Abstract
The main cause of death in melanoma patients is widespread metastases. Staging of melanoma is based on the primary tumor thickness, ulceration, lymph node and distant metastases. Metastases develop in regional lymph nodes, as satellite or in-transit lesions, or in distant organs. Lymph flow and chemotaxis is responsible for the homing of melanoma cells to different sites. Standard pathologic evaluation of sentinel lymph nodes fails to find occult melanoma in a significant proportion of cases. Detection of small numbers of malignant melanoma cells in these and other sites, such as adjacent to the primary site, bone marrow or the systemic circulation, may be enhanced by immunohistochemistry, reverse transcription PCR, evaluation of lymphatic vessel invasion and proteomics. In the organs to which melanoma cells metastasize, extravasation of melanoma cells is regulated by adhesion molecules, matrix metalloproteases, chemokines and growth factors. Melanoma cells may travel along external vessel lattices. After settling in the metastatic sites, melanoma cells develop mechanisms that protect them against the attack of the immune system. It is thought that one of the reasons why melanoma cells are especially resistant to killing is the fact that melanocytes (cells from which melanoma cells derive) are resistant to such noxious factors as ultraviolet light and reactive oxygen species. Targeted melanoma therapies are, so far, largely unsuccessful, and new ones, such as adjuvant inhibition of melanogenesis, are under development.
Keywords: biotherapy, chemokine, integrin, melanoma, metastasis, sentinel lymph node
Melanoma occurrence, staging & detection
Before we review several concepts related to mechanisms of melanoma metastasis and treatment, we will briefly describe the currently available data about melanoma occurrence, the currently used system that divides melanoma patients into groups with different survival rates and the methods used to detect melanoma cells beyond the primary cutaneous tumor site. The American Cancer Society predicted that 59,940 new cases of melanoma occurred in 2007 (4% of all cancer cases). Of those cases, 8100 patients (13%) died, predominantly because of widespread metastases (1–2% of all cancer deaths) [201]. The Melanoma Staging Committee of the American Joint Committee on Cancer established a new evidence-based staging system for cutaneous melanoma in 2003 [1]. These criteria have subsequently been approved by several entities including the Union Against Cancer/Union International Contre le Cancer TNM (tumor, lymph nodes and metastsis) Committee, the WHO Melanoma Program and the European Organization for Research and Treatment of Cancer Melanoma Group [1]. Stages I and II of this system are comprised of patients without regional or distant metastases. Stage III patients have metastases either in the regional lymph nodes or in intralymphatic sites (further defined as satellite or in-transit). Stage IV patients have metastases in distant sites. Survival of patients in all groups depends on different predictors. The thickness and ulceration of melanomas are important criteria in assessing survival in patients with localized disease (stages I and II) (Figure 1). Patients with the least advanced disease (other than melanoma in situ), that is, in stage IA disease (i.e., T1aN0M0, lesion less than 1 mm thick, without ulceration and extending into reticular dermis [Clark level II/III]) have a 94% 5-year and an 86% 10-year survival. Patients with stage IIC disease (i.e., T4bN0M0, lesion more than 4 mm thick with ulceration) have a 53% 5-year and a 41% 10-year survival [1]. The number of metastatic lymph nodes and the overall quantity of neoplastic cells are greatest in patients with stage III disease (Figure 2). Specifically, detection of lymph nodes by palpation as opposed to detection of neoplastic cells in lymph nodes by light microscopy was linked to a shorter survival. Patients with stage IIIA disease (i.e., T1–4aN1a-2aM0, lesion of any thickness, without ulceration, with microscopic metastasis) have a 67% 5-year survival rate. Patients with stage IIIC disease (any TN3M0, four or more metastatic lymph nodes, matted lymph nodes, in-transit metastasis with metastatic lymph nodes) have a 28% 5-year survival rate (Figure 3) [2]. In patients with stage IV disease, the site of metastasis and level of lactate dehydrogenase are the most important predictors of survival. Patients that have metastases to distant skin or subcutanesous sites or distant lymph nodes are categorized in group M1a. These patients have a 1-year survival rate of 59%. Patients with metastases to the lungs are categorized in group M1b and have a 1-year survival rate of 57%. Patients with metastases to any other visceral sites have the worst prognosis with a 1-year survival rate of 41% [2]. Two or more elevated lactate dehydrogenase levels drawn more than 24 h apart will upgrade a patient to group M1c, regardless of the site of metastases. Of note, the number of metastases, although previously recognized as a prognostic determinant, is not incorporated as part of the current staging system. This is due to existing differences, such as lack of standardization of imaging modalities in different centers [1].
Figure 1. Breslows tumor thickness and ulceration are currently the American Joint Committee on Cancer criteria for staging of primary cutaneous melanoma and the most predictive factor for occult metastasis at the time of diagnosis.
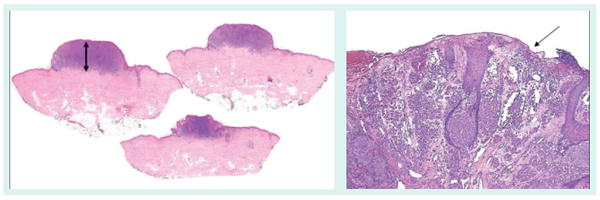
Figure 2. Histologic and clinical findings that classify the tumor as American Joint Committee on Cancer stage III.
(A) Microscopic, immunohistochemistry positive (MART1) sentinel lymph node, (B) clinically and pathogically positive lymph node and (C) peritumoral and in transit metastasis (right).
Figure 3. Factors that are predictive of metastasis in the primary.
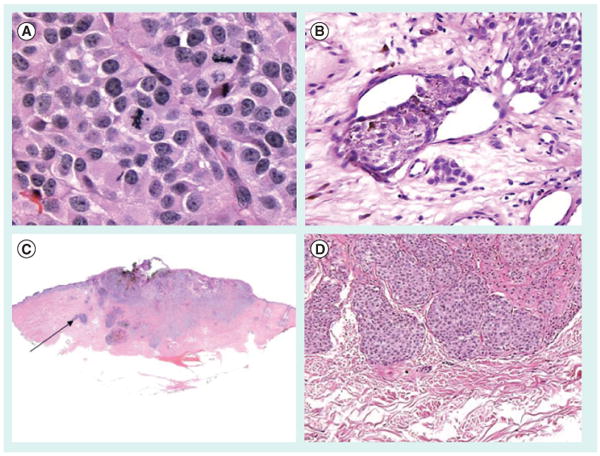
(A) Mitotic rate, (B) vascular invasion, (C) absence of a tumor-infiltrating lymphocyte host response and (D) microsatellites, whose presence upgrades the melanoma to American Joint Committee on Cancer stage IIIc and are essentially in-transit metastasis.
Since metastasis is the most important predictor of the patient's prognosis, there is a lot of effort directed at unequivocal determination of their presence in the adjacent epidermis, sentinel lymph nodes, circulation and distant sites (Box 1).
Box 1. Detection of melanoma: summary of different aspects of occult melanoma detection.
| Aspect: | |
| • ‘Field cells’ detection | [3,4] |
| • Local lymphatic invasion | [5–7] |
| • Sentinel lymph node | [8] |
| • Sentinel lymph node with reverse transcription PCR | [9–12] |
| • Microarray analysis | [13] |
| • Immune environment in the sentinel lymph node | [17–20] |
| • Circulating melanoma cells | [21–29] |
| • Proteomic analysis | [35] |
| • Imaging studies | [36–41] |
Before melanoma cells metastasize they extend into the adjacent epidermis. ‘Field cells’ were characterized by Bastian et al. [3]. Epidermis adjacent to the acral lentiginous melanoma can harbor cells with a high level of DNA amplifications (11q13, 5p15) that can be detected by fluorescent in situ hybridization. Genetic analysis of these cells suggests that they precede melanoma in situ. They also extend significantly into normal skin without a correlation to tumor thickness or size [4].
Another potential step in the evolution of a primary tumor into its metastasis to lymph nodes is local lymphatic invasion. This is usually only assessed in the excisions of the primary tumor on hematoxylin and eosin slides. In a recent study, immunohistochemical stains with antibodies against podoplanin (specific for lymphatic vessels) and S-100 (specific for melanoma) [5] were combined with multispectral imaging analysis. This increased the sensitivity of detection of lymphatic invasion sevenfold. Dadras et al. used the antibody against lymphatic endothelial hyaluronan receptor (LYVE)-1 to decorate lymphatic vessels in the tumor and its close proximity (within 100 μm) to its border [6]. Additionally, they assessed expression of VEGF-C and VEGF-D in melanomas. As it turned out, VEGF-C and not VEGF-D correlated with a higher frequency of melanoma metastases to sentinel lymph nodes. The relative area of vascular invasion of primary melanomas [7] correlated with metastasis to sentinel lymph nodes to a greater extent (p = 0.00008) than did tumor thickness (p = 0.005), as determined by the Wilcoxon rank test.
The choice of which lymph nodes to dissect by the method of elective lymph node dissection was based on the fact that patients with similar tumors presented with metastases in certain regional locations. This approach resulted in patients being overtreated and significant unnecessary morbidity. Sentinel lymph node biopsy is based on the pre- and intraoperative radiological imaging and injection of a marker dye that is distributed towards the closest lymph node that might harbor metastasis. The multicenter Selective Lymphadenectomy Trial-1 (MLST-1) has assessed the validity of this approach. In the MLST-1, 2001 patients with primary cutaneous melanomas greater than 1 mm in thickness and a Clark's level III stage or greater, or less than 1 mm in thickness and at least a Clark's level IV stage, were randomly divided into two groups. Patients in the first group underwent a wide excision of their tumors and their clinical status was subsequently observed. Patients in the second group underwent wide excision of their tumors with sentinel lymph node biopsy followed by a complete lymph node dissection if metastasis was found in the sentinel lymph node. The first interim results of this trial were published in 2006 [8]. The mean estimated 5-year disease-free survival rate for the population was 78.3 ± 1.6% in the biopsy group and 73.1 ± 2.1% in the observation group (hazard ratio [HR] for recurrence [corrected]: 0.74; 95% confidence interval [CI]: 0.59–0.93; p = 0.009). Further analysis of MLST-1 are awaited and a new MLST-2 project has been initiated. The goals of the MLST-2 project are to discover if complete lymph node dissection is necessary after positive sentinel lymph node and, also, whether the determination of melanoma-specific mRNAs by RT-PCR in the sentinel lymph nodes correlates with an unfavorable prognosis in the patients [9]. According to systematic review and meta-analysis by Mocellin et al., positive reverse transcription PCR status correlated with both TNM stage (stage I or II vs III; PCR positivity, 95.1 vs 46.6%; p < 0001) and disease recurrence (PCR positive vs negative; relapse rate, 16.8 vs 8.7%; p < 0001). PCR positivity was also associated with a worse overall prognosis (HR: 5.08; 95% CI: 1.83–14.08; p = 002) and disease-free survival (HR: 0.41; 95% CI: 1.86–6.24; p < 0.0001) [10]. There is much debate and ongoing research regarding the molecular approach to the sentinel lymph node diagnosis. Several studies indicate that approximately 10% of sentinel lymph node-negative (as determined by hematoxylin and eosin and immunohistochemical staining) patients may experience local or widespread metastases [11]. A recent article presents results of combined studies of sentinel lymph nodes by three techniques: staining with hematoxylin and eosin, staining with antibodies against S-100 and HMB-45, and detection of tyrosinase mRNA by RT-PCR [12]. In this study, the 45 patients who were negative for melanoma by all three techniques did not have a recurrence in over 2 years of follow-up. Nevertheless, a very limited number of patients were studied (60 individuals). The conceptual problem related to the detection of tyrosinase and Melan-A by RT-PCR is that these genes are expressed by both melanoma and nevus cells. In addition, melanoma cells express different antigens with variable intensity. There is an effort to find antigens that would make RT-PCR both more sensitive and specific. Analysis of multiple markers (tyrosinase, melanoma antigen recognized by T cell [MART]-1, tyrosinase-related protein [TRP]1, TRP2, melanoma antigen [MAGE]-3, N-acetylgalactosaminyltransferase, Pax3, glycoprotein [gp]100 [10]) is one approach to increase sensitivity. Microarray technology has been employed to find new and possibly more specific antigens. Soikelli et al. identified 22 genes that differentiated lymph nodes in patients without melanoma from sentinel lymph nodes in patients with melanoma [13]. Subsequent prospective studies of Melan-A and tyrosinase expression confirmed their possible significance in the determination of patients' survival. Moreover, osteopontin (SPP1) and preferentially expressed antigen in melanoma (PRAME) were identified as melanoma-specific markers that differentiate melanoma cells from nevus cells in the sentinel lymph nodes. SPP1 is a phosphoprotein that binds CD44 and integrins, and regulates cell growth, adhesion, motility and angiogenesis [14]. PRAME is a melanoma-associated antigen recognized by cytotoxic lymphocytes [15]. Its function is unknown and, although it was discovered several years ago, it is still not used for differential diagnosis between benign and malignant melanocytic lesions. Proteins such as Skp2 and p27(kip1), which are implicated in melanoma progression, are another possibility [16]. The other potential proteins that may be used for sentinel lymph node study may include those that characterize their immunological microenvironment (i.e., expression of pro- and anti-inflammatory cytokines). Expression levels of IL-13, leptin, lymphotoxin-β receptor and macrophage inflammatory protein (MIP)1b is significantly higher, and expression level of IL-11Ra is lower in melanoma-positive compared with melanoma-negative sentinel lymph nodes [17]. Determination of the level of expression of these genes may aid in the assessment of sentinel lymph nodes. The other possibility is the expression of OX40 (CD134) on sentinel lymph node CD4 T cells. Expression of this gene decreased with more advanced features (e.g., thickness and ulceration) of primary cutaneous melanoma [18]. Immune cells and mediators also offer a possible explanation as to why melanoma cells mainly metastasize via of the lymphatic system. It is known that melanoma cells themselves are highly immunogenic. The immune system must be suppressed locally or systemically before metastases will develop. Patients with advanced melanoma have a high percentage of FOXp3-positive, IL-10-producing Treg lymphocytes [19]. It is possible that melanoma cells produce cytokines, such as TGFβ2, which might convert peripheral dendritic cells into cells with a tolerant phenotype [20]. These cells would migrate towards lymph nodes that neighbor the primary site of cutaneous melanoma. Lymph nodes with such an immunotolerant microenvironment would be natural harbors of spreading melanoma cells.
Molecular technology not only allows for more sensitive and specific assessment of sentinel lymph nodes, but also makes it possible to detect cancer cells in the circulation. The sole fact of the existence of circulating cancer cells seems to be only one of the prerequisites for the development of metastasis; nevertheless, it seems to be an important one. Markers used to detect circulating melanoma cells used in different studies included tyrosine, Melan-A, MAGE-3, melanoma cell-adhesion molecule-18, p97 and gp100 [21–24]. Most of these studies (∼60%) [22] found a correlation between the presence of circulating melanoma cells and poor outcome in the patients. Already, measurement of tyrosinase mRNA levels was a significant adverse prognostic factor for disease-free survival [25]. Several markers, such as Melan-A, gp100, MAGE-3, melanoma-inhibiting activity (MIA) and tyrosinase were used in the studies by Arenberger et al. [26]. Of those, MAGE-3 correlated the best with disease progression. Three markers were detected in 39% of the patients as opposed to one marker in 33% of the patients during disease progression. MAGEA-plex, MART-1 and tyrosinase delineated circulating melanoma cells most efficiently in studies by Xi et al. [27] because they had at least a 1000-fold higher expression in the melanoma cells within the peripheral blood. Of note, new methods are being developed that might allow for more effective yield of cancer cells from blood [28]. In addition, it seems that not the number of circulating single tumor cells but rather cell clusters correlate with the development of metastases [29]. Hence, PCR methods might not correlate as well with the development of metastases as previously thought.
Adequacy of several proteins is also being tested as markers of melanoma progression. TA90 immune complex (TA90IC) and MIA protein predicted survival in patients with stage III disease [30]. There are conflicting data regarding the usefulness of S-100β [30,31]. VEGF, VEGF receptor (VEGFR)1, TGF-β1 and Bcl-2 but not matrix metalloprotease (MMP)-3, angiogenin or VEGFR2 in serum correlated in some way with advancing stages in melanoma [32–34]. Proteomic analyses offer a promising alternative. In a study by Mian et al., 82% of samples from patients with stage III disease were correctly identified as having been derived from high-risk patients [35].
Search for the distant metastases in patients includes physical examination, chest radiography, ultrasonography, computed tomography (CT), MRI, lymphoscintigraphy and PET. (18F) fluorodeoxyglucose used in PET marks malignant cells. Malignant cells accumulate glucose as a result of upregulation of glucose transport (GLUT) molecules on their surface (such as GLUT1) [36]. PET has a superior role in the detection of distant metastases in all sites, except the thorax, iliac lymph nodes, subcutis and psoas muscle [37,38]. It has an overall 74% sensitivity and 67% specificity [37]. Concurrent PET and CT, especially with dedicated CT interpretation increases sensitivity to 98% and specificity to 94% [38]. New methods of detection of metastases were developed, such as melanin-inversion recovery imaging [39]. Radioactively labeled α-melanocyte-stimulating hormone (MSH) (203Pb, 99m cytotoxic T lymphocyte or 111In) may be an alternative probe to use for the detection of distant metastases [40,41].
Mechanisms of metastasis
It is generally believed that a neoplastic process progresses through several stages: tumor initiation, progression, invasion and then metastasis. Mechanisms of tumor (including melanoma) initiation and progression are reviewed elsewhere [42–44]. Of note, recent attention of the scientific (including melanoma) community shifted from progressive changes of neoplastic clones (accumulation of new mutations leading to more aggressive phenotypes) to the concept of neoplastic stem cells (Figure 4) [45–47]. According to this theory, tumors harbor stable stem cells that give rise to subsequent progeny. Stem cells would thus provide an attractive target for antineoplastic therapies. The actual initiating event of metastasis is not known. One concept suggests that the fusion of cancer cells with macrophages or other migratory bone marrow-derived cells underlies metastasis [48]. Fusion would result in activation of master regulatory genes that activate multiple pathways, particularly related to epithelial–mesenchymal transition, such as SNAIL, SLUG, SPARC and TWIST. Electron microscopy studies of spontaneous PADA melanoma showed that melanin is located in autophagosomes and not in the cytoplasm, as it is normally packaged in the melanocytes [49]. This provides one of the many clues that cancer cells can acquire the phenotype of macrophages.
Figure 4. Melanomas are characterized (similar to most metastatic tumors) as a heterogenous population of tumor cells that are genetically unstable and lead to clonal divergence and acquisition of metastatic phenotype.
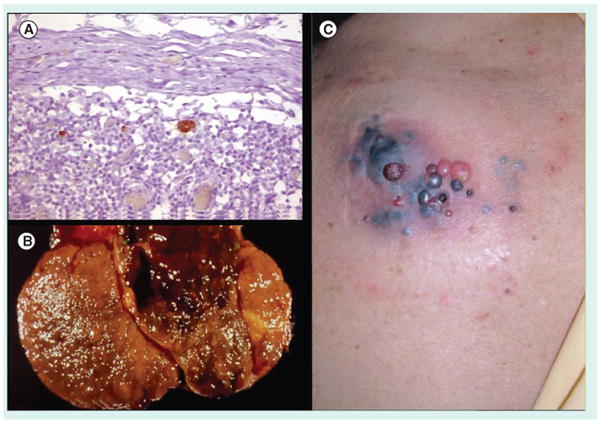
A desmoplastic melanoma that developed a dark papule that lead to clinical and pathologic recognition is shown. The patient developed a lung metastasis with a similar population of cells.
The process of metastasis consists of several phases, as described by Nguyen and Massague [42], including vessel formation, evasion of immune surveillance, embolism, capillary adhesion, extravasation and organ-specific colonization. The spread of metastasis can occur through the vessel lumina, but also via extracellular lattices. Melanoma cells are characterized by an intrinsic, cell-specific phenotype inherited from their cells of origin. We will describe these phenomena more closely.
Routes of metastasis
Melanoma cells follow different patterns of metastatic spread. Some mechanisms responsible for interaction of melanoma cells with specific target organs have been elucidated. An excellent analysis was performed by the German Central Malignant Melanoma Registry, who recorded the data of patients treated in Tuebingen University Department of Dermatology in the years 1976–1996 [50]. Of the 3001 patients who presented with primary cutaneous melanoma, 466 developed metastases. Of these patients, 50% developed regional lymph node metastases, 22% developed satellite or in-transit metastases and 28% developed distant metastases, including distant skin metastases (Figure 5). Of the patients with distant metastases, 57% died. Of several possible risk factors (patient sex, age, tumor location, histology, thickness and level of invasion), primary tumor location predicted most strongly where metastases would develop. As a whole, primary melanomas metastasize most frequently to the regional lymph nodes. Tumors located in the extremities and trunk had the strongest predilection to develop satellite or in-transit metastases (Figure 6). Tumors located in the head and neck areas tended to develop metastases via all three routes. Statistical analyses showed certain gender-related predilections that were related to the actual primary melanoma site. Of interest, tumor thickness was also related to certain patterns of metastasis development. Tumors less than 0.76 mm and more than 1.5 mm thick developed preferentially satellite or in-transit metastases. Tumors of average thickness (0.75–1.5 mm) showed the highest rate of development of distant metastases. Time from initial presentation to the development of metastases was significantly longer for distant metastases. Accordingly, the mean period to development of distant metastases was 25 months, satellite/in-transit metastases was 17 months and lymph node metastases was 16 months. Other studies showed a similar distribution of routes of metastases and time of presentation [51–53].
Figure 5. Melanoma metastatic to subcutaneous tissue.

Distant subcutaneous melanoma metastases are a common phenomenon. Illustrated here is a well-circumscribed subcutaneous melanoma nodule from the scalp. Resection of distant metastases (metastatectomy), such as subcutaneous metastases, in selected melanoma patients is associated with improved 5-year survival [160].
Figure 6. In-transit melanoma metastasis of the lower extremity.
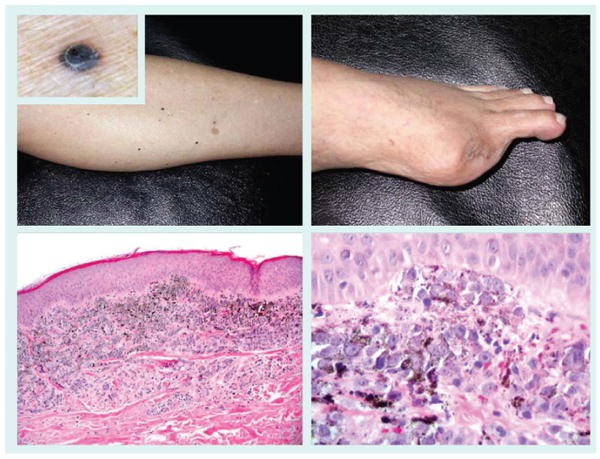
Extremity melanomas are often associated with a protracted course characterized by in-transit metastasis slowly progressing to the regional lymph node basin and beyond. The metastases can be epidermotropic and mimic a primary melanoma. Amputation of an acral melanoma of the first toe (top right panel) was followed by the acquisition of numerous ascending in-transit metastases, seen as small dark papules (top left panel and inset). Biopsy revealed well-defined superficial dermal nodules (bottom left panel) of melanoma cells abutting the epidermis (bottom right panel).
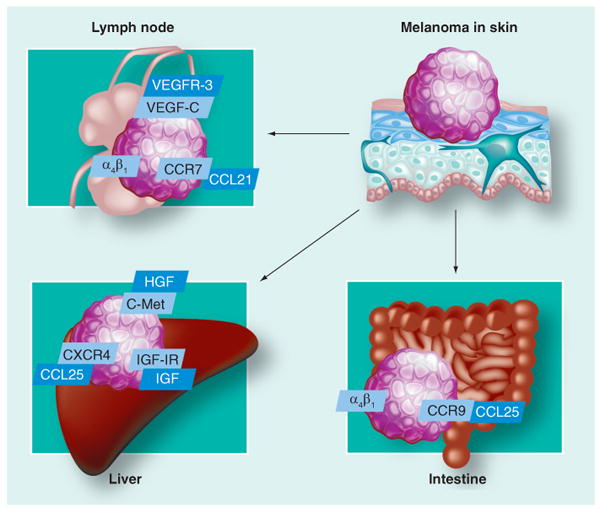
The old concept of ‘seed and soil’, that is, random dissemination of cancer cells, was recently challenged with the new concept of receptor–ligand interactions. How tumor cells reach lymphatic vessels is not yet fully elucidated [54]. Neoplastic cells may simply follow an interstitial pressure gradient or may secrete cytokines that induce growth of lymphatics towards the tumor or within the tumor. Tumor cells that invade the extracellular matrix (ECM) reach lymphatic vessels. Malignant cells then flow within the lymphatics to reach the subcapsular sinus of the lymph nodes. VEGF-R2 and VEGF-R3 are predominantly expressed on lymphatic endothelial cells. VEGF is a family of peptides that includes VEGF-A, VEGF-B, VEGF-C and VEGF-D, which bind to several receptors: VEGFR-1, VEGFR-2 and VEGFR-3. VEGF-C and VEGF-D, which bind to VEGFR-3, act on lymphatic endothelial cells to induce the production of lymphatic vessels [55]. It is known that melanoma cells can secrete VEGF-C, which induces lymphangiogenesis in the tumor [56]. However, only VEGFR tyrosine kinase inhibitors, and not antibodies (which target all VEGF receptors) reduced melanoma lymph node metastasis [57]. High VEGF-C levels in serum are related to deep lymph node involvement [58]. However, neither VEGFR-3 nor CD31 expression in primary cutaneous melanoma predicted lymph node involvement [59]. To our knowledge, there are no comprehensive studies to determine mechanisms or gene expression in melanomas that tend to metastasize to regional versus distant sites. Some melanomas express ligands/receptors that would cause preferential homing of melanoma cells to lymph nodes versus ligands/receptors associated with adherence of melanoma cells to tissues in distant sites. Chemokine/chemokine receptors and adhesion molecules are potential candidates of such ligand/receptor pairs. Chemokines are structurally related, small (8–14 kDa) polypeptide signaling molecules that activate serpentine G-protein-coupled receptors. The chemokine receptors are divided into CXC, CC, C and CX3C subgroups. Melanoma cells can express chemokine receptors chemokine (C-X-C motif) receptor (CXCR)4, CXCR7, CC-chemokine receptor (CCR)9, CCR10 and chemokines CXCL8, chemokine (C-X-C motif) ligand (CXCL)1–3, chemokine CC-ligand (CCL) 5 and CCL2 (Table 1) [60]. The study by Takeuchi et al. [61] showed a heterogeneous expression of CCR7 by melanoma cells. This receptor is normally expressed on naive T cells and dendritic cells. The CCL21/secondary lymphoid tissue chemokine (SLC) produced in lymph nodes causes homing of CCR7-bearing naive T cells and dendritic cells to the lymph nodes. In the aforementioned study, CCR7-bearing melanoma cells migrated preferentially in response to CCL21/SLC. The other potential chemokine/chemokine receptor is CXCL12 and CXCR4. The HR of melanoma relapse and death in patients whose melanoma cells expressed CXCR4 was 2.5 (95% CI: 1.2–6.1) as compared with patients with CXCR4-negative tumors [62]. The ligand for another chemokine receptor (CCR10) expressed on melanoma cells, CCL27, is expressed by normal keratinocytes and may stimulate melanoma development [63]. There is a difference in melanomas expressing different integrins. Melanomas that express integrin αvβ3 tend to develop lung metastases [64]. On the other hand, melanomas expressing integrin α4β1 develop lymph node metastases [65]. As far as distant metastases are concerned, expression of p75 NGF receptor (NGF-R) correlates with brain metastases and survival of brain-metastatic cell lines of epitheliod melanomas (Figure 7) [66]. NGF-R (low-affinity neurotrophin receptor) and TrkC (high-affinity neurotrophin receptor) are expressed in melanoma cells [66,67]. Their stimulation results in the enhancement of melanoma invasion through the stimulation of heparanase activity [68,69]. Axelsen et al. have identified thousands of genes that characterize normal tissues and different types of solid neoplasms (brain, breast, colon, endometrium, kidney, liver, lung, ovary, prostate, thyroid and melanoma) [70]. As expected, only a minority of genes were differentially expressed in cancers compared with their tissues of origin. Interestingly, different neoplasms were characterized by different levels of gene expression from normal tissues other than their tissue of origin. Melanomas expressed the highest number of genes that characterize the normal brain. This overexpression might contribute, in part, to the mechanism of metastases of melanoma to the brain, or just be related to the neural crest origin of melanomas [71]. Cutaneous melanoma metastasizes most commonly to the lungs (Figure 8) [72]. A single focus of pulmonary metastasis is associated with a better survival than the presence of multiple foci [73]. There are also data indicating that advanced age is associated with a better survival in mice with pulmonary metastasis as opposed to other sites of metastasis [74]. Tumor-doubling time might help determine which patients with pulmonary metastatic melanoma will benefit from surgery [75,76]. The liver is the most common (and often, the only) site of systemic metastasis of uveal melanoma [77]. Compared with normal melanocytes, uveal melanoma cells express more c-Met, IGF-IR and CXCR4. There is a high expression of corresponding ligands (HGF, IGF and CXCL12, respectively) in the liver [78]. Of all cancers, cutaneous melanoma is the most common type to metastasize to the submucosa of the small intestine (Figure 9) [79]. Migration to the small intestine is thought to be directed by CCL25, which is produced by the epithelium of the small intestine and attracts CCR9-bearing melanoma cells [80]. Integrin α4β1, but not β7, facilitates this process [80]. Examples of well-characterized receptor–ligand interactions in melanoma metastasis are summarized in Figure 10.
Table 1. Chemokines and melanoma: summary of chemokine/chemokine receptor pairs expressed on melanoma cells.
| Chemokine receptor | Chemokine | Ref. |
|---|---|---|
| CCR7 | CCL21/SLC | [61] |
| CXCR4 | CXCL12 | [62,78] |
| CCR10 | CCL27 | [63] |
| CCR9 | CCL25 | [80] |
CCR: CC-chemokine receptor; CCL: Chemokine CC-ligand.
Figure 7. Melanoma brain metastasis.
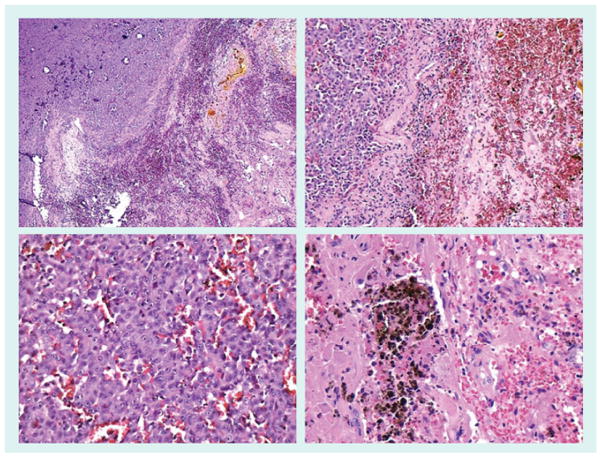
Brain metastases are common in melanoma and often hemorrhagic [161]. The top left panel depicts a cerebral metastases (top left) bordering a necrotic and hemorrhagic area (bottom right). The bottom left panel shows extravasated red blood cells and small vessels surrounding a nest of melanoma cells. The top right panel shows cerebral metastases (left side) adjacent to a large area of hemorrhage (right side). The bottom right panel illustrates both hemosiderin and hemorrhage in brain parenchyma.
Figure 8. Melanoma lung metastasis.

The lung is the most common sight of cutaneous melanoma visceral metastasis. Depicted in the left panel is replacement of most of the lung parenchyma by metastases. The top right panels shows the melanoma cells infiltrating alveolar spaces. The bottom right panel shows a metastasis with variable melanization of tumor cells.
Figure 9. Melanoma small intestine metastasis.
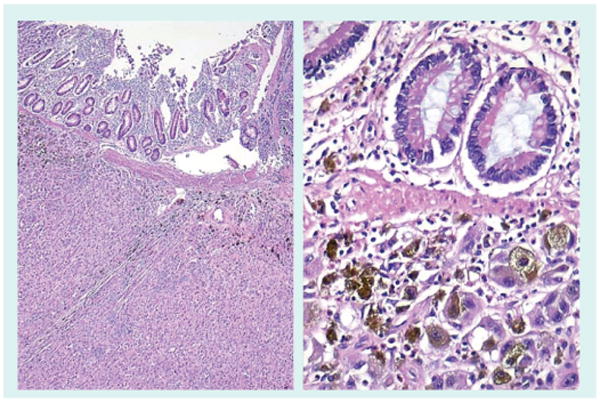
Metastasis to small intestine are rare and most often due to melanoma. The left panel shows submucosal small intestinal metastases. The right panel illustrates a small cluster of melanoma cells in the lamina propria.
Figure 10. Selected mechanisms of chemotaxis of melanoma cells to lymph nodes and distant organs.
Vessel formation
Formation of new vessels in primary and metastatic tumors is of extreme importance. This is thought to occur through the recapitulation of embryonic vessel formation (i.e., vasculogenic mimicry). This is defined more strictly as endothelial-like features being acquired by tumor cells. The tumor microenvironment, that is, interstitial fluid pressure, pH, cytokines, laminin, collagens, growth factors, nutrients and oxygen, is thought to stimulate angiogenesis through different mechanisms. Melanocytes of the epidermis are located in the milieu of decreased oxygen concentration which is thought to be responsible for their possible neoplastic transformation through stabilization of hypoxia-inducible factor (HIF)-1α and Akt-dependent pathway [81,82]. Hypoxia is known to increase expression of CXCR-4 on melanoma cells [83]. Vasculogenic mimicry was the major pattern of new vessel formation in the skeletal muscle and endothelium-dependent vessel formation in the abdominal cavity [84]. Difference in interstitial pressure offers a plausible explanation of why different mechanisms are in place. Melanoma cells developing in the dense structure of skeletal muscle require a more aggressive, enzyme-dependent mechanism for their growth and vessel formation. With regard to melanoma metastases in other sites, the number of microvessels in melanoma metastases in the brain was lower than the number seen in metastases to the breast or lung, and their diameter was intermediate when compared with these two types of metastases [85]. This finding was unexpected, since brain melanoma metastases are characterized by extremely frequent hemorrhage.
VEGF-A is the most potent stimulant of angiogenesis; however, data regarding its correlation with melanoma metastases are conflicting [32,86]. VEGF-A has multiple isoforms that are formed by alternative splicing of single mRNA [87,88]. One of the classes of isoforms of VEGF, VEGFxxxb, is known to have actual antiangiogenic properties [89]. VEGFxxxb expression is decreased in metastatic melanoma, which suggests switching from anti-angiogenic to proangiogenic isoforms during the formation of melanoma and metastasis [90].
Extravasation
Although melanoma is considered to metastasize mainly via the lymphatic route, the process of melanoma cells adhering to endothelial cells and the subsequent invasion into ECM has been studied extensively. Extravasation is the movement of cancer cells from the vessels to the surrounding tissue. Important parts of this process are adhesion to endothelial cells, and then degradation of components of the ECM (e.g., proteoglycans, including heparin sulphate). Migration of leukocytes through the endothelial cell layer involves interaction of β2-integrins LFA-1 or Mac-1 with their ligand ICAM-1. Most tumor cells do not express β2-integrins. Neutrophils may enhance the adhesion of melanoma cells to the endothelium and, subsequently, their extravasation. This is achieved by the binding of ICAM-1 onto melanoma cells and β2-integrins onto neutrophils [91]. This process is regulated by IL-8 that, in turn, is controlled by B-Raf [92]. B-Raf encodes a Ras-regulated kinase that regulates cell proliferation and is mutated in a specific site (glutamic acid for valine substitution at codon 600 in exon 15) [93]. On the other hand, some melanoma cells express other potential candidates that could mediate adhesion: αvβ3 or α4β1 integrins [94]. However, only highly metastatic melanoma cells expressing α4β1 bind to vascular cell-adhesion molecule (VCAM)-1 on endothelial cells as opposed to non-metastatic melanoma cells expressing similar levels of α4β1 [94]. This suggests an ancillary rather than primary role of this integrin in the adhesion. Lactadherin was recently shown to enhance phagocytosis of apoptotic melanoma cells [95]. This may have significance, not only in tumor development but also in adhesion of melanoma cells to endothelial cells. Both neutrophils and melanoma cells transiently express heparinase during invasion [96]. Production of metalloproteases by cancer cells is of paramount importance since tumor cells have to degrade collagens of the ECM in the setting of increasing interstitial pressure during tumor growth. It was shown that aggressive melanomas produce laminin-5 (Ln-5, γ2-chain), and membrane-type matrix metalloproteinase (MMP)-1, -2, -9 and -14 [97]. Ln-5 can then render poorly aggressive melanoma cells more aggressive. Melanoma cells metastasizing to the skeletal muscle overexpress MMP-2 and MMP-9, CK-18 and HIF-1α compared with melanoma cells metastasizing to the abdominal cavity [84]. Metalloproteases cleave triple helical collagen only at specific sites. Cathepsins B and L cleave collagen at specific extrahelical regions. Cathepsin K cleaves collagen both at intra- and extrahelical sites. Expression of all cathepsins have been documented in melanoma [98,99]. These enzymes not only cut collagens, but also elastin. Melanoma develops in the skin, an organ with a particular abundance of elastin. Elastin-derived peptides, such as VGVAPG or VAPG, bind to receptors on melanoma: galectin-3, integrin αVβ3 and the elastin-binding protein. Moreover, after binding of these peptides, melanoma cells become more aggressive and express more CXCR-4, MMP-2, MMP-3, VEGF-C, CD44, ICAM1 and neural cell adhesion molecule (NCAM) [100]. Extravasation is also promoted by autocrine production of VEGF-A acting on VEGFR-1 and -2 on melanoma cells [101]. Osteopontin, a gene overexpressed in melanoma as evidenced by microarray analyses, increases expression of several proteinases and may serve as an additional prognostic marker [102–104].
Evasion of immune surveillance
Melanoma cells develop mechanisms that prevent them from being attacked by immune cells. Natural killer (NK) cells are one of the most potent modes of organism defense against cancers. NKG2D ligands activate NKD2D (receptors) on NK cells. Intracellular retention of the NKG2D ligand MCH-I chain-related gene A (MICA) is responsible for evasion of melanoma cells from the effects of NK cells [105]. Melanoma cell proliferation is inhibited by cytokines, such as IL-2, IL-12, IFN-α, IFN-γ, TNF-α and IL-6. Oncostatin M is one of the families of IL-6-related cytokines that act through gp130 receptors and Janus kinase–signal transducers and activators of transcription pathways. Metastatic melanomas developed resistance to these cytokines through the modification receptor of oncostatin M. More specifically, hypoacetylation of the promoter of β-subunit rendered this receptor less responsive to oncostatin M [106].
Embolism
It is known that prothrombotic status promotes metastasis, whereas anticoagulation prevents its formation [107]. Neoplastic cells in circulation form complexes with leukocytes and platelets [108]. Platelets are thought to form a protective barrier that is an additional protective factor against the immune system [109]. In general, several molecules have been linked to metastasis formation, such as thrombin, tissue factor, P-selectin, fibrinogen and lypophosphatidic acid [108]. Congenital prothrombotic disorders predispose to the formation of lung metastases [110]. In one study, protease-activated thrombin receptor (PAR)-1 and not fibrin, tissue factor or VEGF correlated with occurrence of metastases [111]. Protease-activated thrombin receptor is a receptor with serpentine structure, activated through the cleavage of the extracellular amino terminus by thrombin. Cleaved terminus binds to the second extracellular domain of the receptor, resulting in an intracellular signal [112]. The platelet-specific receptor gp Ib-IX has a primary role in tethering the platelets to thrombus-forming surfaces [113]. gp Ib-IX, more specifically the α unit of gp Ib, is important in the formation of melanoma metastases in the lung [114]. Platelet factor ([PF]-4/CXCL4) has a role in thrombus formation [115]. A variant of PF-4, PF-4var/CXCL4L1, inhibits angiogenesis, development and metastasis of melanoma [116].
Angiotropism
Most models of metastasis assume that tumor cells intravasate into lymphatic or vascular circulation and then extravasate. Interestingly, an alternative concept has been developed with regards to melanoma metastasis [117]. It is known that melanoma spreads along nerves and skin appendages. As noted previously, the presence of melanoma cells in the circulation does not predict metastasis. It was noted that melanoma cells are located under endothelium entangled in matrix-containing laminin [118]. This concept suggests that melanoma cells travel along external surfaces of vessels rather than in their lumen (Figure 11). This model was called ‘extravascular migratory metastasis’ or ‘angiotropism’ [117]. γ1-aminin enhances melanoma pulmonary metastases [119]. Specific γ1-laminin-derived peptide, C16 (KAFDITYVRLKF), increased angiotropism without intravasation in the shell-less chick chorioallantoic membrane model [117]. This provides some evidence of angiotropism as a possible mechanism of melanoma spread.
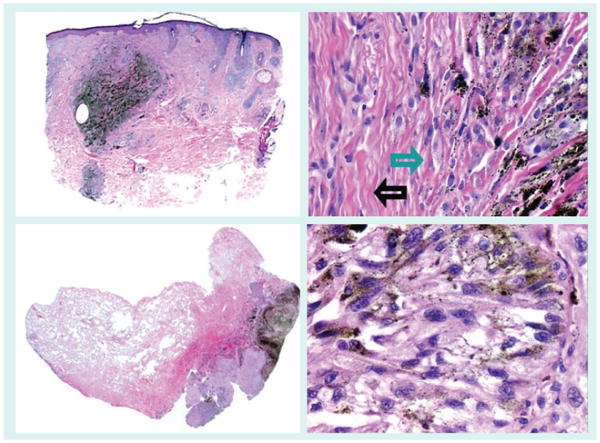
Figure 11. Melanoma angiotropism.
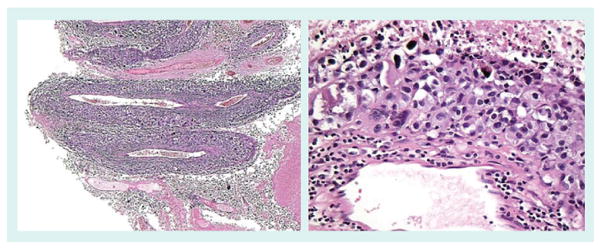
Angiotropism, perivascular cuffing by melanoma cells of the external surface of vessels, is a recently described phenomenon [162], which has been shown to independently predict for local recurrence and in-transit metastasis [163]. The left panel shows the expansion of the media and adventitia of several small muscular vessels by melanoma cells. The right panel demonstrates a small vessel surrounded by a cuff of melanoma cells.
Melanocyte as the ‘source cell’ of melanoma
Melanoma is known to be more aggressive than most other cancers. Introduction of the Ras oncogene into normal melanocytes resulted in significantly more metastasizing melanomas than similar introduction into normal fibroblasts or epithelial cells [120]. This provides evidence that an intrinsic feature of the melanocyte might be responsible for the rapid development of metastatic disease. Since melanocytes are derived from the neural crest, they are characterized by expression of the motility-associated genes that not only mediate the neural crest but also tumor cell migration. Some of those genes are transcription factor Slug, endothelin receptor B, ERBB3, CD44 and Nodal [120,121]. Alternatively, the melanogenesis process that defines the melanocyte, might be the culprit [122]. Formation of melanin consists of transformation of l-tyrosine to melanin pigment through several oxidative–reduction reactions [123]. Melanogenesis thus forms oxidative environment and some of its intermediates (quinones and semiquinones) are directly toxic and mutagenic. Melanin scavenges biomolecules and oxygen. All these processes might significantly enhance the effects of typical oncogenes. Inhibition of melanogenesis should therefore decrease melanoma aggressiveness and act as an enhancer of current therapy protocols [124]. We have recently shown that the inhibition of melanogenesis by phenylthiourea and d-penicillamine enhanced cytoxicity of cyclophosphamide and IL-2-activated lymphocytes against melanoma cells [125].
Therapy
Treatment of melanoma in its early stages is predominantly surgical and consists of excision of the primary tumor with a 1–2-cm margin, and radical lymphadenectomy if the sentinel lymph nodes harbor metastasis. Patients with four or more positive lymph nodes, or melanoma extending beyond the lymph node capsule, may be treated with radiotherapy. Focal recurrent or in-transit tumors in the extremities can be treated with perfusion of limbs with hyperthermia and melphalan [72]. Patients with primary melanoma thicker than 2 mm with ulceration, thicker than 4 mm, regional lymph node involvement, and certainly those with metastatic disease can be offered an adjuvant therapy, possibly in a clinical trial setting with the use of IFN-α2b or IL-2. Alternative treatment options include combinations of dacarbazine (DTIC), tamoxifen, temozolomide and/or thalidomide. Several other options are either in research, preclinical or clinical trial phases [72,126].
So-called targeted melanoma therapy protocols (Table 2) focus on different antigens, receptors or signal transduction pathways-associated proteins such as BRAF, VEGFR, PDGFR, Raf kinases, mitogen-activated protein kinase kinase, Bcl-2, Kit, FLT-3, Cdk4/6, mTOR, PTEN–PI(3)K–Akt, Ras, NF-κB, αvβ3 integrin and heat-shock protein 90 [127]. Sorafenib (Nexavar®, BAY 43–9006) attacks the ATP-binding site of BRAF kinase and also CRAF, VEGFR-2, PDGFR-β, p38, fit-3 and c-kit [128]. Treatment with sorafenib alone resulted in stable disease in only 19% of the patients [129]. In Phase I/II trials, 54% of patients who received a combination of sorafenib with carboplatin and paclitaxel achieved remission lasting longer than 3 months [130]. However, a recent trial showed no difference in overall survival [131]. Treatment of patients with renal cell carcinoma or melanoma with a combination of sorafenib and IFN-α2b resulted in stable disease in 61.5% of patients [132]. Overall disease control rates to sorafenib in different trials ranged from 33 to 92% [133]. Monoclonal anti-CTLA-4 antibodies (MDX-010 and CP-675,206) target cytotoxic T-lymphocyte antigen-4. Cytotoxic T-cell-mediated anti-tumor activity consists of recognition of antigen associated with tumor by T-cell receptor (TCR) of cytotoxic T lymphocyte. This activity is enhanced by subsequent binding of CD80/86 to CD28 on T c. This second step can be impeded by CTLA-4 and, thus, inhibition of CTL-4 can be used as therapy [134]. In total, 11% of melanoma patients treated with anti-CTLA-4 antibody responded clinically [135]. Patients who received combination therapy with anti-CTLA-4 antibody and peptide vaccinations achieved a 17% response rate [136]. Combination therapy with anti-CTLA-4 and IL-2 achieved a 22% response rate [137]. Anti-BCL-2 antisense oligonucleotide (oblimersen sodium) inhibits antiapoptotic protein BCL-2 (Figure 12) [138]. Combination therapy with anti-BCL-2 antisense oligonucleotide and dacarbazine achieved a 13.5% overall response rate [139]. Monoclonal antibody against αVβ3 integrin (MEDI-522) targets a molecule important in endothelial cell proliferation and function that is also expressed in melanomas [140]. Patients treated with combination therapy of MEDI-522 and DTIC had a median survival of 12.6 months [141]. Stimulation of TLR9 activates plasmacytoid dendritic cells, thus inducing an innate immune response. Overall 15% of melanoma patients treated with TLR9 activating nucleotide (PF-3512676) achieved stable disease [142]. Midostaurin (PKC412A) inhibits several targets, including PKCα, VEGFR-2, Kit, PDGFR and FLT-3. In total, 12% of melanoma patients treated with this inhibitor achieved stable disease [143]. Angiozyme is a ribozyme that targets VEGFR-1 [144], a receptor related to angiogenesis. A total of 25% of melanoma patients treated with angiozyme achieved stable disease [145]. Addition of an anti-inflammatory agent, such as rofecoxib, increases modestly but significantly the effect of chemotherapy with trofosfamide [146]. There was an increase in 1-year survival rate of 9% and the HR (therapy augmented with rofecoxib versus chemotherapy alone) was 1.9 (95% CI: 1.17–3.06).
Table 2. Melanoma target therapies: summary of recent attempts of targeted melanoma therapies.
| Drug | Response* (%) | Ref. |
|---|---|---|
| Anti-BRAF kinase | 19–92 | [127–131] |
| Anti-CTLA-4 antibodies | 11–22 | [132–135] |
| Anti-BCL-2 antisense oligonucleotide | 13.5 | [136] |
| Anti-αvβ3 antibody | n/a | [138–139] |
| TLR-9 activating nucleotide | 15 | [140] |
| PKCα inhibitor | 12 | [141] |
| Anti-VEGFR-1 ribozyme | 25 | [142,143] |
See text and references for definitions of response rates.
n/a: Not available; PK: Protein kinase; TLR: Toll-like receptor; VEGFR: VEGF receptor.
Figure 12. Selection of therapy and phenotyping of melanoma metastases.
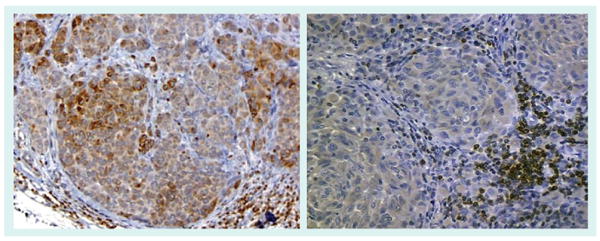
Determination of the phenotype and genotype of metastatic melanoma may play a new role in pathology to aid in the selection of targeted therapy. The antiapoptotic protein Bcl-2 is suspected to influence the treatment responsiveness of melanoma. The lack of Bcl-2 expression demonstrated in the melanoma metastasis in the right panel (lymphocytes are internal positive controls) has been associated with a higher response rate to chemoimmunotherapy in comparison to a diffuse and focal pattern of Bcl-2 expression found in the melanoma metastasis depicted in the left panel [164].
Since results of most clinical trials are very unsatisfactory, there is constant search for new molecular targets and treatment protocols. Potential targets include MCR-1 and CRH-R1 [147,148]. MCR-1 is a most important positive regulator of melanogenesis with stimulating α-MSH, adrenocorticotropin and inhibitory agouti ligands [123,149]. 177Lu-labeled DOTA conjugated to α-MSH prolonged survival of B16/F1 melanoma-bearing C57 mice [150]. CRH-R1 responds to CRH with stimulation of production and release of pro-opiomelanocortin-derived peptides by the pituitary and by the peripheral organs, including skin [151,152]. CRH inhibits proliferation of melanoma cells in vitro [153] and in vivo [154]. Other targets may include modification of steroid production and signaling [155–157] or other endocrine molecules [158,159].
Key issues
The current melanoma staging system is based on melanoma thickness, ulceration and presence of local lymph node and distant metastases.
In patients with stage IV disease, the site of metastasis and the level of lactate dehydrogenase are the most important predictors of survival.
The number of metastases is not currently included in the staging system but may be included in the future when imagining protocols are standardized.
Metastases most commonly first present at regional lymph nodes but approximately a third of them present directly at distant sites.
Melanoma cells reach regional lymph nodes most probably owing to interstitial tissue gradients and/or due to interaction of chemical mediators.
Chemokine/chemokine receptors are thought to be responsible for homing of melanoma cells to distant organs.
The best characterized examples of such chemokine/chemokine receptor pairs are: C-C chemokine receptor (CCR)7 and chemokine (C-C motif) ligand (CCL)21, CCR10, CCL27, CXCR4 and CXCL12.
Assessment of ‘field cells’ and local lymphatic vessel invasion may be additional parameters to be assessed in the excisions of primary cutaneous melanomas.
Assessment of the clinical utility of determination of melanoma-specific gene products, such as tyrosinase mRNA by reverse transcription PCR in sentinel lymph nodes, is underway.
Other more specific melanoma gene products are being discovered and may become new targets in diagnostic protocols.
Assessment of sentinel lymph node microenvironment (suppression of immune surveillance) is emerging as another diagnostic possibility.
Data regarding predictive value of determination of circulating melanoma cells or associated proteins are currently not encouraging.
Computed tomography/PET is emerging as the distant metastasis imaging diagnostic modality of choice.
Vasculogenic mimicry is a pattern of new vessel formation in tumors metastatic to skeletal muscle and endothelium-dependent mechanisms in the abdominal cavity.
VEGF plays a role in angiogenesis in metastatic melanomas.
Extravasation of melanoma cells from the vessels is aided by adhesion molecules, interactions with neutrophils, lactadherin, heparanase, metalloproteases, cathepsins, laminin and elastin-derived peptides.
Intracellular retention of the natural killer (NK)G2D ligand major histocompatibility complex I chain-related gene is responsible for evasion of melanoma cells from attack by NK cells.
Modification of the receptor of oncostatin M on melanoma cells renders it resistant to the action of antiproliferative cytokine.
Prothrombotic proteins, such as protease-activated thrombin receptor-1, glycoprotein 1b-IX and platelet factot-4 have a role in the formation of melanoma metastases.
‘Extravascular migratory metastasis’ is an alternative mechanism of metastasis spread.
Slug, endothelin receptor B, ERBB3, CD44, Nodal and apparatus, substrates and products of melanogenesis are probable causes of melanoma aggressiveness.
Melanoma therapy protocols target molecules such as BRAF, VEGF receptor, PDGF receptor, Raf kinases, MEK, Bcl-2, Kit, FLT-3, Cdk4/6, mTOR, PTEN-PI(3)K-Akt, Ras, nuclear factor-κB, αVβ3 integrin and heat-shock protein 90 and, so far, are largely unsuccessful.
Expert commentary
In our opinion, one of the most important things yet to learn in melanoma metastasis biology are the mechanisms of homing of melanoma cells to target metastatic sites. Available data is rather scant and only partly based on experimental models. Pertinent clinical data in regards to mechanisms of routing of melanoma sites to different organs is practically nonexistent. Correlation of homing of melanoma cells with the homing of cells of immune origin is an entirely legitimate approach and has provided us some information on that aspect. Nevertheless, other receptor–ligand mechanisms should be explored. Searching for the presence of receptors on melanoma cells that would bind to tissue-specific ligands is one possible alternative. The other aspect of melanoma metastasis biology is the formation of metastatic cells. This does not have to be a Darwinian event as previously thought, but may occur through other mechanisms. Fusion of cancer cells with macrophages is a possible alternative that should be an object of more intense research and scrutiny in the melanoma community.
Five-year view
The current staging system of melanoma is effective in predicting patient prognosis. Nevertheless, the most important part of the diagnostic process (i.e., assessment of the presence of metastases in the sentinel lymph nodes) is still far from perfect. Evaluation of the expression of genes characteristic of melanoma cells, such as tyrosinase, in the potentially affected lymph nodes may be the most effective tool and become a diagnostic standard in the foreseeable future. Imaging techniques such as CT/PET are currently being intensively developed and if standardized, will become the most efficient tool to assess for the presence of distant metastases. Although traditional melanoma markers, such as S-100 or Melan-A, have a primary role in current diagnostic protocols, markers such as SPP1 and PRAME may become important ancillary tools. There is quite a lot of information about the interaction of melanoma cells with endothelium. Since melanoma cells tend to metastasize via the lymphatic route, there should be more efforts to elucidate the mechanism of interaction of melanoma cells with lymphatic vessels, such as survival in lymph, adhesion and extravasation. Migration of melanoma cells along the external walls of lymphatic vessels is an interesting alternative that should be assessed more closely. In spite of tremendous ongoing efforts, therapies directed at different molecular targets are largely unsuccessful. Combination therapies yield only modestly better effects. There are some efforts that focus on specific melanocyte-derived phenotype of melanoma cells. Melanocytes are not only resistant to UV light and toxins but melanoma cells are even more resistant to radio-, chemo- and biotherapies.
Acknowledgments
This work was supported by the National Institutes of Health grants AR047079 and AR052190 to AS.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Blazej Zbytek, Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, 930 Madison Avenue, Memphis, TN 38163, USA, Tel.: +1 901 448 6300, Fax: +1 901 448 6979, bzbytek@utmem.edu.
J Andrew Carlson, Department of Pathology and Laboratory Medicine, Albany Medical Center, 47 New Scotland Avenue, Albany, NY, USA, Tel.: +1 518 262 8099, Fax: +1 518 262 8092, carlsoa@mail.amc.edu.
Jacqueline Granese, Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, 930 Madison Avenue, Memphis, TN 38163, USA, Tel.: +1 901 448 6300, Fax: +1 901 448 6979, jackiegranese@yahoo.com.
Jeffrey Ross, Department of Pathology and Laboratory Medicine, Albany Medical Center, Albany, NY, 47 New Scotland Avenue, Albany, NY, USA, Tel.: +1 518 262 5461, Fax: +1 518 262 8092, rossj@mail.amc.edu.
Martin C Mihm, Jr, Dermatopathology Unit, Massachusetts General Hospital, 55 Fruit Street, WRN 225, Boston, MA 02114, USA, Tel.: +1 617 726 2967, Fax: +1 617 726 7474, mmihm@partners.org.
Andrzej Slominski, Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, 930 Madison Avenue, Memphis, TN 38163, USA, Tel.: +1 901 448 6300, Fax: +1 901 448 6979, aslominski@utmem.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interes
- 1.Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54(3):131–149. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Buzaid AC, Soong SJ et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]; • Current melanoma staging system.
- 3.Bastian BC, Kashani-Sabet M, Hamm H, et al. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res. 2000;60(7):1968–1973. [PubMed] [Google Scholar]
- 4.North JP, Kageshita T, Pinkel D, Leboit PE, Bastian BC. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol. 2008;128(8):2024–2030. doi: 10.1038/jid.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X, Gimotty PA, Guerry D, et al. Lymphatic invasion revealed by multispectral imaging is common in primary melanomas and associates with prognosis. Hum Pathol. 2008;39(6):901–909. doi: 10.1016/j.humpath.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dadras SS, Lange-Asschenfeldt B, Velasco P, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18(9):1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 7.Dadras SS, Paul T, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162(6):1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton DL, Thompson JF, Cochran AJ et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]; •• Results of important trial assessing sentinel lymph node biopsy.
- 9.Cochran AJ, Ohsie SJ, Binder SW. Pathobiology of the sentinel node. Curr Opin Oncol. 2008;20(2):190–195. doi: 10.1097/CCO.0b013e3282f46d70. [DOI] [PubMed] [Google Scholar]
- 10.Mocellin S, Hoon DS, Pilati P, Rossi CR, Nitti D. Sentinel lymph node molecular ultrastaging in patients with melanoma: a systematic review and meta-analysis of prognosis. J Clin Oncol. 2007;25(12):1588–1595. doi: 10.1200/JCO.2006.09.4573. [DOI] [PubMed] [Google Scholar]
- 11.Fincher TR, McCarty TM, Fisher TL, et al. Patterns of recurrence after sentinel lymph node biopsy for cutaneous melanoma. Am J Surg. 2003;186(6):675–681. doi: 10.1016/j.amjsurg.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Denninghoff VC, Falco J, Kahn AG, Trouchot V, Curutchet HP, Elsner B. Sentinel node in melanoma patients: triple negativity with routine techniques and PCR as positive prognostic factor for survival. Mod Pathol. 2008;21(4):438–444. doi: 10.1038/modpathol.3801020. [DOI] [PubMed] [Google Scholar]; • Recent article regarding the use of reverse transcription-PCR in the analysis of sentinel lymph nodes.
- 13.Soikkeli J, Lukk M, Nummela P, et al. Systematic search for the best gene expression markers for melanoma micrometastasis detection. J Pathol. 2007;213(2):180–189. doi: 10.1002/path.2229. [DOI] [PubMed] [Google Scholar]
- 14.Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90(10):1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda H, Lethe B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6(2):199–208. doi: 10.1016/s1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Murphy M, Ross J, Sheehan C, Carlson JA. Skp2 and p27kip1 expression in melanocytic nevi and melanoma: an inverse relationship. J Cutan Pathol. 2004;31(10):633–642. doi: 10.1111/j.0303-6987.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 17.Torisu-Itakura H, Lee JH, Scheri RP, et al. Molecular characterization of inflammatory genes in sentinel and nonsentinel nodes in melanoma. Clin Cancer Res. 2007;13(11):3125–3132. doi: 10.1158/1078-0432.CCR-06-2645. [DOI] [PubMed] [Google Scholar]
- 18.Sarff M, Edwards D, Dhungel B, et al. OX40 (CD134) expression in sentinel lymph nodes correlates with prognostic features of primary melanomas. Am J Surg. 2008;195(5):621–625. doi: 10.1016/j.amjsurg.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Cesana GC, DeRaffele G, Cohen S, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24(7):1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 20.Polak ME, Borthwick NJ, Gabriel FG, et al. Mechanisms of local immunosuppression in cutaneous melanoma. Br J Cancer. 2007;96(12):1879–1887. doi: 10.1038/sj.bjc.6603763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson JA, Slominski A, Linette GP, Mihm MC, Jr, Ross JS. Biomarkers in melanoma: staging, prognosis and detection of early metastases. Expert Rev Mol Diagn. 2003;3(3):303–330. doi: 10.1586/14737159.3.3.303. [DOI] [PubMed] [Google Scholar]; •• Review of biomarkers in melanoma.
- 22.Palmieri G, Casula M, Sini MC, Ascierto PA, Cossu A. Issues affecting molecular staging in the management of patients with melanoma. J Cell Mol Med. 2007;11(5):1052–1068. doi: 10.1111/j.1582-4934.2007.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slominski A, Wortsman J, Carlson JA, Matsuoka LY, Balch CM, Mihm MC. Malignant melanoma. Arch Pathol Lab Med. 2001;125(10):1295–1306. doi: 10.5858/2001-125-1295-MM. [DOI] [PubMed] [Google Scholar]
- 24.Carlson JA, Ross JS, Slominski A et al. Molecular diagnostics in melanoma. J Am Acad Dermatol. 2005;52(5):743–775. doi: 10.1016/j.jaad.2004.08.034. quiz 775–748. [DOI] [PubMed] [Google Scholar]
- 25.Visus C, Andres R, Mayordomo JI, et al. Prognostic role of circulating melanoma cells detected by reverse transcriptase-polymerase chain reaction for tyrosinase mRNA in patients with melanoma. Melanoma Res. 2007;17(2):83–89. doi: 10.1097/CMR.0b013e3280a60878. [DOI] [PubMed] [Google Scholar]
- 26.Arenberger P, Arenbergerova M, Vohradnikova O, Kremen J. Early detection of melanoma progression by quantitative real-time RT-PCR analysis for multiple melanoma markers. Keio J Med. 2008;57(1):57–64. doi: 10.2302/kjm.57.57. [DOI] [PubMed] [Google Scholar]
- 27.Xi L, Nicastri DG, El-Hefnawy T, Hughes SJ, Luketich JD, Godfrey TE. Optimal markers for real-time quantitative reverse transcription PCR detection of circulating tumor cells from melanoma, breast, colon, esophageal, head and neck, and lung cancers. Clin Chem. 2007;53(7):1206–1215. doi: 10.1373/clinchem.2006.081828. [DOI] [PubMed] [Google Scholar]
- 28.Tveito S, Maelandsmo GM, Hoifodt HK, Rasmussen H, Fodstad O. Specific isolation of disseminated cancer cells: a new method permitting sensitive detection of target molecules of diagnostic and therapeutic value. Clin Exp Metastasis. 2007;24(5):317–327. doi: 10.1007/s10585-006-9052-8. [DOI] [PubMed] [Google Scholar]
- 29.Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36(3):889–894. [PubMed] [Google Scholar]
- 30.Faries MB, Gupta RK, Ye X, et al. A comparison of 3 tumor markers (MIA, TA90IC, S100B) in stage III melanoma patients. Cancer Invest. 2007;25(5):285–293. doi: 10.1080/07357900701208634. [DOI] [PubMed] [Google Scholar]
- 31.Domingo-Domenech J, Castel T, Auge JM et al. Prognostic implications of protein S-100β serum levels in the clinical outcome of high-risk melanoma patients. Tumour Biol. 2007;28(5):264–272. doi: 10.1159/000110424. [DOI] [PubMed] [Google Scholar]; • Significance of S-100β serum protein levels in melanoma patients.
- 32.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Circulating levels of vascular endothelial growth factor (VEGF), matrix metalloproteinase-3 (MMP-3), and BCL-2 in malignant melanoma. Med Oncol. 2008 doi: 10.1007/s12032-008-9058-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma Res. 2006;16(5):405–411. doi: 10.1097/01.cmr.0000222598.27438.82. [DOI] [PubMed] [Google Scholar]
- 34.Bolander A, Wagenius G, Larsson A, et al. The role of circulating angiogenic factors in patients operated on for localized malignant melanoma. Anticancer Res. 2007;27(5A):3211–3217. [PubMed] [Google Scholar]
- 35.Mian S, Ugurel S, Parkinson E et al. Serum proteomic fingerprinting discriminates between clinical stages and predicts disease progression in melanoma patients. J Clin Oncol. 2005;23(22):5088–5093. doi: 10.1200/JCO.2005.03.164. [DOI] [PubMed] [Google Scholar]; • Significance of proteomics in melanoma.
- 36.Som P, Atkins HL, Bandoypadhyay D, et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-d-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med. 1980;21(7):670–675. [PubMed] [Google Scholar]
- 37.Prichard RS, Hill AD, Skehan SJ, O'Higgins NJ. Positron emission tomography for staging and management of malignant melanoma. Br J Surg. 2002;89(4):389–396. doi: 10.1046/j.0007-1323.2002.02059.x. [DOI] [PubMed] [Google Scholar]
- 38.Strobel K, Dummer R, Husarik DB, Perez Lago M, Hany TF, Steinert HC. High-risk melanoma: accuracy of FDG PET/CT with added CT morphologic information for detection of metastases. Radiology. 2007;244(2):566–574. doi: 10.1148/radiol.2442061099. [DOI] [PubMed] [Google Scholar]; • Recent article about the significance of PET in metastatic melanoma.
- 39.Crean AM, Juli C. Diagnosis of metastatic melanoma to the heart with an intrinsic contrast approach using melanin inversion recovery imaging. J Comput Assist Tomogr. 2007;31(6):924–930. doi: 10.1097/RCT.0b013e31804b213b. [DOI] [PubMed] [Google Scholar]
- 40.Miao Y, Figueroa SD, Fisher DR, et al. 203Pb-labeled {α}-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. J Nucl Med. 2008;49(5):823–829. doi: 10.2967/jnumed.107.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao Y, Benwell K, Quinn TP. 99mTc- and 111In-labeled α-melanocyte-stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48(1):73–80. [PubMed] [Google Scholar]
- 42.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8(5):341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]; •• Excellent review about general mechanisms of cancer metastasis.
- 43.Gaggioli C, Sahai E. Melanoma invasion – current knowledge and future directions. Pigment Cell Res. 2007;20(3):161–172. doi: 10.1111/j.1600-0749.2007.00378.x. [DOI] [PubMed] [Google Scholar]; •• Excellent review about melanoma invasion.
- 44.Crowson AN, Magro C, Miller A, Mihm MC., Jr The molecular basis of melanomagenesis and the metastatic phenotype. Semin Oncol. 2007;34(6):476–490. doi: 10.1053/j.seminoncol.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Sabatino M, Zhao Y, Voiculescu S, et al. Conservation of genetic alterations in recurrent melanoma supports the melanoma stem cell hypothesis. Cancer Res. 2008;68(1):122–131. doi: 10.1158/0008-5472.CAN-07-1939. [DOI] [PubMed] [Google Scholar]
- 46.Grichnik JM, Burch JA, Schulteis RD, et al. Melanoma, a tumor based on a mutant stem cell? J Invest Dermatol. 2006;126(1):142–153. doi: 10.1038/sj.jid.5700017. [DOI] [PubMed] [Google Scholar]
- 47.Wang E, Voiculescu S, Le Poole IC, et al. Clonal persistence and evolution during a decade of recurrent melanoma. J Invest Dermatol. 2006;126(6):1372–1377. doi: 10.1038/sj.jid.5700193. [DOI] [PubMed] [Google Scholar]
- 48.Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nat Rev Cancer. 2008;8(5):377–386. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]; •• Concept of macrophage and melanoma fusion hybrids.
- 49.Pawelek JM, Chakraborty AK, Rachkovsky ML, Orlow SJ, Bolognia JL, Sodi SA. Altered N-glycosylation in macrophage x melanoma fusion hybrids. Cell Mol Biol (Noisy-le-grand) 1999;45(7):1011–1027. [PubMed] [Google Scholar]; •• Concept of macrophage and melanoma fusion hybrids.
- 50.Meier F, Will S, Ellwanger U, et al. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002;147(1):62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- 51.Markowitz JS, Cosimi LA, Carey RW, et al. Prognosis after initial recurrence of cutaneous melanoma. Arch Surg. 1991;126(6):703–707. doi: 10.1001/archsurg.1991.01410300045006. [DOI] [PubMed] [Google Scholar]
- 52.Reintgen DS, Cox C, Slingluff CL, Jr, Seigler HF. Recurrent malignant melanoma: the identification of prognostic factors to predict survival. Ann Plast Surg. 1992;28(1):45–49. doi: 10.1097/00000637-199201000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Soong SJ, Harrison RA, McCarthy WH, Urist MM, Balch CM. Factors affecting survival following local, regional, or distant recurrence from localized melanoma. J Surg Oncol. 1998;67(4):228–233. doi: 10.1002/(sici)1096-9098(199804)67:4<228::aid-jso4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 54.Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98(2):413–423. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 55.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25(7):387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Skobe M, Hamberg LM, Hawighorst T, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159(3):893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sini P, Samarzija I, Baffert F, et al. Inhibition of multiple vascular endothelial growth factor receptors (VEGFR) blocks lymph node metastases but inhibition of VEGFR-2 is sufficient to sensitize tumor cells to platinum-based chemotherapeutics. Cancer Res. 2008;68(5):1581–1592. doi: 10.1158/0008-5472.CAN-06-4685. [DOI] [PubMed] [Google Scholar]
- 58.Vihinen PP, Hilli J, Vuoristo MS, Syrjanen KJ, Kahari VM, Pyrhonen SO. Serum VEGF-C is associated with metastatic site in patients with malignant melanoma. Acta Oncol. 2007;46(5):678–684. doi: 10.1080/02841860600965020. [DOI] [PubMed] [Google Scholar]
- 59.Wobser M, Siedel C, Schrama D, Brocker EB, Becker JC, Vetter-Kauczok CS. Expression pattern of the lymphatic and vascular markers VEGFR-3 and CD31 does not predict regional lymph node metastasis in cutaneous melanoma. Arch Dermatol Res. 2006;297(8):352–357. doi: 10.1007/s00403-005-0633-1. [DOI] [PubMed] [Google Scholar]
- 60.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118(6):915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]; • Reviews the roles fulfilled by chemokines in melanoma.
- 61.Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res. 2004;10(7):2351–2358. doi: 10.1158/1078-0432.ccr-03-0195. [DOI] [PubMed] [Google Scholar]
- 62.Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11(5):1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 63.Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25(4):345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]; • Reviews the roles fulfilled by chemokines in metastases.
- 64.Hieken TJ, Ronan SG, Farolan M, Shilkaitis AL, Das Gupta TK. Molecular prognostic markers in intermediate-thickness cutaneous malignant melanoma. Cancer. 1999;85(2):375–382. doi: 10.1002/(sici)1097-0142(19990115)85:2<375::aid-cncr15>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 65.Hieken TJ, Ronan SG, Farolan M, Shilkaitis AL, Das Gupta TK. β1, integrin expression: a marker of lymphatic metastases in cutaneous malignant melanoma. Anticancer Res. 1996;16(4B):2321–2324. [PubMed] [Google Scholar]
- 66.Marchetti D, Murry B, Galjour J, Wilke-Greiter A. Human melanoma TrkC: its association with a purine-analog-sensitive kinase activity. J Cell Biochem. 2003;88(5):865–872. doi: 10.1002/jcb.10473. [DOI] [PubMed] [Google Scholar]
- 67.Marchetti D, Aucoin R, Blust J, Murry B, Greiter-Wilke A. p75 neurotrophin receptor functions as a survival receptor in brain-metastatic melanoma cells. J Cell Biochem. 2004;91(1):206–215. doi: 10.1002/jcb.10649. [DOI] [PubMed] [Google Scholar]
- 68.Denkins Y, Reiland J, Roy M, et al. Brain metastases in melanoma: roles of neurotrophins. Neuro Oncol. 2004;6(2):154–165. doi: 10.1215/S115285170300067X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walch ET, Albino AP, Marchetti D. Correlation of overexpression of the low-affinity p75 neurotrophin receptor with augmented invasion and heparanase production in human malignant melanoma cells. Int J Cancer. 1999;82(1):112–120. doi: 10.1002/(sici)1097-0215(19990702)82:1<112::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 70.Axelsen JB, Lotem J, Sachs L, Domany E. Genes overexpressed in different human solid cancers exhibit different tissue-specific expression profiles. Proc Natl Acad Sci USA. 2007;104(32):13122–13127. doi: 10.1073/pnas.0705824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cattell E, Kelly C, Middleton MR. Brain metastases in melanoma: a European perspective. Semin Oncol. 2002;29(5):513–517. doi: 10.1053/sonc.2002.35246. [DOI] [PubMed] [Google Scholar]
- 72.Abeloff MD. Clinical Oncology. Elsevier Churchill Livingstone; PA, USA: 2004. [Google Scholar]
- 73.Neuman HB, Patel A, Hanlon C, Wolchok JD, Houghton AN, Coit DG. Stage-IV melanoma and pulmonary metastases: factors predictive of survival. Ann Surg Oncol. 2007;14(10):2847–2853. doi: 10.1245/s10434-007-9448-y. [DOI] [PubMed] [Google Scholar]
- 74.Chen YM, Wang PS, Liu JM, et al. Effect of age on pulmonary metastases and immunotherapy in young and middle-aged mice. J Chin Med Assoc. 2007;70(3):94–102. doi: 10.1016/S1726-4901(09)70338-7. [DOI] [PubMed] [Google Scholar]
- 75.Carlson JA. Tumor doubling time of cutaneous melanoma and its metastasis. Am J Dermatopathol. 2003;25(4):291–299. doi: 10.1097/00000372-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 76.Ollila DW, Stern SL, Morton DL. Tumor doubling time: a selection factor for pulmonary resection of metastatic melanoma. J Surg Oncol. 1998;69(4):206–211. doi: 10.1002/(sici)1096-9098(199812)69:4<206::aid-jso3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]; • Original article about the concept of tumor-doubling time in melanoma.
- 77.Lorigan JG, Wallace S, Mavligit GM. The prevalence and location of metastases from ocular melanoma: imaging study in 110 patients. Am J Roentgenol. 1991;157(6):1279–1281. doi: 10.2214/ajr.157.6.1950883. [DOI] [PubMed] [Google Scholar]
- 78.Bakalian S, Marshall JC, Logan P, et al. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res. 2008;14(4):951–956. doi: 10.1158/1078-0432.CCR-06-2630. [DOI] [PubMed] [Google Scholar]
- 79.Agrawal S, Yao TJ, Coit DG. Surgery for melanoma metastatic to the gastrointestinal tract. Ann Surg Oncol. 1999;6(4):336–344. doi: 10.1007/s10434-999-0336-5. [DOI] [PubMed] [Google Scholar]
- 80.Amersi FF, Terando AM, Goto Y, et al. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin Cancer Res. 2008;14(3):638–645. doi: 10.1158/1078-0432.CCR-07-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8(6):443–454. doi: 10.1016/j.ccr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. Influence of the microenvironment on melanoma cell fate determination and phenotype. Cancer Res. 2006;66(16):7833–7836. doi: 10.1158/0008-5472.CAN-06-0731. [DOI] [PubMed] [Google Scholar]
- 83.Schutyser E, Su Y, Yu Y, et al. Hypoxia enhances CXCR4 expression in human microvascular endothelial cells and human melanoma cells. Eur Cytokine Netw. 2007;18(2):59–70. doi: 10.1684/ecn.2007.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun B, Zhang S, Zhang D, Gu Y, Zhang W, Zhao X. The influence of different microenvironments on melanoma invasiveness and microcirculation patterns: an animal experiment study in the mouse model. J Cancer Res Clin Oncol. 2007;133(12):979–985. doi: 10.1007/s00432-007-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salgado KB, Toscani NV, Silva LL, Hilbig A, Barbosa-Coutinho LM. Immunoexpression of endoglin in brain metastasis secondary to malignant melanoma: evaluation of angiogenesis and comparison with brain metastasis secondary to breast and lung carcinomas. Clin Exp Metastasis. 2007;24(6):403–410. doi: 10.1007/s10585-007-9077-7. [DOI] [PubMed] [Google Scholar]
- 86.Bayer-Garner IB, Hough AJ, Jr, Smoller BR. Vascular endothelial growth factor expression in malignant melanoma: prognostic versus diagnostic usefulness. Mod Pathol. 1999;12(8):770–774. [PubMed] [Google Scholar]
- 87.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77(7):527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 88.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48(11):2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 89.Woolard J, Wang WY, Bevan HS, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64(21):7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 90.Pritchard-Jones RO, Dunn DB, Qiu Y, et al. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. Br J Cancer. 2007;97(2):223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang S, Slattery MJ, Wagner D, Simon SI, Dong C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann Biomed Eng. 2008;36(4):661–671. doi: 10.1007/s10439-008-9445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang S, Sharma A, Peng HH, Robertson G, Dong C. Targeting mutant (V600E) B-Raf in melanoma interrupts immunoediting of leukocyte functions and melanoma extravasation. Cancer Res. 2007;67(12):5814–5820. doi: 10.1158/0008-5472.CAN-06-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62(23):6997–7000. [PubMed] [Google Scholar]
- 94.Klemke M, Weschenfelder T, Konstandin MH, Samstag Y. High affinity interaction of integrin α4β1 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances migration of human melanoma cells across activated endothelial cell layers. J Cell Physiol. 2007;212(2):368–374. doi: 10.1002/jcp.21029. [DOI] [PubMed] [Google Scholar]
- 95.Fens MH, Mastrobattista E, de Graaff AM, et al. Angiogenic endothelium shows lactadherin-dependent phagocytosis of aged erythrocytes and apoptotic cells. Blood. 2008;111(9):4542–4550. doi: 10.1182/blood-2007-06-094763. [DOI] [PubMed] [Google Scholar]
- 96.Komatsu N, Waki M, Sue M, et al. Heparanase expression in B16 melanoma cells and peripheral blood neutrophils before and after extravasation detected by novel anti-mouse heparanase monoclonal antibodies. J Immunol Methods. 2008;331(1–2):82–93. doi: 10.1016/j.jim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 97.Seftor RE, Seftor EA, Koshikawa N, et al. Cooperative interactions of laminin 5 γ2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61(17):6322–6327. [PubMed] [Google Scholar]
- 98.Quintanilla-Dieck MJ, Codriansky K, Keady M, Bhawan J, Runger TM. Cathepsin K in melanoma invasion. J Invest Dermatol. 2008;128(9):2281–2288. doi: 10.1038/jid.2008.63. [DOI] [PubMed] [Google Scholar]
- 99.Frohlich E, Schlagenhauff B, Mohrle M, Weber E, Klessen C, Rassner G. Activity, expression, and transcription rate of the cathepsins B, D, H, and L in cutaneous malignant melanoma. Cancer. 2001;91(5):972–982. [PubMed] [Google Scholar]
- 100.Pocza P, Suli-Vargha H, Darvas Z, Falus A. Locally generated VGVAPG and VAPG elastin-derived peptides amplify melanoma invasion via the galectin-3 receptor. Int J Cancer. 2008;122(9):1972–1980. doi: 10.1002/ijc.23296. [DOI] [PubMed] [Google Scholar]
- 101.Lacal PM, Ruffini F, Pagani E, D'Atri S. An autocrine loop directed by the vascular endothelial growth factor promotes invasiveness of human melanoma cells. Int J Oncol. 2005;27(6):1625–1632. [PubMed] [Google Scholar]
- 102.Zhou Y, Dai DL, Martinka M, et al. Osteopontin expression correlates with melanoma invasion. J Invest Dermatol. 2005;124(5):1044–1052. doi: 10.1111/j.0022-202X.2005.23680.x. [DOI] [PubMed] [Google Scholar]
- 103.Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor-κ B-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001;276(48):44926–44935. doi: 10.1074/jbc.M103334200. [DOI] [PubMed] [Google Scholar]
- 104.Rangel J, Nosrati M, Torabian S, et al. Osteopontin as a molecular prognostic marker for melanoma. Cancer. 2008;112(1):144–150. doi: 10.1002/cncr.23147. [DOI] [PubMed] [Google Scholar]
- 105.Fuertes MB, Girart MV, Molinero LL, et al. Intracellular retention of the NKG2D ligand MHC class I chain-related gene A in human melanomas confers immune privilege and prevents NK cell-mediated cytotoxicity. J Immunol. 2008;180(7):4606–4614. doi: 10.4049/jimmunol.180.7.4606. [DOI] [PubMed] [Google Scholar]
- 106.Lacreusette A, Nguyen JM, Pandolfino MC, et al. Loss of oncostatin M receptor β in metastatic melanoma cells. Oncogene. 2007;26(6):881–892. doi: 10.1038/sj.onc.1209844. [DOI] [PubMed] [Google Scholar]
- 107.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 108.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Pictures in molecular medicine: three-dimensional visualization of intravascular tumor cells in mice. Trends Mol Med. 2001;7(8):377. doi: 10.1016/s1471-4914(01)02094-9. [DOI] [PubMed] [Google Scholar]
- 109.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300. [PubMed] [Google Scholar]
- 110.Bruggemann LW, Versteeg HH, Niers TM, Reitsma PH, Spek CA. Experimental melanoma metastasis in lungs of mice with congenital coagulation disorders. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00316.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Excellent article examining the effect of coagulation disorders on melanoma metastasis.
- 111.Depasquale I, Thompson WD. Prognosis in human melanoma: PAR-1 expression is superior to other coagulation components and VEGF. Histopathology. 2008;52(4):500–509. doi: 10.1111/j.1365-2559.2008.02978.x. [DOI] [PubMed] [Google Scholar]
- 112.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 113.Bergmeier W, Piffath CL, Goerge T, et al. The role of platelet adhesion receptor GPIbα far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc Natl Acad Sci USA. 2006;103(45):16900–16905. doi: 10.1073/pnas.0608207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jain S, Zuka M, Liu J, et al. Platelet glycoprotein Ib α supports experimental lung metastasis. Proc Natl Acad Sci USA. 2007;104(21):9024–9028. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eslin DE, Zhang C, Samuels KJ, et al. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood. 2004;104(10):3173–3180. doi: 10.1182/blood-2003-11-3994. [DOI] [PubMed] [Google Scholar]
- 116.Struyf S, Burdick MD, Peeters E, et al. Platelet factor-4 variant chemokine CXCL4L1 inhibits melanoma and lung carcinoma growth and metastasis by preventing angiogenesis. Cancer Res. 2007;67(12):5940–5948. doi: 10.1158/0008-5472.CAN-06-4682. [DOI] [PubMed] [Google Scholar]
- 117.Lugassy C, Barnhill RL. Angiotropic melanoma and extravascular migratory metastasis: a review. Adv Anat Pathol. 2007;14(3):195–201. doi: 10.1097/PAP.0b013e31805048d9. [DOI] [PubMed] [Google Scholar]
- 118.Lugassy C, Eyden BP, Christensen L, Escande JP. Angio-tumoral complex in human malignant melanoma characterised by free laminin: ultrastructural and immunohistochemical observations. J Submicrosc Cytol Pathol. 1997;29(1):19–28. [PubMed] [Google Scholar]
- 119.Kuratomi Y, Nomizu M, Tanaka K, et al. Laminin γ 1 chain peptide, C-16 (KAFDITYVRLKF), promotes migration, MMP-9 secretion, and pulmonary metastasis of B16-F10 mouse melanoma cells. Br J Cancer. 2002;86(7):1169–1173. doi: 10.1038/sj.bjc.6600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gupta PB, Kuperwasser C, Brunet JP et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37(10):1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Examines the melanocyte as the source of aggressiveness of melanoma.
- 121.Topczewska JM, Postovit LM, Margaryan NV, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12(8):925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 122.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 123.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]; •• Review of mammalian pigmentation.
- 124.Slominski A, Paus R, Mihm MC. Inhibition of melanogenesis as an adjuvant strategy in the treatment of melanotic melanomas: selective review and hypothesis. Anticancer Res. 1998;18(5B):3709–3715. [PubMed] [Google Scholar]
- 125.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2008 doi: 10.1002/ijc.24005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer. 2007;109(3):455–464. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- 127.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 128.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 129.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95(5):581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Flaherty KT, Brose M, Schucter L. Phase I/II trial of BAY 43–9006, carboplatin (C) and paclitaxel (P) demonstrates antitumor activity in the expansion cohort of patients with metastatic melanoma. J Clin Oncol. 2004;22(Suppl):14S. [Google Scholar]
- 131.McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized Phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008;26(13):2178–2185. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 132.Escudier B, Lassau N, Angevin E, et al. Phase I trial of sorafenib in combination with IFN α2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res. 2007;13(6):1801–1809. doi: 10.1158/1078-0432.CCR-06-1432. [DOI] [PubMed] [Google Scholar]
- 133.Takimoto CH, Awada A. Safety and anti-tumor activity of sorafenib (Nexavar) in combination with other anti-cancer agents: a review of clinical trials. Cancer Chemother Pharmacol. 2008;61(4):535–548. doi: 10.1007/s00280-007-0639-9. [DOI] [PubMed] [Google Scholar]
- 134.O'Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110(12):2614–2627. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 135.Maker AV, Yang JC, Sherry RM, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29(4):455–463. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22 Pt 1):6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a Phase I/II study. Ann Surg Oncol. 2005;12(12):1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mohammad R, Giri A, Goustin AS. Small-molecule inhibitors of Bcl-2 family proteins as therapeutic agents in cancer. Recent Patents Anticancer Drug Discov. 2008;3(1):20–30. doi: 10.2174/157489208783478676. [DOI] [PubMed] [Google Scholar]
- 139.Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 140.Kuphal S, Bauer R, Bosserhoff AK. Integrin signaling in malignant melanoma. Cancer Metastasis Rev. 2005;24(2):195–222. doi: 10.1007/s10555-005-1572-1. [DOI] [PubMed] [Google Scholar]
- 141.Hersey P, Sosman J, O'Day S. A Phase II, randomized, open-label study evaluating the antitumor activity of MEDI-522, a humanized monoclonal antibody directed against the human metastatic melanoma (MM) Proc Am Soc Clin Oncol. 2005;23:711S. [Google Scholar]
- 142.Pashenkov M, Goess G, Wagner C, et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24(36):5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 143.Millward MJ, House C, Bowtell D, et al. The multikinase inhibitor midostaurin (PKC412A) lacks activity in metastatic melanoma: a Phase IIA clinical and biologic study. Br J Cancer. 2006;95(7):829–834. doi: 10.1038/sj.bjc.6603331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pavco PA, Bouhana KS, Gallegos AM, et al. Antitumor and antimetastatic activity of ribozymes targeting the messenger RNA of vascular endothelial growth factor receptors. Clin Cancer Res. 2000;6(5):2094–2103. [PubMed] [Google Scholar]
- 145.Weng DE, Masci PA, Radka SF, et al. A Phase I clinical trial of a ribozyme-based angiogenesis inhibitor targeting vascular endothelial growth factor receptor-1 for patients with refractory solid tumors. Mol Cancer Ther. 2005;4(6):948–955. doi: 10.1158/1535-7163.MCT-04-0210. [DOI] [PubMed] [Google Scholar]
- 146.Reichle A, Vogt T, Coras B, et al. Targeted combined anti-inflammatory and angiostatic therapy in advanced melanoma: a randomized Phase II trial. Melanoma Res. 2007;17(6):360–364. doi: 10.1097/CMR.0b013e3282f1d2c8. [DOI] [PubMed] [Google Scholar]
- 147.Carlson JA, Linette GP, Aplin A, Ng B, Slominski A. Melanocyte receptors: clinical implications and therapeutic relevance. Dermatol Clin. 2007;25(4):541–557. viii–ix. doi: 10.1016/j.det.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007:265–266. 143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Slominski A. Coming of age of melanogenesis-related proteins. Arch Pathol Lab Med. 2002;126(7):775–777. doi: 10.5858/2002-126-0775-COAOMR. [DOI] [PubMed] [Google Scholar]
- 150.Miao Y, Shelton T, Quinn TP. Therapeutic efficacy of a 177Lu-labeled DOTA conjugated α-melanocyte-stimulating hormone peptide in a murine melanoma-bearing mouse model. Cancer Biother Radiopharm. 2007;22(3):333–341. doi: 10.1089/cbr.2007.376.A. [DOI] [PubMed] [Google Scholar]
- 151.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162(1–2):97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 152.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiolog Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 153.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206(3):780–791. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carlson KW, Nawy SS, Wei ET, et al. Inhibition of mouse melanoma cell proliferation by corticotropin-releasing hormone and its analogs. Anticancer Res. 2001;21(2A):1173–1179. [PubMed] [Google Scholar]
- 155.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;455(3):364–366. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 156.Slominski A, Zbytek B, Szczesniewski A, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol. 2005;288(4):E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 157.Slominski A, Zjawiony J, Wortsman J, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271(21):4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Slominski A, Zjawiony J, Paus R, Elias PM, Tobin DJ, Feingold K. Skin as an endocrine organ: implications for its function. Drug Discov Today Dis Mech. 2008 doi: 10.1016/j.ddmec.2008.04.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Slominski A, T D, Zmijewski M, Wortsman J, Paus R. Cutaneous melatonin: production, metabolism and phenotypic effects. Trends End Metab. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 160.Karakousis CP, Velez A, Driscoll DL, Takita H. Metastasectomy in malignant melanoma. Surgery. 1994;115(3):295–302. [PubMed] [Google Scholar]
- 161.Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol. 1983;1(4):313–317. doi: 10.1007/BF00165714. [DOI] [PubMed] [Google Scholar]
- 162.Lugassy C, Barnhill RL. Angiotropic melanoma and extravascular migratory metastasis: a review. Adv Anat Pathol. 2007;14(3):195–201. doi: 10.1097/PAP.0b013e31805048d9. [DOI] [PubMed] [Google Scholar]
- 163.Van Es SL, Colman M, Thompson JF, McCarthy SW, Scolyer RA. Angiotropism is an independent predictor of local recurrence and in-transit metastasis in primary cutaneous melanoma. Am J Surg Pathol. 2008;32(9):1396–403. doi: 10.1097/PAS.0b013e3181753a8e. [DOI] [PubMed] [Google Scholar]
- 164.Vlaykova T, Talve L, Hahka-Kemppinen M, et al. Immunohistochemically detectable bcl-2 expression in metastatic melanoma: association with survival and treatment response. Oncology. 2002;62(3):259–268. doi: 10.1159/000059574. [DOI] [PubMed] [Google Scholar]
Website
- 201.American Cancer Society; GA, USA: 2008. Cancer facts and figures 2007. www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf. [Google Scholar]


