Summary
The amygdala plays an important role in the emotional-affective component of pain and in pain modulation. Group III metabotropic glutamate receptors (mGluRs) regulate pain-related activity in the amygdala, but the behavioral consequence and contribution of individual subtypes are not known yet. The present study determined the effects of mGluR7 and mGluR8 activation in the central nucleus of the amygdala (CeA) on nocifensive and affective pain responses and on pain-related anxiety-like behavior of adult rats. The pain state was induced by intraarticular injections of kaolin/carrageenan into one knee joint to produce a localized monoarthritis. Subtype-selective agonists were administered into the CeA by microdialysis in normal rats and in rats with arthritis. An mGluR7-selective agonist (N,NI-dibenzyhydryl-ethane-1,2-diamine dihydrochloride, AMN082, 25 µM) decreased spinal withdrawal reflex thresholds and increased audible and ultrasonic vocalizations evoked by brief (15 s) compression of the knee. AMN082 also decreased the open-arm preference in the elevated plus maze (EPM) test, suggesting anxiety-like behavior. In arthritic animals, however, AMN082 failed to modulate the increased spinal reflexes and vocalizations and anxiety-like behavior. An mGluR8-selective agonist (S-3,4-dicarboxyphenylglycine, S-3,4-DCPG, 10 µM) had no effect in normal animals but inhibited the increased spinal reflex responses and audible and ultrasonic vocalizations of arthritic rats. S-3,4-DCPG also increased the open-arm choices of arthritic rats, suggesting anxiolytic effects. The results suggest that under normal conditions mGluR7, but not mGluR8, facilitates pain responses and has anxiogenic properties whereas mGluR8, but not mGluR7, can inhibit nocifensive and affective behaviors and anxiety in a model of arthritic pain.
Keywords: amygdale, arthritis, pain, vocalization, anxiety, mGluR, microdialysis
1. Introduction
Pain carries a negative affective valence and is closely linked to anxiety and depression (Gallagher and Verma, 2004; Rhudy and Meagher, 2003; Rome and Rome, 2000). The amygdala is now recognized as an important neural substrate for the emotional-affective dimension of pain (Ji et al., 2007; Kulkarni et al., 2007; Neugebauer et al., 2004; Pedersen et al., 2007; Rhudy and Meagher, 2001). The amygdala is composed of several distinct nuclei. The laterocapsular division of the central nucleus (CeLC) has been termed “nociceptive amygdala” because it is the target of the spino-parabrachio-amygdaloid pain pathway and most CeLC neurons process pain-related information (Gauriau and Bernard, 2002; Hunt and Mantyh, 2001; Neugebauer et al., 2004).
Our previous studies demonstrated central sensitization (Han et al., 2005b; Ji and Neugebauer, 2007; Li and Neugebauer, 2006; Neugebauer and Li, 2003) and synaptic plasticity (Bird et al., 2005; Fu and Neugebauer, 2008; Neugebauer et al., 2003) in the CeLC in arthritic pain. Synaptic plasticity in the CeLC was also shown in chronic neuropathic pain and correlated positively with pain behavior (Ikeda et al., 2007). Conversely, deactivation of the amygdala decreased pain responses in models of arthritic (Fu and Neugebauer, 2008; Han et al., 2005b; Han and Neugebauer, 2005; Ji et al., 2007), visceral (Tanimoto et al., 2003) and neuropathic pain (Pedersen et al., 2007) and in the prolonged phase of the formalin test (Carrasquillo and Gereau, 2007).
Pain-related changes in the amygdala are regulated by metabotropic glutamate receptors (mGluRs; see Neugebauer, 2007a). Eight mGluR subtypes have been cloned and are classified into groups I (mGluRs 1 and 5), II (mGluRs 2 and 3) and III (mGluRs 4, 6, 7 and 8) based on sequence homology, pharmacology and signal transduction mechanisms (Neugebauer, 2007a; Schoepp et al., 1999). A group III agonist (LAP4) inhibited the increased responses of sensitized CeLC neurons in the arthritis pain model (Li and Neugebauer, 2006) through a mechanism that involved presynaptic inhibition of glutamatergic transmission (Han et al., 2004). A group III antagonist increased the responses of sensitized CeLC neurons in arthritis (Li and Neugebauer, 2006), suggesting that group III mGluRs in the amygdala are activated endogenously in this pain model. The contribution of individual mGluR subtypes and their effects on pain behavior remain to be determined.
Group III mGluRs have also emerged as novel targets for anxiety disorders (Neugebauer, 2008; Swanson et al., 2005) since the central administration of group III agonists produced anxiolytic-like effects (Palucha et al., 2004). However, studies using mGluR7 (Cryan et al., 2003; Masugi et al., 1999) and mGluR8 (Duvoisin et al., 2005; Linden et al., 2002) knockout mice and subtype-selective agonists for mGluR7 (N,NI-dibenzyhydryl-ethane-1,2-diamine dihydrochloride, AMN082) (Mitsukawa et al., 2005) and mGluR8 (S-3,4-dicarboxyphenylglycine, S-3,4-DCPG) (Schmid and Fendt, 2006) suggest that mGluR7 and mGluR8 have opposing anxiogenic and anxiolytic functions, respectively.
A recent study also provided evidence for divergent roles of mGluR7 and mGluR8 in pain modulation (Marabese et al., 2007). In the brainstem S-3,4-DCPG and AMN082 had anti- and pro-nociceptive effects, respectively, through differential effects on neuronal responses and transmitter release. The present study sought to determine if mGluR7 and mGluR8 in the amygdala also have opposing pain-related functions.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (250–300 g) were housed in a temperature controlled room and maintained on a 12 h day/night cycle. Water and food were available ad libitum. Behavioral data were obtained from untreated normal rats and rats with a localized arthritis in one knee (5–6 h after induction). All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch (UTMB) and conformed to the guidelines of the International Association for the Study of Pain (IASP) and of the National Institutes of Health (NIH).
2.2. Arthritis pain model
An experimental monoarthritis was induced in the left knee joint of adult rats as described in detail previously (Neugebauer et al., 2007). A kaolin suspension (4%, 80–100 µl) was injected into the joint cavity through the patellar ligament with a syringe (1 ml) and needle (25 G5/8). After repetitive flexions and extensions of the knee for 15 min, a carrageenan solution (2%, 80–100 µl) was injected into the knee joint cavity, and the leg was flexed and extended for another 5 min. This treatment paradigm reliably leads to inflammation of the knee within 1–3 h, which reaches a maximum plateau at 5–6 h, and persists for days (Neugebauer et al., 2007). Therefore, behavior measurements of arthritis pain-related changes were made at the 5–6 h time point (plateau phase).
2.3. Experimental protocol
On day 1, a guide cannula for drug (or ACSF vehicle) application by microdialysis was stereotaxically inserted into the CeA. On day 2 behavior measurements (spinal withdrawal reflexes, audible and ultrasonic vocalizations, and anxiety-like behavior in the elevated plus maze test) were measured before and during (15–20 min) drug administration (or ACSF vehicle control). Animals tested on day 2 were either normal (no arthritis) or arthritic (5–6 h postinduction).
2.4. Microdialysis and drug administration
As described in detail previously (Fu and Neugebauer, 2008; Han et al., 2005b; Han and Neugebauer, 2005; Ji et al., 2007) rats were anaesthetized with pentobarbital sodium (50 mg/kg, i.p.) on day 1. A small hole was drilled into the parietal bone on the right, i.e., contralateral to the left knee joint in which the arthritis would be induced later. The rationale for drug administration into the right CeA was that anatomical and electrophysiological data suggest a strong contralateral projection of the spino-parabrachio-amygdaloid pain pathway (Gauriau and Bernard, 2002; Neugebauer et al., 2004). Our previous studies showed significant behavioral effects of drug administration into the right CeA (Fu and Neugebauer, 2008; Han et al., 2005b; Han and Neugebauer, 2005; Ji et al., 2007). A guide cannula was implanted stereotaxically (David Kopf Instruments), on the dorsal margin of the CeA, using the following coordinates (Paxinos and Watson, 1998): 2.3 mm caudal to bregma, 4.0 mm lateral to midline, 7.0 mm depth. The cannula was fixed to the skull with dental acrylic (Plastics One, Roanoke, VA). Antibiotic ointment was applied to the exposed tissue to prevent infection.
On the day of the behavioral experiment (day 2), a microdialysis probe (CMA/11; membrane diameter, 250 µm; membrane length, 1 mm; 6 kD cut-off; CMA/Microdialysis, Solna, Sweden) was inserted into the CeA through the guide cannula so that the probe protruded by 1 mm. Using polyethylene-50 tubing, the probe was connected to a Harvard infusion pump (Harvard Apparatus, Holliston, MA) and perfused with ACSF (2 µl/min) containing the following (in mM): 125.0 NaCl, 2.6 KCl, 2.5 NaH2PO4, 1.3 CaCl2, 0.9 MgCl2, 21.0 NaHCO3, and 3.5 glucose, oxygenated and equilibrated to pH 7.4. The use of microdialysis for drug administration into the amygdala has been described in detail previously (Fu and Neugebauer, 2008; Han et al., 2005b; Han and Neugebauer, 2005; Ji et al., 2007). Behavior was measured before (predrug) and at 15–20 min during continued drug administration.
2.5. Drugs
The following selective receptor agonists were used: N,NI-dibenzyhydryl-ethane-1,2-diamine dihydrochloride (AMN082; mGluR7) and (S)-3,4-dicarboxyphenylglycine (S-3,4-DCPG; mGluR8) both purchased from Tocris Bioscience (Ellisville, MO). AMN082 and S-3,4-DCPG were dissolved in ACSF (vehicle) on the day of the experiment at a concentration 100 times that predicted to be needed in the tissue based on data in the literature (Marabese et al., 2007; Mitsukawa et al., 2005; Thomas et al., 2001) and our own in vitro studies in brain slices (Palazzo and Neugebauer, unpublished observations) because of the concentration gradient across the dialysis membrane (Fu and Neugebauer, 2008; Han et al., 2005b; Han and Neugebauer, 2005; Ji et al., 2007). The numbers given in this article refer to the drug concentrations in the microdialysis fiber. ACSF administered alone served as a vehicle control. Drugs were administered into the CeA at a rate of 2 µl/min for at least 15 min to establish equilibrium in the tissue. Behavior was measured at 15–20 min during drug application.
2.6. Spinal reflexes
Thresholds of spinally organized hindlimb withdrawal reflexes evoked by mechanical stimulation of the knee joint were measured as described in detail previously (Fu and Neugebauer, 2008; Han et al., 2005b; Han and Neugebauer, 2005; Neugebauer et al., 2007). Mechanical stimuli of continuously increasing intensity were applied to the knee joint using a calibrated forceps equipped with a force transducer whose output was displayed (in g) on a liquid crystal display screen. The area of tissue compressed by the tip of the forceps was 30 mm2. Withdrawal threshold was defined as the minimum stimulus intensity that evoked a reflex response. The test was repeated three times (5 min intervals) and the values were averaged to calculate the threshold (force in g/30 mm2).
2.7. Audible and ultrasonic vocalizations
Vocalizations were recorded and analyzed as described in detail previously (Fu and Neugebauer, 2008; Han et al., 2005b; Han and Neugebauer, 2005; Neugebauer et al., 2007). The experimental setup (U.S. Patent 7,213,538) included a custom designed recording chamber, a condenser microphone (audible range, 20 Hz to 16 kHz) connected to a preamplifier, an ultrasound detector (25 ± 4 kHz), filter and amplifier (UltraVox 4-channel system; Noldus Information Technology, Leesburg, VA), and data acquisition software (UltraVox 2.0; Noldus Information Technology), which automatically monitored the occurrence of vocalizations within user-defined frequencies. Number and duration of digitized events (audible and ultrasonic vocalizations) were recorded on a personal computer for online and offline analysis and storage. This computerized recording system was set to suppress nonrelevant audible sounds (background noise) and to ignore ultrasounds outside the defined frequency range. Animals were placed in the recording chamber for habituation (> 30 min on 2 days) and for acclimation 1 h before the vocalization measurements. The recording chamber ensured the stable positioning of the animal at a fixed distance (6 cm) from the sound detectors. The chamber contained openings for mechanical stimulation (compression) of the knee and for drug administration into the amygdala through the microdialysis probe inserted into the implanted guide cannula.
Brief (15 s) mechanical stimuli of innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) intensities were applied to the knee, using a calibrated forceps with force transducer (see 2.6.). The total duration of vocalizations (arithmetic sum of the duration of individual events) was recorded for 1 min, starting with the onset of the mechanical stimulus. Audible and ultrasonic vocalizations reflect supraspinally organized nocifensive and affective responses to aversive stimuli (Borszcz and Leaton, 2003; Borta et al., 2006; Calvino et al., 1996; Hodgson et al., 2008; Jourdan et al., 1995; Knutson et al., 2002; Ko et al., 2005; Neugebauer et al., 2007; Oliveira and Barros, 2006; Yu et al., 2002).
2.8. Elevated Plus Maze test
Anxiety-like behavior was determined using the elevated plus maze (EPM) test as described previously (Ji et al., 2007). The EPM (0520R, Columbus Instruments, OH, USA) was constructed from stainless steel to facilitate inter-trial cleaning for elimination of odor cues. A central quadrangle (10 × 10 cm) connected two opposing open arms (50 cm long, 10 cm wide) and two opposing closed arms (50 cm long, 10 cm wide, with 40 cm high walls on both sides and on the distal end) arranged in the shape of a plus. The platform was elevated 70 cm above the floor. An automated photocell system (Multi-Varimex v.1.00; Columbus Instruments, OH, USA) recorded the movements of the animal on a personal computer.
Animals were kept in the test room for 24 h before the experiment. At the beginning of each trial, the animal was placed onto the central quadrangle facing an open arm. Anxiety-like behavior was determined by measuring the open-arm preference (ratio of open-arm entries to the total number of entries expressed as %) for 45 min. The EPM was inside a dark enclosure to minimize anxiety levels in the absence of pain. Each rat was tested before and after administration of drugs (or ACSF vehicle control).
2.9. Histology
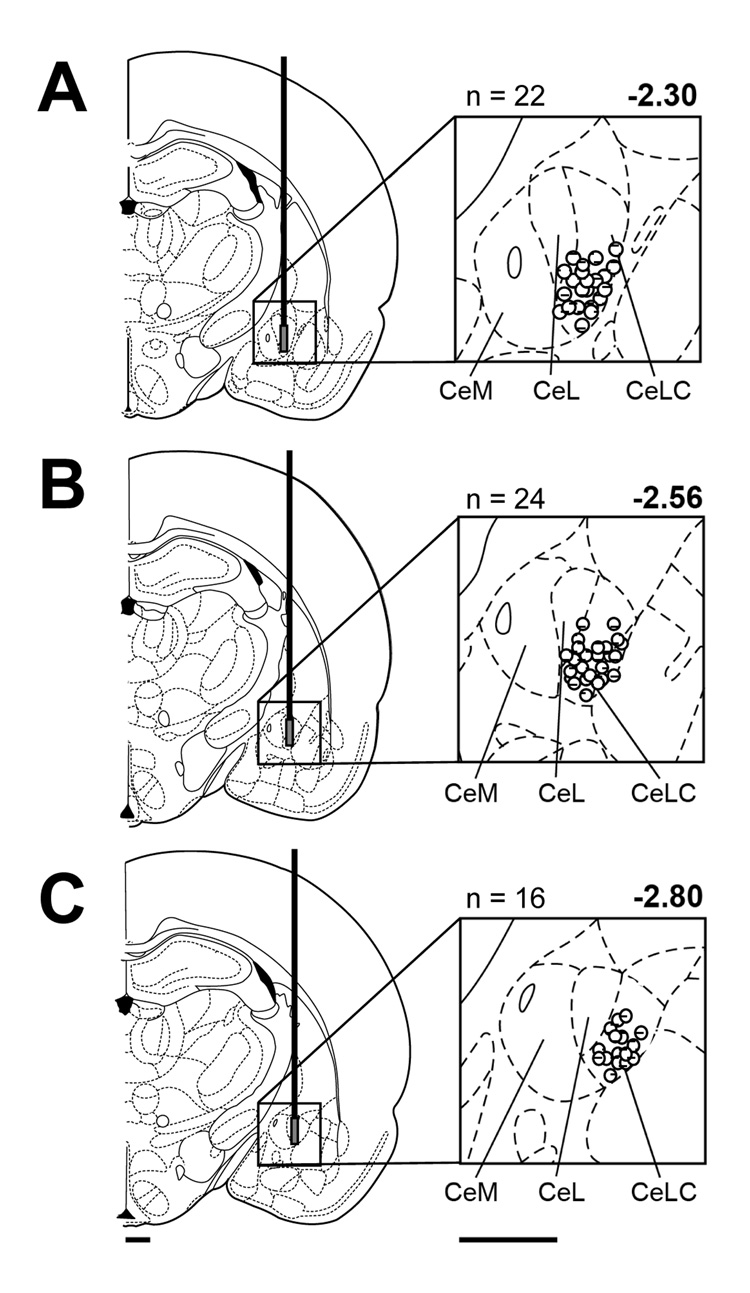
At the end of each experiment, the brain was removed and submerged in 10% Formalin. Tissues were stored in 20% sucrose before they were frozen sectioned at 50 µm. Sections were stained with Neutral Red, mounted on gel-coated slides, and cover-slipped. The position of the microdialysis probe in the CeA was confirmed histologically and plotted on standard diagrams (see Fig. 5).
Figure 5. Histologic verification of drug application sites in the CeA.
Standard diagrams (adapted from Paxinos and Watson, 1998) show coronal sections through the right brain hemisphere at different levels posterior to bregma (−2.30 to −2.80 mm). Symbols show the positions of the tips of the microdialysis membranes in the CeA based on the histologic analysis (see Methods 2.9). The number of lesion sites (n) is indicated above each diagram. CeM, CeL, CeLC, medial, lateral, and laterocapsular divisions of the central nucleus of the amygdala. Calibration bars: 1 mm.
2.10. Data analysis and statistics
All averaged values are given as the mean ± SEM. Spinal reflex threshold is shown as the averaged compression force (g/30 mm2) that evoked the withdrawal of the hindlimb. Duration of audible and ultrasonic vocalizations (in s) is defined as the arithmetic sum (total amount) of the durations of individual vocalization events during the recording period (1 min). Open-arm choice in the EPM was calculated as the ratio of open-arm entries to the total number of entries (expressed as %). The paired t-test was used to evaluate the significance of drug effects on behavior in the same animal (GraphPad Prism 3.0, GraphPad Software, San Diego, CA). Statistical significance was accepted at the level P < 0.05.
3. Results
3.1. Experimental groups
A total of 62 adult male rats were used. Spinal reflexes and vocalizations were measured in the same animals (n = 32) whereas a separate set of animals was used for the study of anxiety-like behavior in the elevated plus maze (EPM) test (n = 30). The behavioral effects of drugs administered into the CeA were investigated in six groups of animals: normal rats that received ACSF (vehicle control), normal rats treated with AMN082 (25 µM), normal rats treated with S-3,4-DCPG (10 µM), arthritic rats that received ACSF, arthritic rats treated with AMN082 (25 µM), and arthritic rats treated with S-3,4-DCPG (10 µM). Numbers refer to drug concentrations in the microdialysis fiber (see 2.5.).
3.2. Spinal reflexes
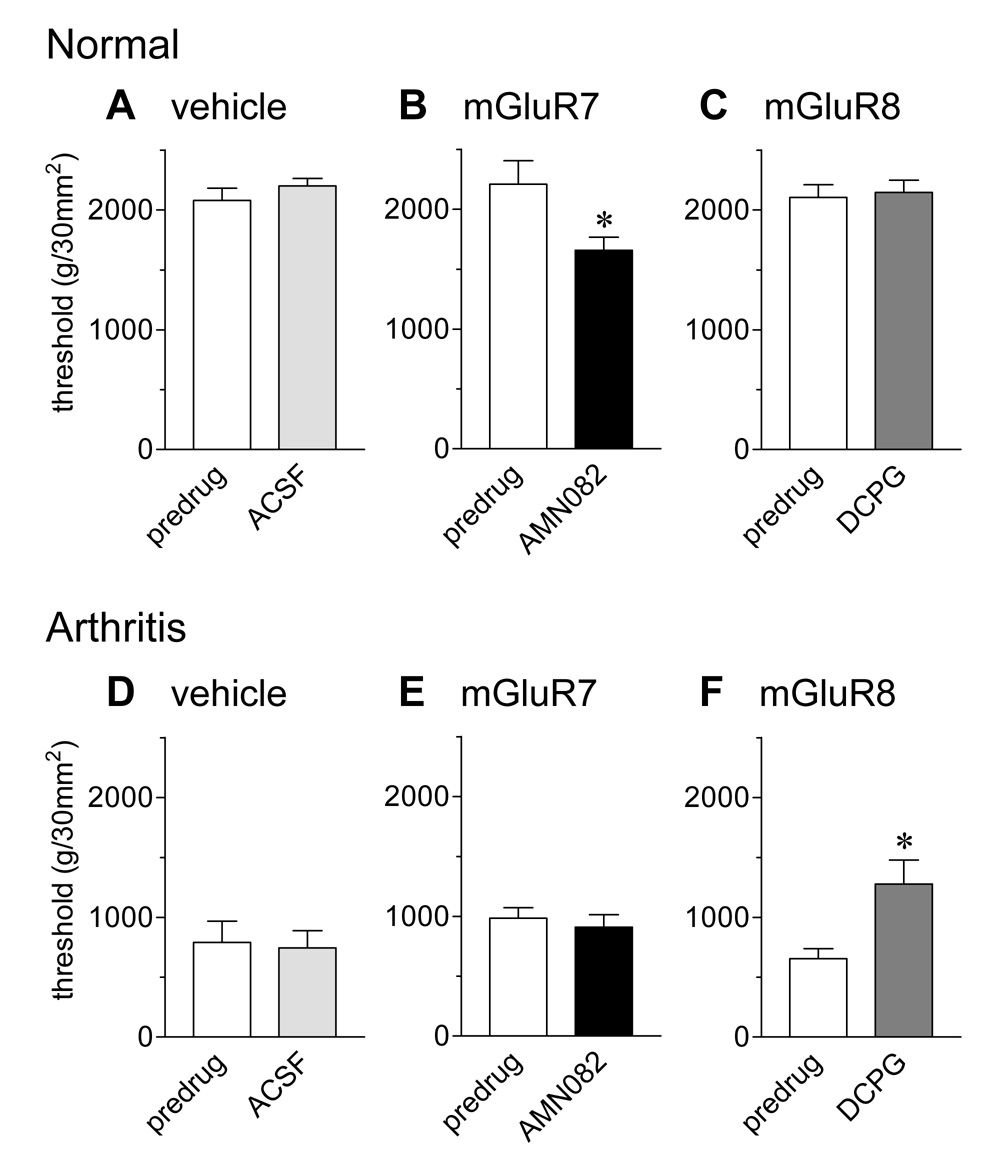
Thresholds of nocifensive hindlimb withdrawal reflexes evoked by mechanical compression of the knee joint with a calibrated forceps were measured before and during drug or vehicle (ACSF) administration into the CeA (Fig. 1). In normal animals, an mGluR7-selective agonist (AMN082; 25 µM, 15–20 min, n = 6; 1B) decreased the withdrawal threshold significantly (P < 0.05, paired t-test), indicating pro-nociceptive effects. In contrast, ACSF (15 min, n = 4; 1A) and an mGluR8-selective agonist (S-3,4-DCPG; 10 µM, 15–20 min, n = 4; 1C) had no effect on reflex thresholds.
Figure 1. An mGluR7 agonist increases spinal nocifensive reflexes of normal animals whereas an mGluR8 agonist inhibits nocifensive reflexes of arthritic animals.
Hindlimb withdrawal thresholds in response to compression of the knee joint were measured in normal animals (A–C) and in animals with a knee joint arthritis (5–6 h postinduction; D–F). Arthritic animals had lower withdrawal thresholds than normal animals, reflecting mechanical hypersensitivity. Administration of ACSF into the CeA had no effect in normal (n = 4; A) and arthritic (n = 4; D) animals. Administration of AMN082 (25 µM) into the CeA significantly decreased withdrawal thresholds in normal animals (n = 6; B) but not in arthritis (n = 6; E). S-3,4-DCPG (10 µM) had no effect in normal animals (n = 4; C) but partially reversed the decreased mechanical thresholds of arthritic animals (n = 4; F). Drugs were administered for 15–20 min. Numbers refer to concentrations in the microdialysis fiber. Bar histograms and error bars show mean ± SE. * P < 0.05 (paired t-test).
Arthritic animals (5–6 h postinduction; Fig. 1D–F) showed decreased withdrawal thresholds for compression of the arthritic knee, indicating mechanical hypersensitivity. S-3,4-DCPG (10 µM, 15–20 min, n = 4; 1F) increased the withdrawal thresholds of arthritic animals significantly (P < 0.05, paired t-test), partially reversing the mechanical hypersensitivity. ACSF (15 min, n = 4; 1D) and AMN082 (25 µM, 15–20 min, n = 6; 1E) had no significant effect in arthritic animals.
3.3. Audible vocalizations
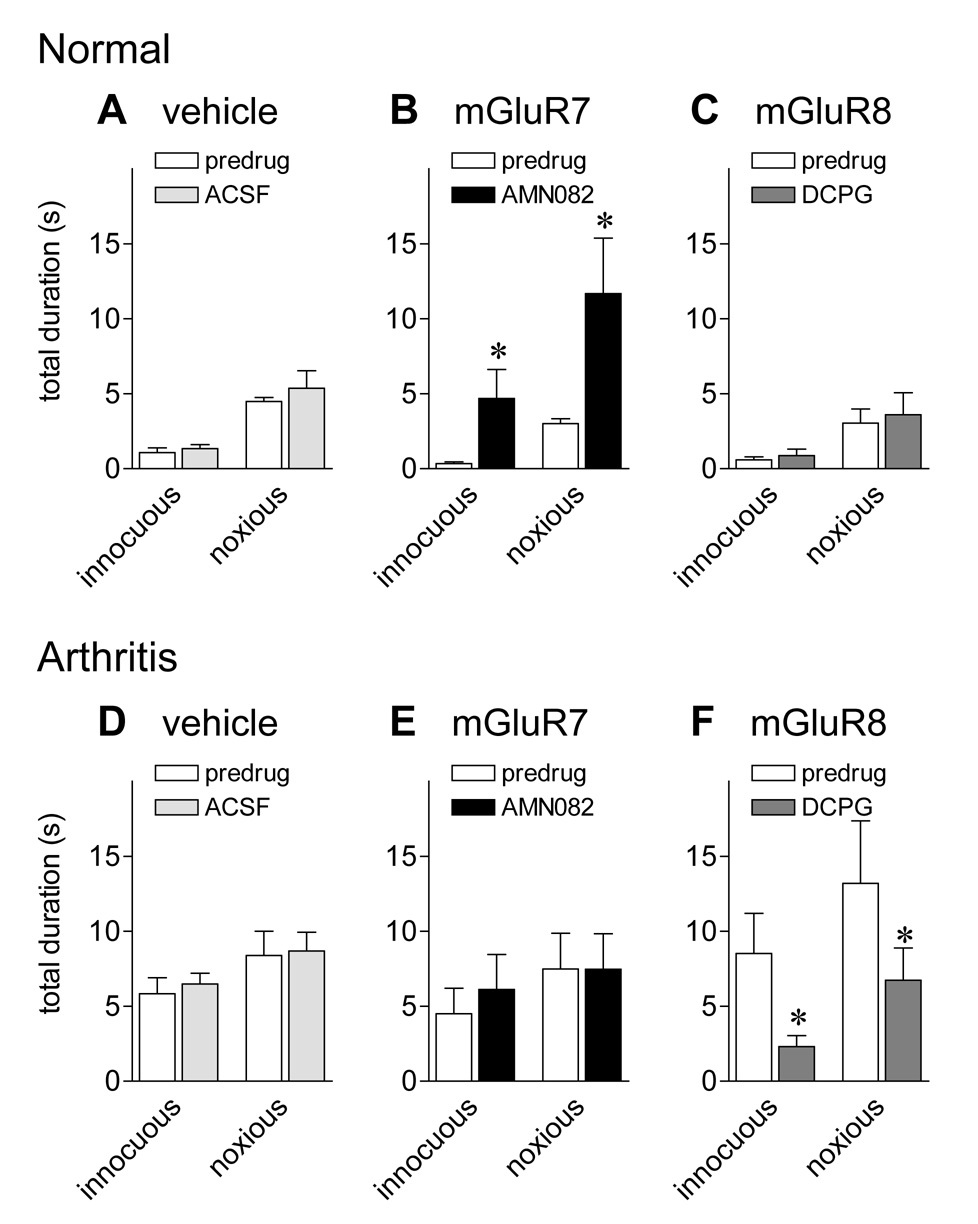
Audible squeaks of rats evoked by noxious stimuli indicate a nocifensive response (Borszcz and Leaton, 2003; Calvino et al., 1996; Jourdan et al., 1995; Neugebauer et al., 2007; Yu et al., 2002). Audible vocalizations to innocuous (500g/30 mm2) and noxious (2000g/30 mm2) compression of the knee (see 2.7.) were measured before and during administration of drugs or ACSF (vehicle control) into the CeA (Fig. 2). Vocalizations and reflexes 3.2. were measured in the same animals.
Figure 2. An mGluR7 agonist increases audible vocalizations of normal animals whereas an mGluR8 agonist inhibits audible vocalizations of arthritic animals.
Audible vocalizations evoked by innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) compression of the knee joint (see 2.7.) were measured in normal animals (A–C) and in arthritic animals (5–6 h postinduction; D–F). Total duration of audible vocalizations in arthritic animals was greater than in normal animals, indicating increased nocifensive responses. Administration of ACSF into the CeA had no effect in normal (n = 4; A) and arthritic (n = 4; D) animals. Administration of AMN082 (25 µM) into the CeA significantly increased the duration of audible vocalizations in normal (n = 6; B) but not arthritic animals (n = 6; E). In contrast, S-3,4-DCPG (10 µM) decreased the audible vocalizations of arthritic (n = 6; F) but not normal animals (n = 6; C). Drugs were administered for 15–20 min. Numbers refer to concentrations in the microdialysis fiber. Bar histograms and error bars show mean ± SE. * P < 0.05 (paired t-test).
Rats did not vocalize spontaneously in a control period of 5–10 min before stimulation. Under normal conditions (Fig. 2A–C) only noxious stimuli evoked a vocalization response. Arthritic animals (5–6 h postinduction; Fig. 2D–F) vocalized to innocuous stimuli and the duration of vocalizations to noxious stimuli was increased compared to normal animals, reflecting a state of allodynia and hyperalgesia, respectively.
In normal animals, an mGluR7-selective agonist (AMN082; 25 µM, 15–20 min, n = 6; 2B) increased audible vocalizations to innocuous and noxious stimuli (P < 0.05, paired t-test), indicating pro-nociceptive effects. In contrast, ACSF (15 min, n = 4; 2A) and an mGluR8-selective agonist (S-3,4-DCPG; 10 µM, 15–20 min, n = 6; 2C) had no effect on audible vocalizations of normal animals.
In arthritic animals (5–6 h postinduction) S-3,4-DCPG (10 µM, 15–20 min, n=6; 2F) inhibited audible vocalizations to innocuous and noxious stimuli significantly (P < 0.05, paired t-test), reversing the effect of arthritis. ACSF (15 min, n = 4; 2D) and AMN082 (25 µM, 15–20 min, n = 6; 2E) had no significant effect in arthritic animals.
3.4. Ultrasonic vocalizations
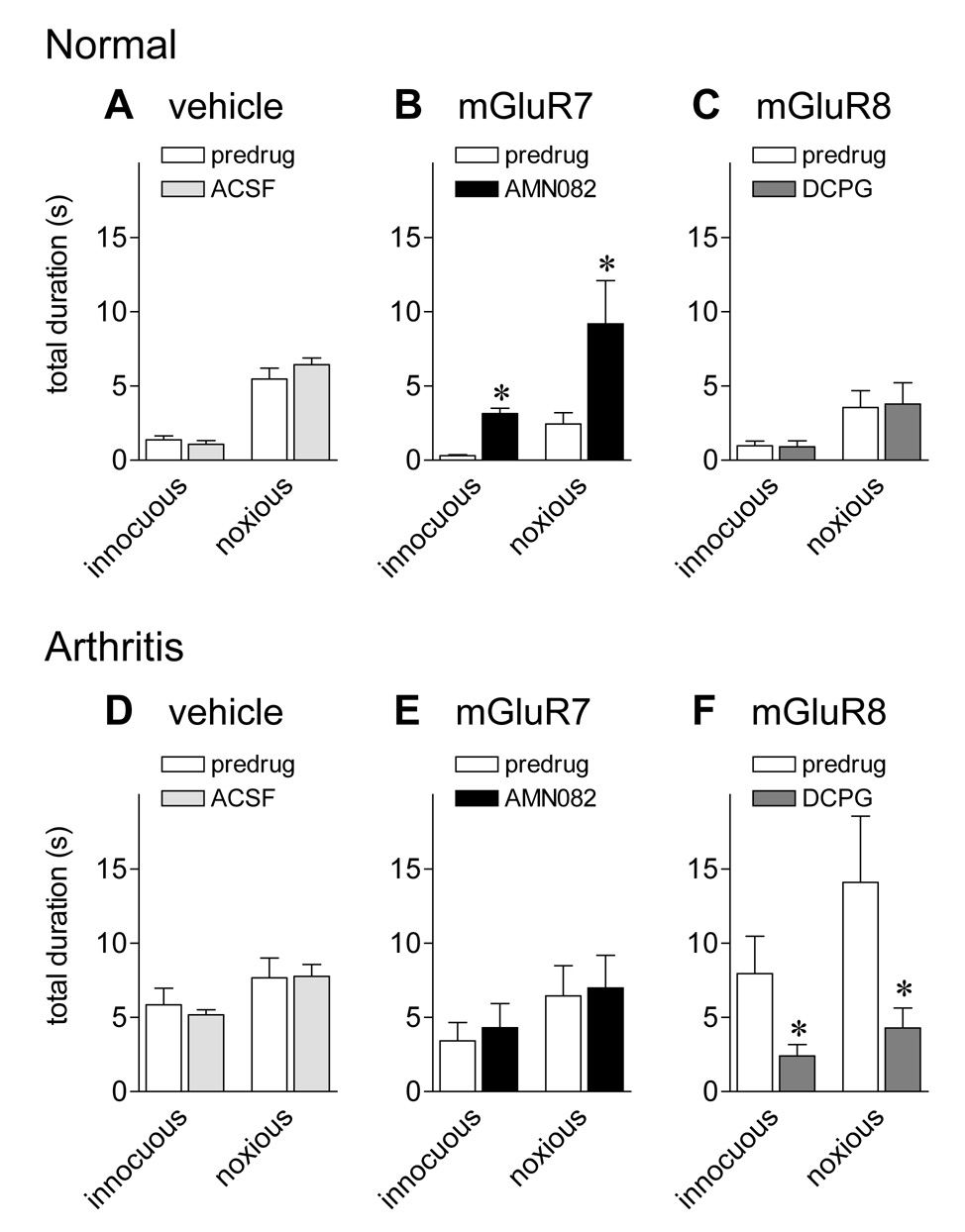
Ultrasonic vocalizations in the 22–25 kHz range are emitted in various unconditioned and conditioned aversive situations and reflect an unpleasant emotional-affective state (Borta et al., 2006; Calvino et al., 1996; Hodgson et al., 2008; Jourdan et al., 1995; Knutson et al., 2002; Ko et al., 2005; Neugebauer et al., 2007; Oliveira and Barros, 2006). Ultrasonic vocalizations evoked by innocuous (500g/30 mm2) and noxious (2000g/30 mm2) stimulation of the knee were measured before and during administration of drugs or vehicle (ACSF) into the CeA (Fig. 3). Ultrasonic and audible vocalizations were measured simultaneously in the same animals.
Figure 3. An mGluR7 agonist increases ultrasonic vocalizations of normal animals whereas an mGluR8 agonist inhibits ultrasonic vocalizations of arthritic animals.
Ultrasonic vocalizations (25 ± 4 kHz) evoked by innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) compression of the knee joint were measured in normal animals (A–C) and in arthritic animals (5–6 h postinduction; D–F). Audible (Fig. 2) and ultrasonic vocalizations (Fig. 3) were measured simultaneously in the same animals (see 2.7.). Total duration of ultrasonic vocalizations was greater in arthritic animals than in normal animals, indicating increased aversive-affective responses. Administration of ACSF into the CeA had no effect in normal (n = 4; A) and arthritic (n = 4; D) animals. AMN082 (25 µM) significantly increased the duration of ultrasonic vocalizations in normal (n = 6; B) but not arthritic animals (n = 6; E). In contrast, S-3,4-DCPG (10 µM) decreased the vocalizations of arthritic (n = 6; F) but not normal animals (n = 6; C). Drugs were administered for 15–20 min. Numbers refer to concentrations in the microdialysis fiber. Bar histograms and error bars show mean ± SE. * P < 0.05 (paired t-test).
Rats did not emit any spontaneous ultrasonic vocalizations in the 25 kHz range in a control period of 5–10 min before stimulation. In normal animals (Fig. 3A–C) only noxious stimuli evoked a vocalization response. Arthritic animals (5–6 h postinduction; Fig. 3D–F) vocalized to innocuous stimuli and showed increased vocalizations to noxious stimuli, reflecting allodynia and hyperalgesia, respectively.
In normal animals, AMN082 (25 µM, 15–20 min, n = 6; 3B) increased ultrasonic vocalizations to innocuous and noxious stimuli (P < 0.05, paired t-test). ACSF (15 min, n = 4; 3A) and S-3,4-DCPG (10 µM, 15–20 min, n = 6; 3C) had no effect on ultrasonic vocalizations of normal animals.
In arthritic animals (5–6 h postinduction) S-3,4-DCPG (10 µM, 15–20 min, n = 6; 3F) inhibited the duration of ultrasonic vocalizations significantly (P < 0.05, paired t-test), reversing the effect of arthritis. ACSF (15 min, n = 4; 3D) and AMN082 (25 µM, 15–20 min, n = 6; 3E) had no significant effect in arthritic animals.
3.5. Anxiety-like behavior (EPM)
Anxiety-like behavior was determined using the elevated plus maze (EPM) test as described previously (Ji et al., 2007). The total number of entries, a general activity indicator, and the open-arm preference (choice), a negative indicator of anxiety-like behavior (see 2.8.), were measured in normal and arthritic animals before and during administration of mGluR7- or mGluR8-selective agonists or vehicle (ACSF) into the CeA (Fig. 4).
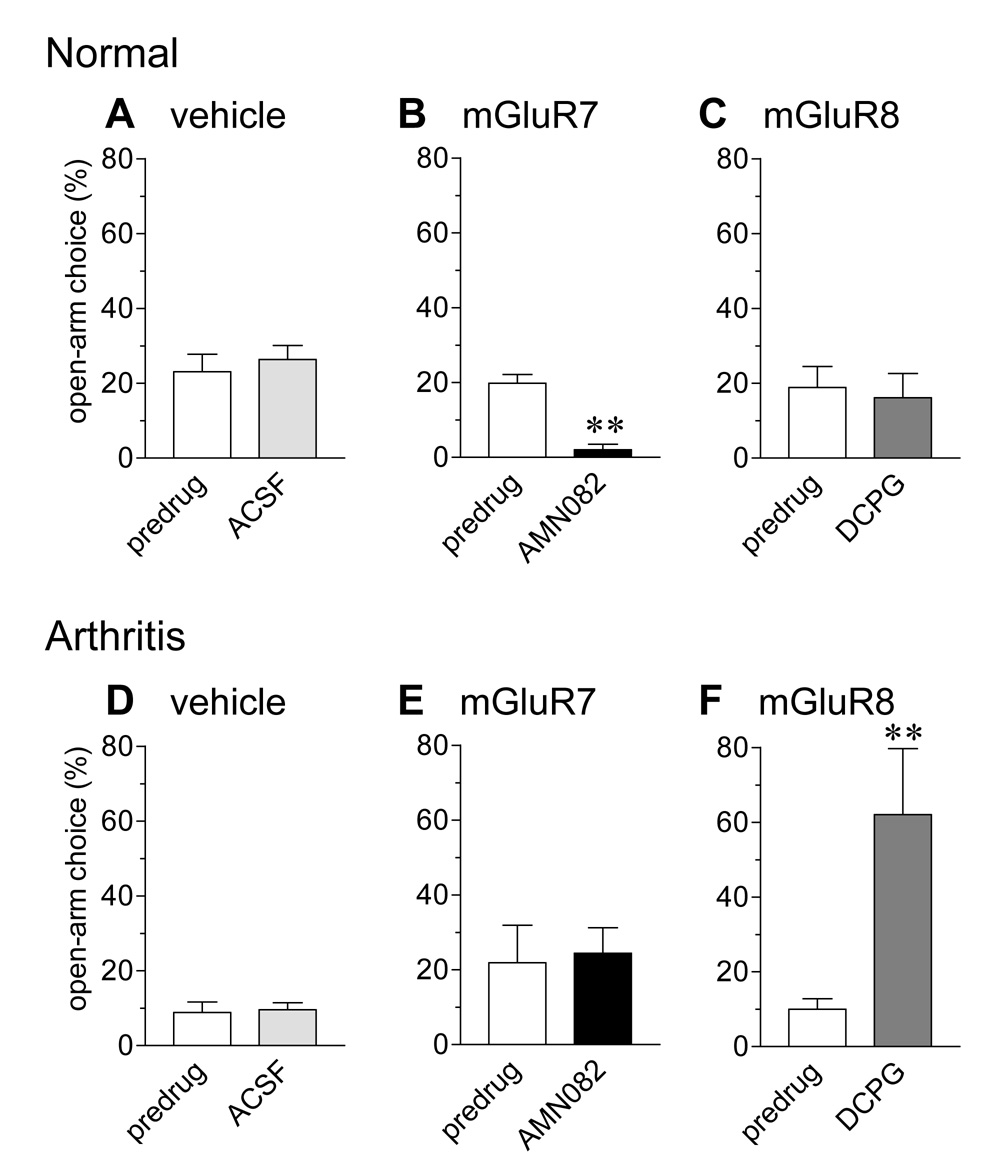
Figure 4. An mGluR7 agonist produces anxiety-like behavior in normal animals whereas an mGluR8 agonist has anxiolytic effects in arthritic animals.
Anxiety-like behavior was determined by measuring the open-arm choice (ratio of open-arm entries to the total number of entries expressed as %) in the elevated plus maze test for 45 min (see 2.8.). The open-arm choice was lower in arthritic animals (5–6 h postinduction; D–F) than in normal animals (A–C), indicating increased anxiety-like behavior in the arthritis pain model. Administration of ACSF into the CeA of normal (n = 5, A) and arthritic animals (n = 5, D) had no significant effect. AMN082 (25 µM) significantly decreased the open-arm choice in normal (n = 5; B) but not arthritic animals (n = 5; E). In contrast, S-3,4-DCPG (10 µM) increased the open-arm choice of arthritic (n = 5; F) but not normal animals (n = 5; C). Drugs were administered for 15–20 min. Numbers refer to concentrations in the microdialysis fiber. Bar histograms and error bars show mean ± SE. ** P < 0.01 (paired t-test).
The open-arm choice of arthritic rats (Fig. 4D–F) was decreased compared to baseline behavior of normal animals (Fig. 4A–C), indicating increased anxiety-like behavior in this pain model. Administration of ACSF (15 min) into the CeA had no effect in normal (n = 5; 4A) and arthritic animals (n = 5; 4D).
Administration of AMN082 (25 µM, 15–20 min) decreased the open-arm choice of normal animals (n = 5; 4B) significantly (P < 0.01, paired t test), mimicking the anxiogenic effect of arthritis. AMN082 (25 µM, 15–20 min) had no significant effect in arthritic animals (n = 5; 4E).
An mGluR8-selective agonist had anxiolytic effects in the arthritis pain state. S-3,4-DCPG (10 µM; 15–20 min) increased the open-arm preference of arthritic animals (n = 5; 4F) significantly (P < 0.01, paired t-test) but had no effect in normal animals (n = 5; 4C).
The total number of entries was significantly lower in arthritic animals (280 ± 27 in 45 min, n = 11) compared to normal controls (90 ± 11, n = 13; P < 0.001, unpaired t-test), which is consistent with reduced exploratory behavior in this pain model (Han et al., 2005a; Neugebauer et al., 2003). However, neither agonist significantly changed the total levels of activity (entries; P > 0.05, one-way ANOVA).
3.6. Histology
Figure 5 shows the histologically verified positions of the tips of the microdialysis probes in the CeA (mostly lateral-capsular division, CeLC).
4. Discussion
This study is the first to investigate the roles of mGluR7 and mGluR8 in the amygdala in pain-related behaviors. Subtype-selective agonists for mGluR7 (AMN082) and mGluR8 (S-3,4-DCPG) (Ayala et al., 2008; Marabese et al., 2007; Mitsukawa et al., 2005; Schmid and Fendt, 2006; Stachowicz et al., 2005; Thomas et al., 2001) were administered into the CeA of normal animals and animals with arthritis induced in one knee by intraarticular injections of kaolin/carrageenan. The key findings are as follows. (1) In agreement with our previous studies (Fu and Neugebauer, 2008; Han et al., 2005b; Han and Neugebauer, 2005; Ji et al., 2007) arthritic rats showed decreased nocifensive reflex thresholds, increased audible and ultrasonic vocalizations, and decreased open-arm choices in the EPM, indicating anxiety-like behavior. (2) Activation of mGluR7 with AMN082 had pro-nociceptive and anxiogenic-like effects in normal animals but not in the arthritis pain state, suggesting that the arthritis-related changes may occlude mGluR7-mediated effects. (3) Activation of mGluR8 with S-3,4-DCPG had anti-nociceptive and anxiolytic-like effects in arthritic animals but not under normal conditions.
The results show for the first time that mGluR7 and mGluR8 in the amygdala play opposite roles in pain modulation, which is similar to the situation in the brainstem (Marabese et al., 2007). Our interpretation is that in the amygdala mGluR7 activation can mimic arthritis pain-related changes whereas mGluR8 counteracts these. Arthritis pain-related changes occlude the effects of mGluR7. mGluR7 has been shown to couple to the inhibition of GABA release in the nucleus accumbens, thereby increasing the glutamatergic tone (Li et al., 2008). The now well documented facilitation of glutamatergic transmission in the amygdala in pain (Neugebauer et al., 2004; Neugebauer, 2007a) might involve a loss of inhibitory transmission, which could render mGluR7 ineffective. The lack of mGluR7 effects in arthritis could also be explained by a mechanism that involves desensitization. AMN082 induces robust internalization of mGluR7 and produces a rapid loss of surface mGluR7 (Pelkey et al., 2007).
On the other hand, mGluR8 appears to require a pathological state such as prolonged pain to become effective. This is different from the effects of a broad-spectrum group III agonist (LAP4) that inhibited not only pain-related plasticity but also baseline transmission in the amygdala (Han et al., 2004; Li and Neugebauer, 2006). Interestingly, S-3,4-DCPG did not affect baseline synaptic transmission in hippocampal slices from adult rats (Ayala et al., 2008) and failed to modify anxiety-like behavior in normal rats after administration into the basolateral amygdala (Stachowicz et al., 2005). Therefore, analgesic and anxiolytic-like effects of mGluR8 activation may depend on neuroplasticity that affects group III mGluRs sensitivity (Neugebauer, 2008).
Direct measurements of glutamate and GABA levels in the amygdala are needed to show that mGluR7 and mGluR8 in fact regulate GABA and glutamate release differentially and these effects are different in the pain state and under normal conditions. It is also important to consider that mGluR7 and mGluR8 may play different roles in more chronic pain states, because mechanisms of amygdala plasticity in acute and chronic pain models appear to be different (Ikeda et al., 2007; Neugebauer, 2007b). On the other hand, enhanced glutamatergic transmission is a key mechanism of amygdala plasticity in the acute (Bird et al., 2005; Neugebauer et al., 2003) as well as the chronic pain model (Ikeda et al., 2007). Therefore, modulators of glutamatergic transmission may have similar functions in acute and chronic pain states.
Some methodological aspects of this study need to be considered. We used highly selective agonists for mGluR7 and mGluR8. AMN082 (EC50 = 64-290 nM) is the very first mGluR7 specific agonist with >300-fold selectivity over other mGluR subtypes (Mitsukawa et al., 2005). S-3,4-DCPG is a potent mGluR8 agonist (EC50 = 31 nM) with 100-fold selectivity over mGluR1–7 (Thomas et al., 2001). The fact that the agonists had opposite effects in normal animals as well as in arthritis argues against non-selective drug effects. We selected drug concentrations identical to those used in a recent study that also demonstrated differential effects of these agonists when administered into the brainstem by microdialysis (Marabese et al., 2007).
Microdialysis offers several advantages, including continued drug delivery and steady-state levels without a volume effect (Stiller et al., 2003). However, the dose delivered by microdialysis is not known. Concentration-response analysis of similar-sized non-peptide molecules such as LAP4 in our previous in vivo (Li and Neugebauer, 2006) and in vitro (Han et al., 2004) studies confirmed that the drug concentration in the microdialysis fiber needed to be 100 times higher than the desired tissue concentration because of the concentration gradient across the dialysis membrane and diffusion in the tissue (see Materials and Methods). Therefore, the concentrations used in this study (AMN082, 25 µM; S-3,4-DCPG, 10 µM) would be consistent with the reported potencies of these drugs.
The spread of drugs beyond the site of administration by microdialysis also needs to be considered. Our previous studies suggested that drug effects are limited to an area of less than 1 mm around the injection site. This was determined using offsite drug applications into nearby brain areas such as the striatum (Fu and Neugebauer, 2008; Han et al., 2005b; Han and Neugebauer, 2005; Ji and Neugebauer, 2008). Another study recorded the neuronal effects of lidocaine and tetrodotoxin (TTX) at different distances from the microdialysis probe and concluded that these compounds have an effective spread (significant inhibition of neuronal activity) of 1 mm and 2 mm, respectively (Boehnke and Rasmusson, 2001). It is therefore unlikely that drugs administered locally by microdialysis produce “systemic” effects and reach distant areas such as the brainstem, where a similar pattern of mGluR7 and mGluR8 function has been described previously (Marabese et al., 2007).
Another issue concerns the analysis and interpretation of audible and ultrasonic vocalizations as measurements of the nocifensive and affective components of pain. Supporting evidence comes from an increasing body of literature about pain-related vocalizations. Audible squeaks in response to noxious stimuli indicate a supraspinally organized nocifensive reflex response (Borszcz and Leaton, 2003; Calvino et al., 1996; Jourdan et al., 1995; Neugebauer et al., 2007; Yu et al., 2002). Ultrasonic vocalizations in the 22–25 kHz range are reliably emitted in aversive situations and are believed to reflect an unpleasant emotional-affective state such as fear and anxiety. Ultrasonic vocalizations are sensitive to drugs affecting anxiety-like behavior such as diazepam and morphine; they correlate with anxiety-like behavior in the EPM test and are organized in the limbic system, including the amygdala (Borta et al., 2006; Calvino et al., 1996; Hodgson et al., 2008; Jourdan et al., 1995; Knutson et al., 2002; Ko et al., 2005; Neugebauer et al., 2007; Oliveira and Barros, 2006). We analyzed the duration (total time spent vocalizing) rather than rate of vocalizations because the duration has been shown to be a more sensitive measure of anxiolytic and antidepressant efficacy (Hodgson et al., 2008).
This study focused on the amygdala because it plays a key role in emotionality and affective disorders (Cardinal et al., 2002; Maren, 2005; Phelps and Ledoux, 2005; Zald, 2003) and has emerged as an important brain center involved in the emotional-affective component of pain and its reciprocal relationship with negative affective states and disorders such as fear and anxiety (Carrasquillo and Gereau, 2007; Gauriau and Bernard, 2002; Ji et al., 2007; Kulkarni et al., 2007; Neugebauer et al., 2004; Pedersen et al., 2007; Rhudy and Meagher, 2001). Glutamate receptors (Neugebauer, 2007a) including group III mGluRs (Neugebauer, 2008) regulate pain-related activity and plasticity in the CeA (Han et al., 2004; Li and Neugebauer, 2006).
Group III mGluRs have antinociceptive (Neugebauer, 2008; Varney and Gereau, 2002) and anxiolytic-/antidepressant-like functions (Palucha et al., 2004) and are considered as novel targets for anxiety disorders (Swanson et al., 2005). Group III mGluRs typically decrease transmitter release and can inhibit glutamatergic and GABAergic synaptic transmission (Neugebauer, 2008; Swanson et al., 2005). Therefore, the overall effect of group III mGluR activation is a balance between facilitatory and inhibitory actions. The roles of individual group III mGluR subtypes in pain and anxiety are only beginning to emerge with the availability of subtype selective agents.
Several studies have shown differential contributions of mGluR7 and mGluR8 to pain and anxiety modulation. Stimulation of mGluR7 or mGluR8 in the periaqueductal grey matter had opposing pro- and anti-nociceptive effects, respectively, which correlated with differential effects on the release of GABA and glutamate and on the activity of OFF and ON cells in the rostral ventromedial medulla (Marabese et al., 2007). Genetic ablation of mGluR7 caused deficits in fear response and conditioned taste aversion (Masugi et al., 1999), impaired stress response (Mitsukawa et al., 2006), and had antidepressant- and anxiolytic-like effects (Cryan et al., 2003; Mitsukawa et al., 2006; Swanson et al., 2005). In contrast, mGluR8 knockout mice showed increased anxiety-like behavior (Duvoisin et al., 2005; Linden et al., 2002). The underlying mechanisms remain to be determined.
In conclusion, the present study shows opposing roles of mGluR7 and mGluR8 in the amygdala (CeA) in pain-related nocifensive and affective behaviors. Activation of mGluR7 had pro-nociceptive and anxiety-like effects in normal animals whereas mGluR8 activation was anti-nociceptive and anxiolytic in the arthritis pain model. The results emphasize the importance of these receptors as potential therapeutic targets, although their roles in more chronic pain states remain to be determined. The better understanding of mechanisms underlying the differential effects of group III mGluR subtypes may not only provide insight into the pathophysiology of chronic pain but also lead to novel therapeutic strategies for the relief of pain and/or anxiety.
Acknowledgments
This work was supported by NIH grants NS-38261 and NS-11255.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayala JE, Niswender CM, Luo Q, Banko JL, Conn PJ. Group III mGluR regulation of synaptic transmission at the SC-CA1 synapse is developmentally regulated. Neuropharmacology. 2008;54:804–814. doi: 10.1016/j.neuropharm.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J. Physiol. 2005;564:907–921. doi: 10.1113/jphysiol.2005.084780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: an electrophysiological study in cerebral cortex. J Neurosci. Methods. 2001;105:133–141. doi: 10.1016/s0165-0270(00)00348-4. [DOI] [PubMed] [Google Scholar]
- Borszcz GS, Leaton RN. The effect of amygdala lesions on conditional and unconditional vocalizations in rats. Neurobiol. Learn. Mem. 2003;79:212–225. doi: 10.1016/s1074-7427(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Borta A, Wohr M, Schwarting RKW. Rat ultrasonic vocalization in aversively motivated situations and the role of individual differences in anxiety-related behavior. Behav. Brain Res. 2006;166:271–280. doi: 10.1016/j.bbr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Calvino B, Besson JM, Boehrer A, Depaulis A. Ultrasonic vocalization (22–28 kHz) in a model of chronic pain, the arthritic rat: effects of analgesic drugs. NeuroReport. 1996;7:581–584. doi: 10.1097/00001756-199601310-00049. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J. Neurosci. 2007;27:1543–1551. doi: 10.1523/JNEUROSCI.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van der PH. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur. J. Neurosci. 2003;17:2409–2417. doi: 10.1046/j.1460-9568.2003.02667.x. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Zhang C, Pfankuch TF, O'Connor H, Gayet-Primo J, Quraishi S, Raber J. Increased measures of anxiety and weight gain in mice lacking the group III metabotropic glutamate receptor mGluR8. Eur. J. Neurosci. 2005;22:425–436. doi: 10.1111/j.1460-9568.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci. 2008;28:3861–3876. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher RM, Verma S. Mood and anxiety disorders in chronic pain. Prog. Pain Res. Management. 2004;27:139–178. [Google Scholar]
- Gauriau C, Bernard J-F. Pain pathways and parabrachial circuits in the rat. Exp. Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Han JS, Bird GC, Li W, Neugebauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J. Neurosci. Meth. 2005a;141:261–269. doi: 10.1016/j.jneumeth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Han JS, Bird GC, Neugebauer V. Enhanced group III mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Neuropharmacology. 2004;46:918–926. doi: 10.1016/j.neuropharm.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Han JS, Fu Y, Bird GC, Neugebauer V. Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Mol. Pain. 2006;2:18–29. doi: 10.1186/1744-8069-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Li W, Neugebauer V. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J. Neurosci. 2005b;25:10717–10728. doi: 10.1523/JNEUROSCI.4112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain. 2005;113:211–222. doi: 10.1016/j.pain.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Guthrie DH, Varty GB. Duration of ultrasonic vocalizations in the isolated rat pup as a behavioral measure: sensitivity to anxiolytic and antidepressant drugs. Pharmacol. Biochem. Behav. 2008;88:341–348. doi: 10.1016/j.pbb.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nature. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127:161–172. doi: 10.1016/j.pain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol. Pain. 2007;3:13–17. doi: 10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J. Neurophysiol. 2007;97:3893–3904. doi: 10.1152/jn.00135.2007. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pro- and Anti-Nociceptive Effects of Corticotropin-Releasing Factor (CRF) in Central Amygdala Neurons Are Mediated Through Different Receptors. J Neurophysiol. 2008;99:1201–1212. doi: 10.1152/jn.01148.2007. [DOI] [PubMed] [Google Scholar]
- Jourdan D, Ardid D, Chapuy E, Eschalier A, Le BD. Audible and ultrasonic vocalization elicited by single electrical nociceptive stimuli to the tail in the rat. Pain. 1995;63:237–249. doi: 10.1016/0304-3959(95)00049-X. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Ko SW, Chatila T, Zhuo M. Contribution of CaMKIV to injury and fear-induced ultrasonic vocalizations in adult mice. Mol. Pain. 2005;1:10. doi: 10.1186/1744-8069-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A, Boyle Y, El-Deredy W, Jones AK. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007;56:1345–1354. doi: 10.1002/art.22460. [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential changes of group II and group III mGluR function in central amygdala neurons in a model of arthritic pain. J. Neurophysiol. 2006;96:1803–1815. doi: 10.1152/jn.00495.2006. [DOI] [PubMed] [Google Scholar]
- Li X, Gardner EL, Xi ZX. The metabotropic glutamate receptor 7 (mGluR(7)) allosteric agonist AMN082 modulates nucleus accumbens GABA and glutamate, but not dopamine, in rats. Neuropharmacology. 2008;54:542–551. doi: 10.1016/j.neuropharm.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden A-M, Johnson BG, Peters SC, Shannon HE, Tian M, Wang Y, Yu JL, Koster A, Baez M, Schoepp DD. Increased anxiety-related behavior in mice deficient for metabotropic glutamate 8 (mGlu8) receptor. Neuropharmacology. 2002;43:251–259. doi: 10.1016/s0028-3908(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Marabese I, Rossi F, Palazzo E, de Novellis V, Starowicz K, Cristino L, Vita D, Gatta L, Guida F, Di Marzo V, Rossi F, Maione S. Periaqueductal Gray Metabotropic Glutamate Receptor Subtype 7 and 8 Mediate Opposite Effects on Amino Acid Release, Rostral Ventromedial Medulla Cell Activities, and Thermal Nociception. J. Neurophysiol. 2007;98:43–53. doi: 10.1152/jn.00356.2007. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic Mechanisms of Associative Memory in the Amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Masugi M, Yokoi M, Shigemoto R, Muguruma K, Watanabe Y, Sansig G, van der PH, Nakanishi S. Metabotropic glutamate receptor subtype 7 ablation causes deficit in fear response and conditioned taste aversion. J. Neurosci. 1999;19:955–963. doi: 10.1523/JNEUROSCI.19-03-00955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsukawa K, Mombereau C, Lotscher E, Uzunov DP, van der PH, Flor PJ, Cryan JF. Metabotropic glutamate receptor subtype 7 ablation causes dysregulation of the HPA axis and increases hippocampal BDNF protein levels: implications for stress-related psychiatric disorders. Neuropsychopharmacology. 2006;31:1112–1122. doi: 10.1038/sj.npp.1300926. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Yamamoto R, Ofner S, Nozulak J, Pescott O, Lukic S, Stoehr N, Mombereau C, Kuhn R, McAllister KH, van der Putten H, Cryan JF, Flor PJ. A selective metabotropic glutamate receptor 7 agonist: Activation of receptor signaling via an allosteric site modulates stress parameters in vivo. PNAS. 2005;102:18712–18717. doi: 10.1073/pnas.0508063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V. Glutamate receptor ligands. Handb. Exp. Pharmacol. 2007a;177:217–249. doi: 10.1007/978-3-540-33823-9_8. [DOI] [PubMed] [Google Scholar]
- Neugebauer V. The amygdala: Different pains, different mechanisms. Pain. 2007b;127:1–2. doi: 10.1016/j.pain.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V. Group III Metabotropic Glutamate Receptors (mGlu4, mGlu6, mGlu7, and mGlu8) In: Gereau RW, Swanson GT, editors. The Glutamate Receptors. Totowa, NJ: Humana Press; 2008. pp. 489–508. [Google Scholar]
- Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol. Pain. 2007;3:8–20. doi: 10.1186/1744-8069-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J. Neurophysiol. 2003;89:716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J. Neurosci. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Oliveira AR, Barros HM. Ultrasonic rat vocalizations during the formalin test: a measure of the affective dimension of pain? Anesth. Analg. 2006;102:832–839. doi: 10.1213/01.ane.0000196530.72813.d9. [DOI] [PubMed] [Google Scholar]
- Palucha A, Tatarczynska E, Branski P, Szewczyk B, Wieronska JM, Klak K, Chojnacka-Wojcik E, Nowak G, Pilc A. Group III mGlu receptor agonists produce anxiolytic- and antidepressant-like effects after central administration in rats. Neuropharmacology. 2004;46:151–159. doi: 10.1016/j.neuropharm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pedersen LH, Scheel-Kruger J, Blackburn-Munro G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain. 2007;127:17–26. doi: 10.1016/j.pain.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Yuan X, Lavezzari G, Roche KW, McBain CJ. mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology. 2007;52:108–117. doi: 10.1016/j.neuropharm.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ledoux JE. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr. Opin. Psychiatry. 2001;14:241–245. [Google Scholar]
- Rhudy JL, Meagher MW. Negative affect: effects on an evaluative measure of human pain. Pain. 2003;104:617–626. doi: 10.1016/S0304-3959(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Rome HP, Rome JD. Limbically augmented pain syndrome (LAPS): kindling, corticolimbic sensitization, and the convergence of affective and sensory symptoms in chronic pain disorders. Pain Med. 2000;1:7–23. doi: 10.1046/j.1526-4637.2000.99105.x. [DOI] [PubMed] [Google Scholar]
- Schmid S, Fendt M. Effects of the mGluR8 agonist (S)-3,4-DCPG in the lateral amygdala on acquisition/expression of fear-potentiated startle, synaptic transmission, and plasticity. Neuropharmacology. 2006;50:154–164. doi: 10.1016/j.neuropharm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Stachowicz K, Klak K, Pilc A, Chojnacka-Wojcik E. Lack of the antianxiety-like effect of (S)-3,4-DCPG, an mGlu8 receptor agonist, after central administration in rats. Pharmacol. Rep. 2005;57:856–860. [PubMed] [Google Scholar]
- Stiller CO, Taylor BK, Linderoth B, Gustafsson H, Warsame Afrah A, Brodin E. Microdialysis in pain research. Adv. Drug Deliv. Rev. 2003;55:1065–1079. doi: 10.1016/s0169-409x(03)00104-2. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M. Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur. J. Neurosci. 2003;18:2343–2350. doi: 10.1046/j.1460-9568.2003.02952.x. [DOI] [PubMed] [Google Scholar]
- Thomas NK, Wright RA, Howson PA, Kingston AE, Schoepp DD, Jane DE. (S)-3,4-DCPG, a potent and selective mGlu8a receptor agonist, activates metabotropic glutamate receptors on primary afferent terminals in the neonatal rat spinal cord. Neuropharmacology. 2001;40:311–318. doi: 10.1016/s0028-3908(00)00169-6. [DOI] [PubMed] [Google Scholar]
- Varney MA, Gereau RW. Metabotropic glutamate receptor involvement in models of acute and persistent pain: prospects for the development of novel analgesics. Current Drug Targets. 2002;1:215–225. doi: 10.2174/1568007023339300. [DOI] [PubMed] [Google Scholar]
- Yu YC, Koo ST, Kim CH, Lyu Y, Grady JJ, Chung JM. Two variables that can be used as pain indices in experimental animal models of arthritis. J. Neurosci. Meth. 2002;115:107–113. doi: 10.1016/s0165-0270(02)00011-0. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]