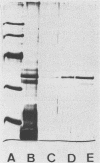
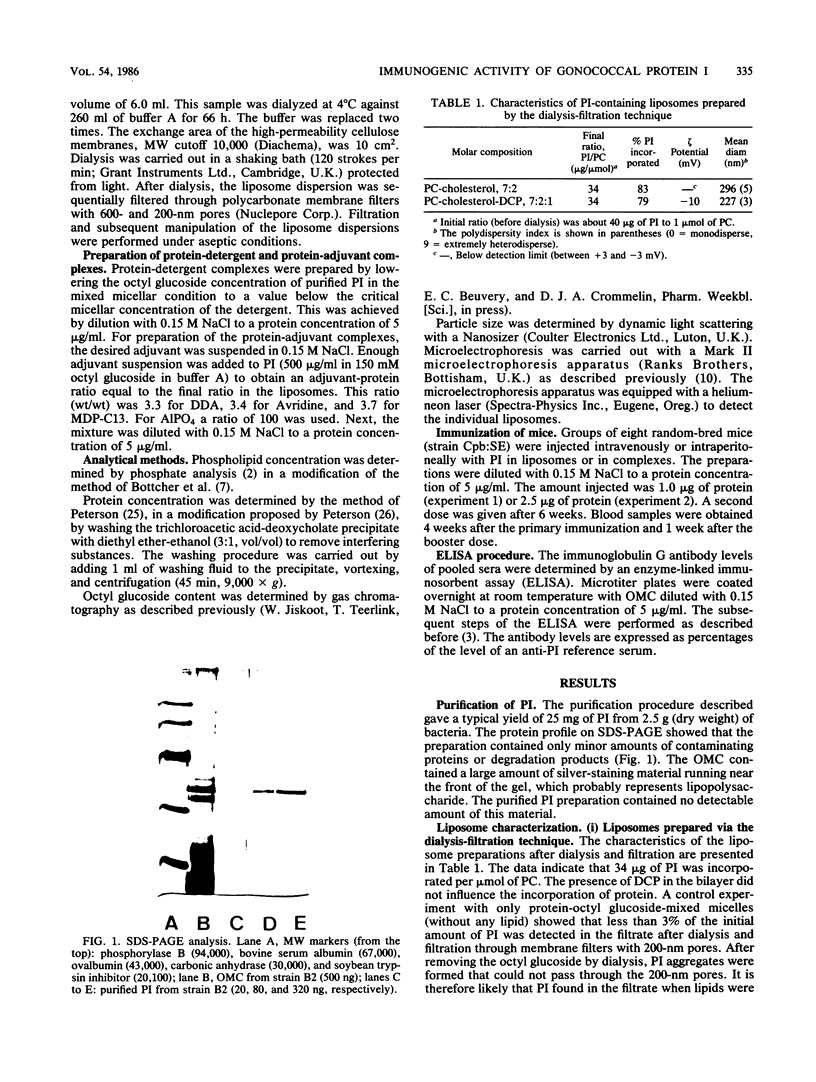
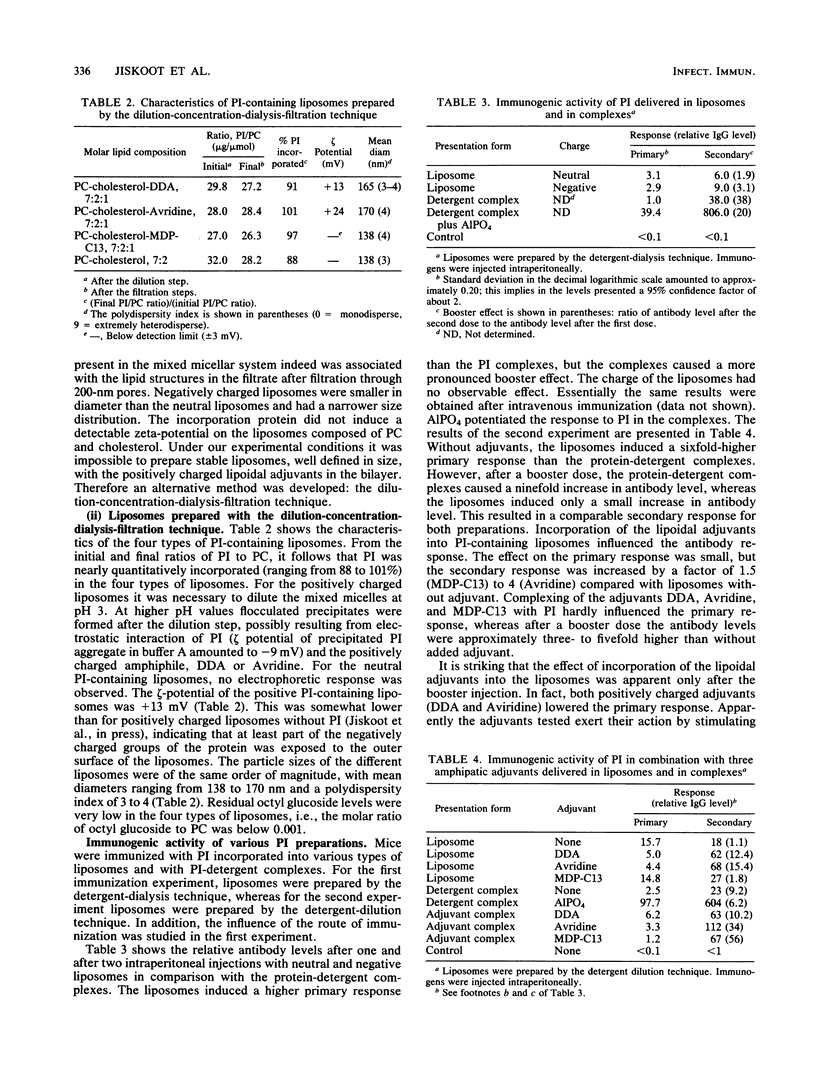
Abstract
For several reasons the major outer membrane protein from Neisseria gonorrhoeae (gonococcal protein [PI]) is an attractive component for a gonococcal vaccine. This paper describes the influence of two different physical forms of PI on its immunogenic activity. To this end PI was delivered in liposomes and in protein-detergent complexes. In both forms PI was present in a multimeric form. The liposomes were composed of phosphatidylcholine and cholesterol. The effect of dicetylphosphate as a negatively charged amphiphile and three lipoidal adjuvants was investigated. Two lipoidal adjuvants (Avridine and dimethyldioctadecylammoniumbromide) were positively charged amphiphiles, whereas the third one (tridecyl N-acetylmuramyl-L-alanyl-D-isoglutaminate) was neutral. The protein-detergent complexes were also tested in the presence of the lipoidal adjuvants and in an AlPO4-adsorbed form. The liposome preparations were characterized for their size, charge, and residual amount of detergent. The immunogenic activity of PI in all forms was tested in mice. The results of the antibody assays showed that PI in the liposomes was more immunogenic than PI in the complexes. A second dose with liposomes induced only a small booster effect, whereas such a dose with the complexes produced pronounced booster effects. The incorporation of the positively charged lipoidal adjuvants in the liposomes resulted in enhanced booster effects. The highest immunogenic activity of PI after two injections, however, was observed in the complexed form adsorbed to AlPO4.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barrera O., Swanson J. Proteins IA and IB exhibit different surface exposures and orientations in the outer membranes of Neisseria gonorrhoeae. Infect Immun. 1984 Jun;44(3):565–568. doi: 10.1128/iai.44.3.565-568.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., Miedema F., van Delft R. W., Haverkamp J., Leussink A. B., te Pas B. J., Teppema K. S., Tiesjema R. H. Preparation and physicochemical and immunological characterization of polysaccharide-outer membrane protein complexes of Neisseria meningitidis. Infect Immun. 1983 Apr;40(1):369–380. doi: 10.1128/iai.40.1.369-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Gonococcal membrane proteins: speculation on their role in pathogenesis. Prog Allergy. 1983;33:298–313. [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984 Feb 1;159(2):452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C., Swanson J. Effects of proteolytic enzymes on the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1981 Jul;33(1):212–222. doi: 10.1128/iai.33.1.212-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crommelin D. J. Influence of lipid composition and ionic strength on the physical stability of liposomes. J Pharm Sci. 1984 Nov;73(11):1559–1563. doi: 10.1002/jps.2600731118. [DOI] [PubMed] [Google Scholar]
- Engel A., Massalski A., Schindler H., Dorset D. L., Rosenbusch J. P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature. 1985 Oct 17;317(6038):643–645. doi: 10.1038/317643a0. [DOI] [PubMed] [Google Scholar]
- Eytan G. D. Use of liposomes for reconstitution of biological functions. Biochim Biophys Acta. 1982 Oct 20;694(2):185–202. doi: 10.1016/0304-4157(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Frantz I. D. Growth Requirements of the Meningococcus. J Bacteriol. 1942 Jun;43(6):757–761. doi: 10.1128/jb.43.6.757-761.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlier D., Bakouche O., Dore J. F. Liposomes as a tool to study the role of membrane presentation in the immunogenicity of a MuLV-related tumor antigen. J Immunol. 1983 Jul;131(1):485–490. [PubMed] [Google Scholar]
- Gotschlich E. C. Development of a gonorrhoea vaccine: prospects, strategies and tactics. Bull World Health Organ. 1984;62(5):671–680. [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Fries E., Kartenbeck J. Reconstitution of Semliki forest virus membrane. J Cell Biol. 1977 Dec;75(3):866–880. doi: 10.1083/jcb.75.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld C. A., Schilham M., Jansen J., Benaissa-Trouw B., Harmsen M., van Houte A. J., Snippe H. The effect of liposomal charge on the neutralizing antibody response against inactivated encephalomyocarditis and Semliki Forest viruses. Clin Exp Immunol. 1984 Jun;56(3):509–514. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marshall T. Detection of protein in polyacrylamide gels using an improved silver stain. Anal Biochem. 1984 Feb;136(2):340–346. doi: 10.1016/0003-2697(84)90227-6. [DOI] [PubMed] [Google Scholar]
- Morein B., Sharp M., Sundquist B., Simons K. Protein subunit vaccines of parainfluenza type 3 virus: immunogenic effect in lambs and mice. J Gen Virol. 1983 Jul;64(Pt 7):1557–1569. doi: 10.1099/0022-1317-64-7-1557. [DOI] [PubMed] [Google Scholar]
- Morein B., Simons K. Subunit vaccines against enveloped viruses: virosomes, micelles and other protein complexes. Vaccine. 1985 Jun;3(2):83–93. doi: 10.1016/0264-410x(85)90055-6. [DOI] [PubMed] [Google Scholar]
- Morein B., Sundquist B., Höglund S., Dalsgaard K., Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. 1984 Mar 29-Apr 4Nature. 308(5958):457–460. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sacci J. B., Jr, Alving C. R., Richardson E. C. Enhancement by lipid A of mucosal immunogenicity of liposome-associated cholera toxin. Rev Infect Dis. 1984 Jul-Aug;6(4):563–566. doi: 10.1093/clinids/6.4.563. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sacci J. B., Jr Enhanced mucosal priming by cholera toxin and procholeragenoid with a lipoidal amine adjuvant (avridine) delivered in liposomes. Infect Immun. 1984 May;44(2):469–473. doi: 10.1128/iai.44.2.469-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Chen K. C., Buchanan T. M. Serology of Neisseria gonorrhoeae: coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules. Infect Immun. 1982 Nov;38(2):462–470. doi: 10.1128/iai.38.2.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Knapp J. S., Buchanan T. B. Serology of Neisseria gonorrhoeae: W-antigen serogrouping by coagglutination and protein I serotyping by enzyme-linked immunosorbent assay both detect protein I antigens. Infect Immun. 1982 Jan;35(1):229–239. doi: 10.1128/iai.35.1.229-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XVIII. 125I-labeled peptide mapping of the major protein of the gonococcal cell wall outer membrane. Infect Immun. 1979 Mar;23(3):799–810. doi: 10.1128/iai.23.3.799-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamauchi H., Tadakuma T., Yasuda T., Tsumita T., Saito K. Enhancement of immunogenicity by incorporation of lipid A into liposomal model membranes and its application to membrane-associated antigens. Immunology. 1983 Dec;50(4):605–612. [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Dancey G. F., Kinsky S. C. Immunogenicity of liposomal model membranes in mice: dependence on phospholipid composition. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1234–1236. doi: 10.1073/pnas.74.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Blake M., Mauro A., Cohn Z. A. Properties of the major outer membrane protein from Neisseria gonorrhoeae incorporated into model lipid membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3831–3835. doi: 10.1073/pnas.80.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]