food for 8 to 12 h each day before the T-maze left/right discrimination training. This training consisted of three stages. In the performance of the training, half of the rats from each group were randomly selected and reinforcement (food reward) placed on the right arm; for the other half of the rats from each group, the reinforcement was placed on the left arm. The reinforced arm then remained consistent throughout the remainder of the training period. The first stage consisted of five trials. In this stage, a guillotine door was placed to close off one arm, and the animal was forced to enter the open arm, which was baited with a food reward that the animal was allowed to eat. For all runs, the animals remained on the maze until 2 mins had elapsed; they were then placed in the start box for 2 mins. The second stage consisted of five trials. In this stage, a guillotine door was placed to close off the same arm as that in the first stage, and the animal was forced to enter the open arm, which was not baited with a food reward. When the animal entered into the open arm, a food reward was given and the animal was allowed to eat the food. The animals remained on the maze for 2 mins and were then placed in the start box for 2 mins. In the third stage, a guillotine door was removed, and the animal could enter into either arm (correct side and incorrect side). If the animal chose the arm on the correct side, the animal received a food reward and was allowed to eat for 2 mins after which it was placed in the start box for 2 mins. If the animal chose the incorrect side arm, the animal was picked up immediately and placed in the start box for 2 mins. The third stage was continued until the animals made four consecutive correct choices or until they had 20 training sessions (the training ceiling). This procedure was performed daily on three successive days.
Left/Right Discrimination Memory Retention Test Session
The retention of left/right discrimination memory was evaluated at 1, 2, 3, 5, 7, 10, and 14 days after the training session. The animals were subjected to 10 trials on each testing day. An entry was defined as all four paws entering the arm. The total number of correct entries was recorded. This paradigm was repeated at 2, 6, and 10 weeks after surgery.
Histopathology
At 90 days after surgery, the animals were anesthetized with sodium pentobarbital (100 mg/kg, intraperitoneally), perfused transcardially with 0.01 mol/L PBS, and perfused with a fixative containing 4% paraformaldehyde in 0.1 mol/L phosphate buffer (PB, pH 7.4). Coronal brain blocks including the caudoputamen or right optic nerve were embedded in paraffin and sectioned (2 μm) for histological examination. Luxol fast blue was used to evaluate the myelin damage. Axonal injury was assessed with a cocktail of mouse monoclonal antibodies directed against nonphosphorylated neurofilaments (SMI 311, Covance Research Products Inc., Berkeley, CA, USA) (Rosenfeld et al, 1987). Coronal brain sections were incubated for 1 h in 0.1 mol/L PBS containing 0.3% Triton X-100 for permeabilization. Ten percent of donkey serum was applied for blocking, followed by incubation overnight in primary antibody (SMI 311) in a dilution of 1:500. The sections were subsequently incubated with a biotinylated anti-mouse IgG raised in donkey (1:2,000; Jackson Immuno Research Labs, West Grove, PA, USA) for 1 h, and then incubated with an avidin–biotin–peroxidase complex solution (1:100; Vector Laboratories, Burlingame, CA, USA) for 1 h. After each incubation, the sections were rinsed for 30 mins with 0.1 mol/L PBS containing 0.3% Triton X-100. The immunoreaction products were visualized with diaminobenzidine (DAB kit; Vector Laboratories). Monochromatic photo images of both sides of the corpus callosum, the caudoputamen traversing fiber bundles bilaterally, and right optic nerve were taken through a microscope with a ×40 objective connected to a digital camera (MetaMorph Image Processing System, Universal Imaging Corp., Downingtown, PA, USA). These images were converted into PICT files by Photoshop (Adobe Systems Inc., San Jose, CA, USA), and the fiber density was analyzed with the NIH image computer program. To account for the variation of the fiber density between right and left sides of the corpus callosum and caudoputamen, fiber densities of both sides were averaged.
For free-floating immunohistochemistry, the rest of the coronal blocks were postfixed for 12 h in 4% paraformaldehyde in 0.1 mol/L PB (pH 7.4), and stored in 20% sucrose in 0.1 mol/L PB (pH 7.4) until used for avidin–biotin–peroxidase complex immunostaining. Endogenous peroxidase was inactivated by immersing the sections in a solution of 0.3% hydrogen peroxide in 10% methanol/0.1 mol/L PBS for 30 mins. To block nonspecific staining, sections were incubated in 5% normal horse serum in 0.1 mol/L PBS containing 0.3% Triton X-100 for 1 h. After blocking, the sections were incubated overnight with the following antibodies (mouse or goat anti-rat) (dilutions in parentheses): against the MHC class II (Ia) antigen (OX 6, 1:100; Serotec, Raleigh, NC, USA), against tumor necrosis factor (TNF) (YC032, 1:800; Yanaihara Institute, Fujinomiya, Shizuoka, Japan), and against E-selectin (5 μg/mL; R&D Systems, Minneapolis, MN, USA). The sections were subsequently incubated with a biotinylated anti-mouse IgG or a biotinylated anti-goat IgG raised in horse (1:200; Vector Laboratories) for 1 h, and then incubated with an avidin-biotin–peroxidase complex solution (1:100; Vector Laboratories) for 1 h. After each incubation other than that for blocking nonspecific staining, the sections were rinsed for 15 mins with 0.1 mol/L PBS containing 0.3% Triton X-100. Finally, the immunoreaction products were visualized with diaminobenzidine (DAB kit; Vector Laboratories). For assessment of nonspecific staining, primary antibodies were replaced with normal mouse or goat IgG. We counted the numerical density of the MHC class II (Ia) antigen immunopositive microglia/macrophages in a 0.075mm2 area in the corpus callosum, and the number of TNF or E-selectin-immunopositive vessels in the total area of the corpus callosum in a stereotactically determined section −2.3mm from bregma. To confirm the cellular source of TNF, double-labeled fluorescent immunohistochemistry was used. After blocking nonspecific staining, the sections were incubated (dilutions in parentheses) overnight with mouse anti-TNF antibody (YC032, 1:200) and rabbit anti-von Willebrand factor antibody (1:2,000; Sigma, St Louis, MO, USA). The sections were subsequently incubated with a Cy3-conjugated anti-mouse IgG and a fluorescein isothiocyanate conjugated anti-rabbit IgG raised in donkey (1:100; Jackson Immuno Research Labs) for 1 h. To evaluate hippocampal damage, 20-μm frozen sections including hippocampus were stained with cresyl violet. See Supplementary information for further details.
Immunoassay
The level of plasma TNF concentration was measured by a Rat TNF US ELISA kit (BioSource International, Camarillo, CA, USA) following the manufacturer’s instructions. Outer diameter values (450 nm) were measured by SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA).
Statistical Analysis
Data are represented as mean±s.d. Differences in mortality rates between groups were determined by Fisher’s exact probability test. Differences in change of ear thickness between groups were determined by unpaired two-tailed Student’s t-test. Differences in proportions of the T-maze spontaneous alternation among each of the three groups were determined by χ2 test. Differences among the groups in the discrimination index of the object recognition test and percentages of correct arm entries in the T-maze left/right discrimination memory retention test were determined by repeated measure analysis of variance (ANOVA) followed by post hoc testing with Fisher’s protected least significant difference procedure (Fisher’s PLSD). Differences in the fiber densities were determined by two-factor ANOVA followed by Fisher’s PLSD post hoc testing. Differences in TNF-immunoreactive vessels, E-selectin-immunoreactive vessels, numerical densities of MHC class II-antigen immunoreactive microglia/macrophages, and in levels of plasma TNF concentration were determined by one-factor ANOVA followed by Fisher’s PLSD post hoc testing. To evaluate the possible effect of optic nerve damage on the object recognition test, a Pearson’s correlation coefficient was calculated between the fiber density of the optic nerve and discrimination index in the E-selectin-treated animals. P < 0.05 was considered significant.
Results
Delayed-Type Hypersensitivity Reaction
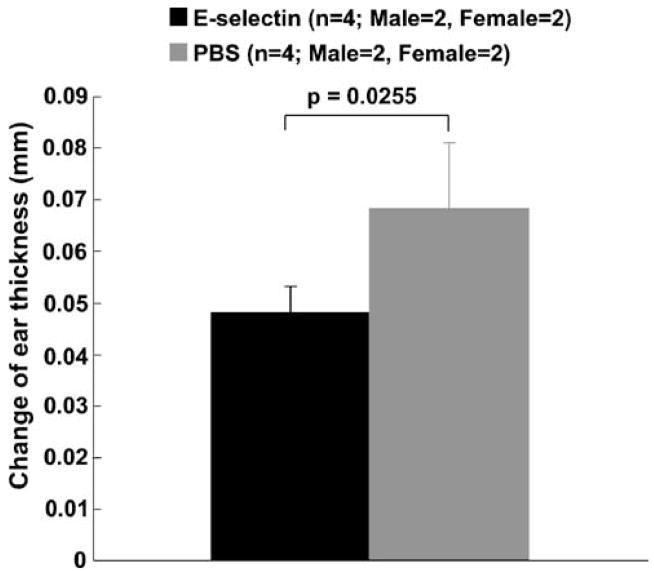
The single E-selectin tolerization schedule significantly suppressed the ear swelling in the delayed-type hypersensitivity suppression study (Figure 2; P = 0.0255). This result conforms to our previous studies (Chen et al, 2003; Takeda et al, 2002) and confirms that immunological tolerance to E-selectin was induced by the intranasal administration of E-selectin.
Figure 2.
DTH reaction in E-selectin-tolerized rats compared with PBS-tolerized rats. E-selectin administration on a single tolerization schedule significantly suppressed ear swelling.
Behavioral Assessment
Object Recognition Test
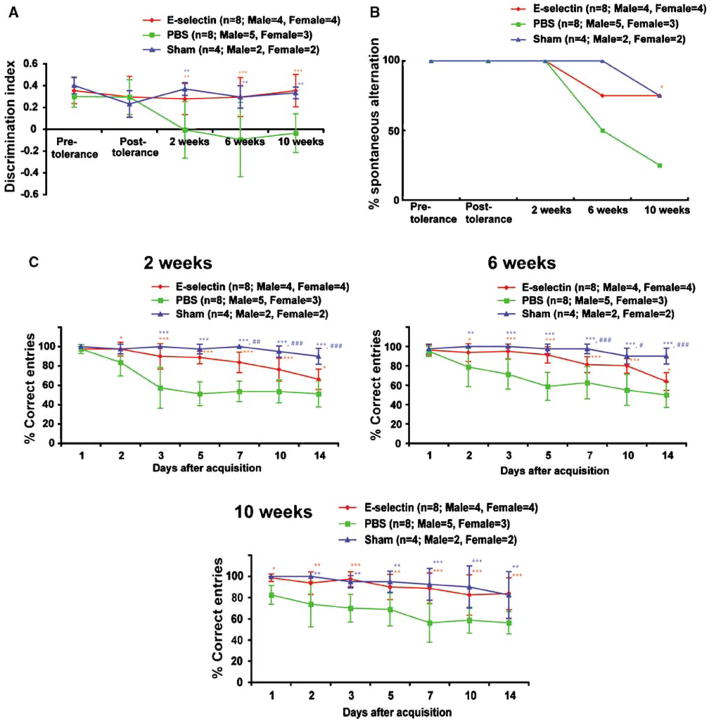
This test evaluates nonspatial working memory related to the frontal subcortical circuits (Ennaceur and Delacour, 1988; Sarti et al, 2002). There were no significant differences in the discrimination index among the E-selectin-tolerized, PBS-treated, and sham-operated groups at baseline (i.e., before surgery). After surgery, the PBS group developed a reduced discrimination index. In contrast, the discrimination indices of the E-selectin and sham groups were maintained at the same baseline levels throughout the experiment. The discrimination indices of the PBS group were significantly decreased as compared with the E-selectin and sham groups (P = 0.0005, P = 0.0059, respectively). There were no significant differences in the discrimination index between the E-selectin and the sham groups (P = 0.7397). Thus, induction and maintenance of mucosal tolerance to E-selectin preserved discrimination ability during protracted forebrain hypoperfusion in contrast to the inability to discriminate between familiar and novel objects noted in the PBS group (Figure 3A).
Figure 3.
Protective effect of mucosal tolerance to E-selectin against behavioral and memory impairments. (A) Graphs of the discrimination index on the object recognition test. The discrimination indices of the E-selectin and sham groups were significantly increased, as compared with the PBS group. *P<0.05, **P<0.01, and P<0.001. (B) Graph of the percent alternation by rats on the T-maze spontaneous alternation test. In the E-selectin-treated animals, the percent of rats that alternated was significantly increased at 10 weeks, as compared with the PBS-treated animals. *P<0.05. (C) Graph of the percentages of correct arm entrance on the T-maze left/right discrimination memory retention test at 2, 6, and 10 weeks after surgery. The percentages of correct arm entries of the E-selectin and sham-operated groups were significantly increased, as compared with the PBS group. Sham or E-selectin versus PBS: *P<0.05, **P<0.01, and P<0.001. Sham versus E-selectin: #P<0.05, ##P<0.01, ###P<0.001.
T-Maze Spontaneous Alternation
This test evaluates spatial working memory related to the frontal subcortical circuits (Sarti et al, 2002). Spontaneous alternation refers to the instinctive behavioral tendency by which rats typically alternate their choices between the arms of the T-maze more often than they repeat their initial choice. There were no significant differences in the percentages of rats showing T-maze spontaneous alternation among the E-selectin, PBS, and sham groups before surgery and at 2 and 6 weeks after surgery. However, by 10 weeks after surgery, the percentage of rats that spontaneously alternated in the PBS group was significantly decreased compared with the E-selectin group (PBS, 25.0%; E-selectin, 75.0%; P = 0.0455). Thus, mucosal tolerization to E-selectin protected against the loss of T-maze spontaneous alternation observed in PBS tolerized rats during protracted forebrain ischemia (Figure 3B).
T-Maze Left/Right Discrimination Memory Retention
This test evaluates spatial and nonspatial reference memory related to the hippocampus and caudoputamen (Oliveira et al, 1997).
Two Weeks after Surgery
The numbers of correct arm entries did not differ among the E-selectin, PBS, and sham groups tested 1 day after the training session. The percentages of correct arm entries were greater than 95%. In the PBS group, the number of correct arm entries decreased over time, and the percentage of correct entries between 3 and 14 days after the training session were diminished to 50 to 60%, which is close to a random choice level. This suggests that animals in this group had lost their left/right discrimination memory. The decrease in the number of correct arm entries was less prominent in the E-selectin group, and the rats tolerized with E-selectin had a higher number of correct entries than the PBS-treated animals by repeated measure ANOVA (PBS: 57.50±21.21% at 3 days and 51.25±13.56% at 14 days; corresponding values for E-selectin: 90.00± 13.09%, 66.25±10.61%; P < 0.0001). In contrast with the PBS and E-selectin groups, the sham group animals retained their left/right discrimination memory at the same level throughout the experiment. By repeated measure ANOVA, the differences between the sham and PBS groups and the sham and E-selectin groups were statistically significant (P < 0.0001 and P = 0.0040, respectively) (Figure 3C).
Six Weeks after Surgery
Similar results to those at 2 weeks after surgery were observed. The E-selectin group had a higher number of correct entries than the PBS group by repeated measure ANOVA (PBS: 71.25±15.53% at 3 days and 50.00±13.09% at 14 days; corresponding values for E-selectin: 95.00±7.56%, 63.75±9.16%; P = 0.008). The differences between the sham and PBS groups and between the sham and E-selectin groups were statistically significant (P < 0.0001; P = 0.0030, respectively) by repeated measure ANOVA (Figure 3C).
Ten Weeks after Surgery
In contrast with 2 and 6 weeks after surgery, both the sham and the E-selectin groups retained their left/right discrimination memory throughout the experiment 10 weeks after surgery, and there were no significant differences in the number of correct entries between these two groups by repeated measure ANOVA (P = 0.6256). The E-selectin and sham groups both had a higher number of correct entries than the PBS group by repeated measure ANOVA (PBS: 70.00±13.09% at 3 days and 56.25± 10.61% at 14 days; corresponding values for E-selectin: 97.50±7.07%, 83.75±15.06%; corresponding values for sham: 95.00±5.77%, 82.50± 22.17%; P = 0.0003; P = 0.0026, respectively) (Figure 3C). Thus, maintenance of mucosal tolerance to E-selectin provided for a delayed recovery of the spatial and nonspatial reference memory in the left/right discrimination task under conditions of permanent bilateral common carotid artery occlusion.
Histopathology
In the sham-operated animals, there was no detectable rarefaction in the white matter. Rarefaction of the white matter was clearly observed in the corpus callosum, in fiber bundles traversing the caudoputamen and in the optic nerve of the PBS-treated rats. The severity of the rarefaction was markedly attenuated in the animals’ tolerized with E-selectin (Figures 4A and 4B). The fiber densities in the E-selectin-tolerized animals were significantly higher than those in the PBS-treated group by two-factor ANOVA and post hoc testing (corpus callosum; P = 0.0063, caudoputamen; P = 0.0440, optic nerve; P < 0.0001; Figure 4C). In contrast, there was no significant difference in the fiber densities between sham and E-selectin groups (P = 0.2026) (Figure 4C). Thus, E-selectin mucosal tolerization had a protective effect against white matter rarefaction induced by protracted hypoperfusion.
Histopathological lesions in the optic nerve and hippocampus did not appear to influence the behavioral assessments. The Pearson’s correlation coefficient between the fiber density of the optic nerve and the discrimination index in the E-selectin-treated animals was −0.470. This correlation was not significantly different from 0 (P = 0.2537). A few dark neurons were detected in the unilateral hippocampus of the three E-selectin-treated (27.3%), three PBS-treated animals (27.3%), and one sham-operated animal (25%). There are no obvious differences in the number of the dark neurons per animal among three groups (data not shown).
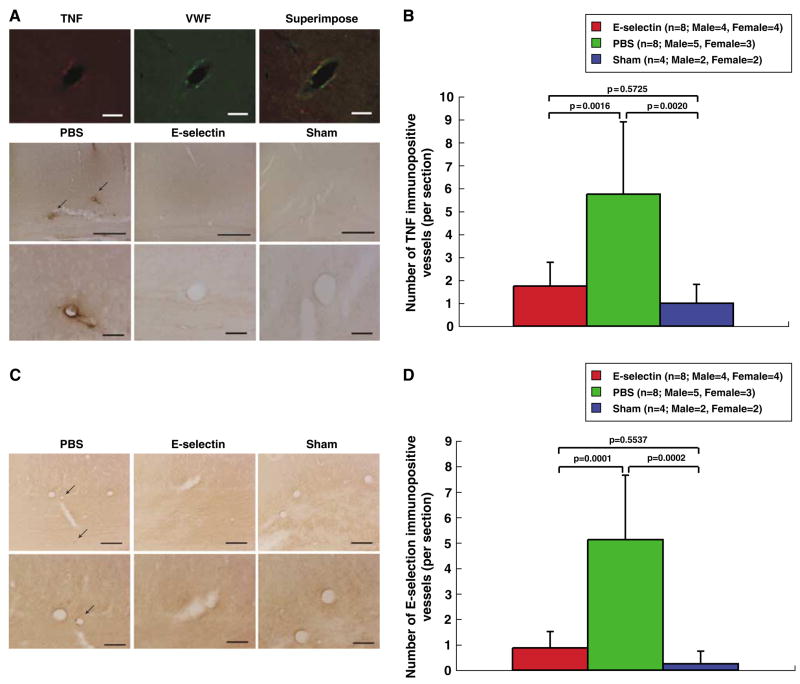
In the white matter of the sham-operated animals, there was positive immunostaining for the MHC class II (Ia) antigen in only a few glial cells. The number of microglia/macrophages that were immunolabeled for theMHC class II (Ia) antigen preferentially in white matter of the corpus callosum and caudoputamen of PBS-treated animals averaged 8.56±7.21 per 0.075mm2; corresponding microglia/macrophage counts in E-selectin-tolerized rats averaged 4.18± 3.53 per 0.075mm2; P = 0.1164. Tumor necrosis factor was expressed in and around endothelial cells of vessels in the brains of the PBS-treated animals (5.75 ±3.15 vessels/section) in accord with our previous observations of activated blood vessels (Ruetzler et al, 2001). This perivascular TNF was prominently expressed in white matter vessels. There were far fewer vessels showing perivascular TNF immunoreactivity in sham-operated animals (1.00±0.82 vessels/section) and similar attenuation in E-selectin- tolerized animals subjected to bilateral permanent common carotid occlusion (1.75±1.04 vessels/section) compared with PBS-treated animals (Figures 5A and 5B). Tumor necrosis factor-immunoreactive vessels were decreased significantly (P = 0.0016) in the E-selectin-tolerized group compared with the PBS-treated group; there was no difference between the number of TNF-immunoreactive vessels per section between the sham and E-selectin groups (P = 0.5725) (Figure 5B). Interestingly, local TNF suppression in brain vessels of E-selectin-tolerized animals was not reflected systemically. Plasma TNF was not decreased (15.42±4.98, 13.81±1.88, 9.87±2.53 pg/ml, ANOVA P = 0.0646, in PBS, E-selectin, and sham groups, respectively) suggesting local TNF immunomodulation in a setting of undiminished systemic TNF production. E-selectin was expressed on endothelial cells of vessels in the brains of the PBS-treated animals (5.13±2.53 per section). The number of E-selectin-immunoreactive vessels was decreased in the E-selectin-tolerized group (0.88± 0.64 per section) as compared with the PBS-treated group (P = 0.0001) (Figures 5C and 5D). In contrast, there were no significant differences in the number of E-selectin-immunoreactive vessels between sham-operated and the E-selectin-tolerized groups (P= 0.5537) (Figure 5D). Thus, induction of mucosal tolerance to E-selectin had a suppressive effect on several indices of vessel activation in forebrain white matter under conditions of protracted hypoperfusion.
Figure 5.
Suppression of vessel activation and local TNF production by mucosal tolerance to E-selectin. (A) Photomicrographs of double-labeled fluorescent immunohistochemistry with anti-TNF and anti-von Willebrand factor antibodies in corpus callosum blood vessels. Immunoreactive TNF was expressed in and around endothelial cells. In the E-selectin-tolerized and sham-operated animals, TNF-immunopositive vessels were markedly less prominent and less frequent than in PBS-treated animals. Scale bars, 50 μm (top); 300 μm (middle), and 50 μm (bottom). (B) Histograms of the numerical density of TNF-immunopositive vessels in the corpus callosum. In the E-selectin-treated and sham-operated animals, the number of TNF-immunopositive vessels was significantly reduced, as compared with the PBS-treated animals. (C) Photomicrographs of the immunohistochemical staining for E-selectin in the corpus callosum. In the E-selectin-treated and sham-operated animals, E-selectin-immunopositive vessels were less prominent and less frequent compared with the PBS-treated animals. Scale bars, 100 μm (top) and 50 μm (bottom). (D) Histograms of the numerical density of E-selectin-immunopositive vessels in the corpus callosum. In the E-selectin- and sham-operated animals, the number of E-selectin-immunopositive vessels was significantly reduced compared with the PBS-treated animals.
Mortality Rates
None of the sham-operated animals died. Of the 11 animals that received E-selectin, two animals (18.2%) died within 7 days after surgery; one more animal (9.1%) succumbed to an anesthetic death during nasal instillation of E-selectin at 9 weeks after surgery. Of the 11 animals that received PBS, three animals (27.3%) died within 7 days after surgery.
Discussion
In this study, we have showed that mucosal tolerance to E-selectin robustly attenuates histological white matter damage and decrements in behavioral assessments that otherwise develop during protracted cerebral hypoperfusion in this VCI model.
It should be pointed out that although the presence of boundary zones and watershed areas between vascular beds can provide a plausible explanation for the location of white matter lesions in cerebral hypoperfusion states that lead to subcortical vascular dementia (Roman, 2004), and there is evidence that chronic hypoperfusion can lead to oligodendroglial apoptosis (Tomimoto et al, 2003), the apparent predilection for white matter damage observed in the present VCI model could have a contribution from gray matter neuron damage as well. In older (9 to 10 months) Sprague–Dawley rats subjected to bilateral common carotid artery occlusion, CA1 hippocampal damage has been reported (Pappas et al, 1996). In contrast, we and others have observed the presence in younger (9 to 12 weeks) Wistar rats of scattered dark neurons in the hippocampus with no clear difference in incidence between animals that have undergone bilateral carotid occlusion and sham-operated controls. Since axonal loss in white matter areas of a cerebral hemisphere can reflect damage to axonal projections from dying neurons that are located both ipsi-laterally and, with commissural connections, contralaterally, the effect of patchy neuronal loss in states of bilateral cerebral hypoperfusion could amplify the appearance of damage in white matter. We cannot, therefore, attribute the beneficial results of this study solely to white matter protection. The key point, however, is that regardless of the relative contributions of white matter and gray matter damage, mucosal tolerization to E-selectin conferred robust protection to the chronically hypoperfused forebrain as manifested by minimization of both functional deficits in memory and detectible histopathology.
The exact mechanisms underlying these protective effects have not been fully elucidated. We have shown previously in an arterial occlusion model that E-selectin tolerization has no effect on residual brain blood flow levels compared with those of PBS-tolerized animals (Chen et al, 2003), so the initial blood flow reduction in the two groups subjected to bilateral common carotid artery occlusion can be considered comparable. Multiple lines of evidence have suggested that the mucosal tolerance to E-selectin induces regulatory T cells and suppresses the activated vessel segments by immunomodulation (Chen et al, 2003; Takeda et al, 2002). The evidence (from E-selectin-tolerized animals) for regulatory T-cell induction includes regulatory T-cell suppression of T-helper type 1 cell-induced-delayed type hypersensitivity, increased transforming growth factor-β1-positive splenocytes, increased interleukin (IL)-10 expression in splenocyte cultures, and showing that adoptive transfer of splenocytes from E-selectin-tolerized donor animals into naïve recipients gave a level of protection from ischemic brain damage that was equivalent to that of animals directly tolerized to E-selectin.
Locally, endothelium integrates and responds to multiple extracellular signals from blood, blood vessel wall, and surrounding parenchymal cells along the vascular tree; local hemostatic potential in each vascular segment fluctuates in phase with the local endothelium as it cycles through activation and inactivation in response to these signals (Rosenberg and Aird, 1999). There is an interplay between local activation of endothelium and systemic activity of the coagulation or complement systems; the coincidence of a systemic inflammatory or procoagulant state and local activation of endothelium can produce focal thrombosis (e.g., local Shwartzman reaction (Hallenbeck et al, 1988)). In this study, TNF- and E-selectin-immunoreactive vessels were increased in the PBS groups, as compared with the sham-operated group even 90 days after surgery. Tumor necrosis factor expressed by vascular and perivascular cells has proinflammatory and procoagulant effects on endothelium (Hallenbeck, 2002; Pober and Cotran, 1990). Since the expression of E-selectin is induced in response to TNF, these findings suggest that endothelial activation and induction of E-selectin expression occurs over a prolonged period under conditions of protracted hypoperfusion. Although the observed attenuation of local TNF and E-selectin in the hypoperfused brains of E-selectin-tolerized animals indicates that local vessel activation was reduced in these animals, the association with reduced white matter injury and preserved function on behavioral tests is correlational and does not establish a causal relationship.
Mucosal tolerance can be achieved through induction of regulatory T cells (Faria and Weiner, 2005) by repetitive administration of low-dose antigen (Chen et al, 1994). Lymphocytes that are tolerized to an antigen and have become antigen-specific regulatory T cells tend to migrate to the locale of the protein molecule to which they have been primed. During endothelial activation in brain ischemia, E-selectin-specific regulatory T cells can be restimulated to produce immunosuppressive cytokines by presentation of E-selectin by local antigen-presenting cells (perivascular macrophages, pericytes, microglia, and dendritic cells) (Balabanov et al, 1999). Lymphocyte restimulation can also occur in deep cervical lymph nodes where both soluble E-selectin, which has been noted to increase in large vessel disease and subcortical vascular encephalopathy (Fassbender et al, 1999), and dendritic cells that have processed E-selectin from ischemic brain accumulate (Karman et al, 2004). In the local draining lymph nodes, antigen presentation can be accompanied by signals that permit homing of activated T cells to brain areas expressing the specific antigen (Karman et al, 2004). In those areas, the regulatory T cells release immunomodulatory cytokines such as TGF-β and IL-10 that counteract the effect of proinflammatory cytokines (including TNF) and suppress inflammation and immune responses after ischemia (Hallenbeck et al, 2005). Since local release of immunological and inflammatory mediators during protracted forebrain hypoperfusion contributes to local vessel activation, local immunosuppression targeted to activating blood vessel segments could protect against development of progressive impairment of microcirculatory perfusion (Dawson et al, 1997). Thus, mucosal tolerance to E-selectin appears to function by suppressing local vessel activation and the surrounding immunological and inflammatory processes.
Other mechanisms may also contribute to the protective effects observed in this study. White matter injury involves glial cells, which are abundant in white matter (Goldberg and Ransom, 2003). Microglial activation with expression of MHC class II antigens has been detected preferentially in the white matter (Farkas et al, 2004; Wakita et al, 1994), and pharmacological suppression of these activated microglia has resulted in an attenuation of the white matter lesions in this model (Wakita et al, 1995). Since TGF-β (Suzumura et al, 1993) and IL-10 (Frei et al, 1994) inhibit the activation of microglia, mucosal tolerization to E-selectin can suppress activated microglia through local production of these cytokines by the regulatory T cells. The number of MHC class II positive-activated microglia/macrophages in the white matter showed a trend toward suppression in the E-selectin group as compared with the PBS group. Activated microglia enhance a variety of inflammatory responses (Gehrmann et al, 1995). Microglia are the major source of proinflammatory cytokines including IL-1 and TNF. Suppression of the microglial activation may inhibit both the expression of E-selectin and other forms of local vessel activation. Activated microglia also release an array of cytotoxic substances that include other proinflammatory cytokines, prostanoids, proteases, reactive oxygen radicals, and nitrogen intermediates. The protective effect of mucosal tolerization to E-selectin may be mediated by suppressing the release of these cytotoxic substances as well. The net effect of these protective E-selectin tolerization mechanisms is to decrease inflammation and to preserve vessel integrity in the hypoperfused brain.
A number of axonal mechanisms of ischemic white matter damage have been clearly showed by extensive studies using rodent optic nerve preparations (Goldberg and Ransom, 2003; Stys, 2004). These data show that disruption of continuous oxygen and glucose delivery (at adequate levels) leads to axonal dysfunction by activation of voltage-dependent channels for sodium and calcium that cause intraaxonal calcium accumulation and activation of calcium-dependent disruptive pathways. Glutamate acting on amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors contributes to this progression of axonal damage. Studies show that the proinflammatory cytokines, TNF and IL-1, can induce an increase in neuronal calcium entry, glutamate release, and AMPA-type glutamate receptor trafficking (Bezzi et al, 2001; Campbell et al, 1998; Leonoudakis et al, 2004; Ogoshi et al, 2005). Further study would be necessary, however, to show that suppression of such proinflammatory cytokine effects is an additional E-selectin tolerization mechanism for the observed white matter cytoprotection.
Since the severity of the damage in the optic nerve was attenuated in the E-selectin-tolerized group as compared with the PBS-treated group, the potential effect of differential visual acuity on behavioral assessments such as the object recognition test needs to be discussed. In this study, the intraindividual discrimination ability preserved by E-selectin tolerization did not correlate with the intraindividual optic nerve fiber density. A possible explanation for this apparent discrepancy is that humans normally base their choices primarily on their memory of visual properties of the sample object. In contrast, when rats explore an object, they sniff it, brush it with vibrissae, and look at it. In rodents, differential exploration of familiar objects and novel objects reflects to some extent their memory for olfactory and tactile properties of the sample object, although visual properties may also be remembered and contribute to discrimination (Mumby, 2001).
The delayed appearance of a protective effect on T-maze left/right discrimination memory conferred by E-selectin tolerization when tested at the 10-week postsurgery time points needs to be addressed. The E-selectin-tolerized group did not display retention of their left/right discrimination memory during the 2- and 6-week study periods after bilateral common carotid artery surgery in contrast with the group’s 10-week postsurgery performance. The left/right discrimination memory impairment noted in E-selectin-tolerized animals during the early postsurgery testing periods is probably to have been caused by decreased levels of residual forebrain blood flow with a cerebral blood flow metabolism mismatch that degraded function, but remained above the threshold for production of permanent cortical white matter damage. Cerebral blood flow in this model remains decreased over a prolonged period and gradually recovers to approach control levels by 8 weeks (Otori et al, 2003). On recovery of cerebral blood flow metabolism coupling, the undamaged brain regions could resume normal function.
In conclusion, this study shows a clear protective effect of mucosal tolerance to E-selectin against memory impairment and white matter (and possibly gray matter) damage during protracted cerebral hypoperfusion. These results support the potential for mucosal tolerization to E-selectin to attenuate progression of subcortical ischemic VCI in at-risk patients.
Supplementary Material
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm).
Acknowledgments
We are indebted to Dr Ronen R Leker (Laboratory of Molecular Biology, National Institute of Neurological Disorders and Stroke, National Institutes of Health) for supplying the T-maze apparatus and helpful advice. We thank Dr Esther Shohami (Department of Pharmacology, Hebrew University of Jerusalem) for helpful advice and Dr Toshiyuki Nakayama for assistance in the induction of tolerance.
This work was supported by the intramural program of NINDS, NIH.
References
- Balabanov R, Beaumont T, Dore-Duffy P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J Neurosci Res. 1999;55:578–87. doi: 10.1002/(SICI)1097-4547(19990301)55:5<578::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–5. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Campbell VA, Segurado R, Lynch MA. Regulation of intracellular Ca2+ concentration by interleukin-1beta in rat cortical synaptosomes: an age-related study. Neurobiol Aging. 1998;19:575–9. doi: 10.1016/s0197-4580(98)00097-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ruetzler C, Pandipati S, Spatz M, McCarron RM, Becker K, Hallenbeck JM. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci USA. 2003;100:15107–12. doi: 10.1073/pnas.2436538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Ruetzler CA, Hallenbeck JM. Temporal impairment of microcirculatory perfusion following focal cerebral ischemia in the spontaneously hypertensive rat. Brain Res. 1997;749:200–8. doi: 10.1016/S0006-8993(96)01166-3. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Donka G, de Vos RA, Mihaly A, Bari F, Luiten PG. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol (Berl) 2004;108:57–64. doi: 10.1007/s00401-004-0864-9. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Bertsch T, Mielke O, Muhlhauser F, Hennerici M. Adhesion molecules in cerebrovascular diseases. Evidence for an inflammatory endothelial activation in cerebral large- and small-vessel disease. Stroke. 1999;30:1647–50. doi: 10.1161/01.str.30.8.1647. [DOI] [PubMed] [Google Scholar]
- Frei K, Lins H, Schwerdel C, Fontana A. Antigen presentation in the central nervous system. The inhibitory effect of IL-10 on MHC class II expression and production of cytokines depends on the inducing signals and the type of cell analyzed. J Immunol. 1994;152:2720–8. [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–87. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Ransom BR. New light on white matter. Stroke. 2003;34:330–2. doi: 10.1161/01.str.0000054048.22626.b9. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–3. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM, Dutka AJ, Kochanek PM, Siren A, Pezeshkpour GH, Feuerstein G. Stroke risk factors prepare rat brain stem tissues for modified local Shwartzman reaction. Stroke. 1988;19:863–9. doi: 10.1161/01.str.19.7.863. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM, Hansson GK, Becker KJ. Immunology of ischemic vascular disease: plaque to attack. Trends Immunol. 2005;26:550–6. doi: 10.1016/j.it.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–8. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Karman J, Ling C, Sandor M, Fabry Z. Dendritic cells in the initiation of immune responses against central nervous system-derived antigens. Immunol Lett. 2004;92:107–15. doi: 10.1016/j.imlet.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Braithwaite SP, Beattie MS, Beattie EC. TNFalpha-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity? Neuron Glia Biol. 2004;1:263–73. doi: 10.1017/S1740925X05000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991;174:791–8. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Ogoshi F, Yin HZ, Kuppumbatti Y, Song B, Amindari S, Weiss JH. Tumor necrosis-factor-alpha (TNFalpha) induces rapid insertion of Ca2+-permeable alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Exp Neurol. 2005;193:384–393. doi: 10.1016/j.expneurol.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Oliveira MG, Bueno OF, Pomarico AC, Gugliano EB. Strategies used by hippocampal- and caudate-putamen-lesioned rats in a learning task. Neurobiol Learn Mem. 1997;68:32–41. doi: 10.1006/nlme.1996.3761. [DOI] [PubMed] [Google Scholar]
- Otori T, Katsumata T, Muramatsu H, Kashiwagi F, Katayama Y, Terashi A. Long-term measurement of cerebral blood flow and metabolism in a rat chronic hypoperfusion model. Clin Exp Pharmacol Physiol. 2003;30:266–72. doi: 10.1046/j.1440-1681.2003.03825.x. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Leys D, Fazekas F, Longstreth WT, Jr, Inzitari D, Wallin A, Filippi M, Scheltens P, Erkinjuntti T, Hachinski V. Role of white matter lesions in cognitive impairment of vascular origin. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S49–54. [PubMed] [Google Scholar]
- Pappas BA, de la Torre JC, Davidson CM, Keyes MT, Fortin T. Chronic reduction of cerebral blood flow in the adult rat: late-emerging CA1 cell loss and memory dysfunction. Brain Res. 1996;708:50–8. doi: 10.1016/0006-8993(95)01267-2. [DOI] [PubMed] [Google Scholar]
- Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–51. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–36. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Roman GC. Brain hypoperfusion: a critical factor in vascular dementia. Neurol Res. 2004;26:454–8. doi: 10.1179/016164104225017686. [DOI] [PubMed] [Google Scholar]
- Rosenberg RD, Aird WC. Vascular-bed-specific hemostasis and hypercoagulable states. New Engl J Med. 1999;340:1555–64. doi: 10.1056/NEJM199905203402007. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J, Dorman ME, Griffin JW, Gold BG, Sternberger LA, Sternberger NH, Price DL. Distribution of neurofilament antigens after axonal injury. J Neuropathol Exp Neurol. 1987;46:269–82. doi: 10.1097/00005072-198705000-00004. [DOI] [PubMed] [Google Scholar]
- Ruetzler CA, Furuya K, Takeda H, Hallenbeck JM. Brain vessels normally undergo cyclic activation and inactivation: evidence from tumor necrosis factor-alpha, heme oxygenase-1, and manganese superoxide dismutase immunostaining of vessels and perivascular brain cells. J Cereb Blood Flow Metab. 2001;21:244–52. doi: 10.1097/00004647-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Sarti C, Pantoni L, Bartolini L, Inzitari D. Persistent impairment of gait performances and working memory after bilateral common carotid artery occlusion in the adult Wistar rat. Behav Brain Res. 2002;136:13–20. doi: 10.1016/s0166-4328(02)00090-6. [DOI] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–30. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Suenaga T, Ohnishi K, Nishimura M, Nakamura S, Akiguchi I, Kimura J. Bundles of amyloid precursor protein-immunoreactive axons in human cerebrovascular white matter lesions. Acta Neuropathol (Berl) 1994;87:450–5. doi: 10.1007/BF00294171. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J Immunol. 1993;151:2150–8. [PubMed] [Google Scholar]
- Takeda H, Spatz M, Ruetzler C, McCarron R, Becker K, Hallenbeck J. Induction of mucosal tolerance to E-selectin prevents ischemic and hemorrhagic stroke in spontaneously hypertensive genetically stroke-prone rats. Stroke. 2002;33:2156–63. doi: 10.1161/01.str.0000029821.82531.8b. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Ihara M, Wakita H, Ohtani R, Lin JX, Akiguchi I, Kinoshita M, Shibasaki H. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol (Berl) 2003;106:527–34. doi: 10.1007/s00401-003-0749-3. [DOI] [PubMed] [Google Scholar]
- Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol (Berl) 1994;87:484–92. doi: 10.1007/BF00294175. [DOI] [PubMed] [Google Scholar]
- Wakita H, Tomimoto H, Akiguchi I, Kimura J. Protective effect of cyclosporin A on white matter changes in the rat brain after chronic cerebral hypoperfusion. Stroke. 1995;26:1415–22. doi: 10.1161/01.str.26.8.1415. [DOI] [PubMed] [Google Scholar]
- Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M, McGeer PL. Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res. 2002;924:63–70. doi: 10.1016/s0006-8993(01)03223-1. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Phillips ML, Rosenbloom CL, Cruz R, Manning A. E-selectin in focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 1996;16:1126–36. doi: 10.1097/00004647-199611000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm).