Abstract
The present study examined the relationship of prenatal cocaine exposure to infant information processing in the first year of life.
In a prospective, longitudinal study of 177 cocaine-exposed and 175 non-exposed infants, the Fagan Test of Infant Intelligence (FTII) was used to measure attention, visual recognition memory and information processing speed at 6.5 and 12 months of age. Groups were compared over time using mixed linear model analyses.
Prenatal cocaine exposure predicted poorer visual recognition memory at 12 months, with exposed infants obtaining lower mean scores and a higher percentage of scores in the risk range. Across exposure groups, information processing speed increased with age, demonstrating a developmental effect. Tobacco and marijuana exposures were related to faster looking times, which did not relate to visual recognition memory.
Cognitive deficits and attentional problems noted in prior studies of cocaine-exposed children at later ages may be detectable in infancy.
Keywords: Cocaine, Infants, Visual recognition, Memory, Fagan Test of Infant Intelligence
1. Introduction
During the 1980s, cocaine use reached epidemic levels in the United States among pregnant women in urban areas, subsequently exposing hundreds of thousands of children to cocaine prenatally (National Institute on Drug Abuse, 1992). Emerging animal and human research suggests that prenatal cocaine exposure may adversely affect child cognitive outcomes (Singer, Arendt, et al., 2002; Singer, Salvator, et al., 2002; Singer et al., 2004; Volpe, 1992), and that, specifically, attentional disorders may be increased (Mayes, 1999). Prenatal cocaine exposure inhibits reuptake of dopamine, norepinephrine, and serotonin in areas of the brain involved in attention and arousal (Stanwood, Washington, Shumsky, & Levitt, 2001), and animals exposed prenatally to cocaine have impaired selective attention (Kosofsky & Wilkins, 1998; Mactutus, 1999; Morgan, Garavan, Mactutus, Booze, & Strupp, 1997). On laboratory-based tasks, cocaine-exposed children have lower rates of selective attention at 4 years of age (Noland et al., 2005; Richardson, Conroy, & Day, 1996) and in later childhood (Leech, Richardson, Goldschmidt, & Day, 1999). One component of visual attention, looking time or look duration, has been conceptualized as an indicator of information processing speed in the first year of life (Colombo, 1993). Shorter look durations during visual processing tasks in the first year of life are indicators of more efficient processing and greater maturity but at older ages or at very low thresholds may reflect distractability and less sustained attention (Colombo, 2002). In one study, prenatal cocaine exposure was related to poorer visual recognition memory, but faster responsiveness on a separate test of visual expectancy (Jacobson, Jacobson, Sokol, Martier, & Chiodo, 1996) suggesting an adverse impact on the attentional system. The study of the outcomes of cocaine-exposed infants has also been complicated by the fact that maternal drug use during pregnancy is associated with a number of factors which also are known to adversely affect child outcomes, including poverty, low socioeconomic status, and prematurity which need to be considered.
The present study examined the effects of prenatal cocaine exposure on speed of information processing and visual recognition memory as indicators of infant cognitive ability in the first year of life. The current study has a larger sample than that of the previous studies of visual recognition memory in cocaine-exposed infants. Further, maternal postpartum psychological functioning was measured and estimates of the severity of cocaine, alcohol, and other drug exposures were made. This study also expanded previous findings by investigating prenatal cocaine exposure effects on attention and information acquisition, measured by the duration of infant looking time. In addition, we assessed the relationship of visual recognition memory to processing speed by infant age and exposure status.
We hypothesized that cocaine-exposed infants would perform more poorly on recognition memory tasks. We also assessed speed of visual processing, expecting that cocaine-exposed infants would show faster information processing speed indicating less sustained attention.
2. Selection and description of participants
2.1. Subjects
A total of 352 infants (177 cocaine-exposed and 175 non-exposed) were participants in this study at 6.5 or 12 months of age (corrected for prematurity) out of a cohort of 415 infants (218 cocaine-exposed, 197 non-exposed) recruited at birth from a large urban county teaching hospital between 1994 and 1996.
2.2. Drug assessments
All recruited mothers and their infants were identified from a high-risk population screened for drug use at birth. Urine toxicology screens were done routinely by the hospital on all mothers who received no prenatal care, appeared to be intoxicated or taking drugs, had a history of involvement with the Department of Human Services in previous pregnancies, self-admitted, or appeared to be at high risk for drug use after an interview with hospital staff. Urine samples were obtained immediately before or after labor and delivery and were analyzed for the presence of cocaine metabolites (benzoylecgonine), cannabinoids, opiates, phencyclidine (PCP), and amphetamines by the Syva Emit method (Syva Company, Palo Alto, CA). The specificity for benzoylecgonine was 99% at 0.3 mg/mL. Follow-up gas chromatography analyses were performed.
Meconium drug analyses were performed for cocaine and its metabolites (benzoylecgonine, meta-hydroxybenzoylecgonine, cocaethylene), cannabinoids, opiates, PCP, amphetamines, and benzodiazepines. A nurse trained in the research protocol collected meconium specimens. In the hospital, infants’ diapers were scraped with a wooden spatula and specimens were placed in a plastic container and refrigerated. Multiple specimens from the same newborn were accumulated and then stirred for 5 min to ensure homogeneity. Further details regarding meconium collection can be found in another publication (Arendt, Singer, Minnes, & Salvator, 1999). Meconium analysis was conducted using gas chromatography—mass spectrography. Screening assays were done with fluorescence polarization immunoassay (United States Drug Testing Laboratories Inc, Des Plaines, IL). Cutoff levels for the drugs of interest were: cocaine and metabolites, 25 ng/g; opiates, 25 ng/g; amphetamines, 100 ng/g; PCP, 25 ng/g; and tetrahydrocannabinol, 25 ng/g. Confirmatory assays were performed with gas chromatography—mass spectrography operated on electron-impact selected-ion monitoring mode.
Infants were identified as cocaine-exposed by a positive response on any one of the following measures: infant meconium, urine, or maternal urine positive for cocaine; maternal report to hospital staff; or maternal self-report during clinical interview. Cocaine-exposed infants were also classified into heavier and lighter severity exposure by dichotomizing the group. Heavier exposure included those infants whose meconium metabolite concentration of benzoylecgonine or maternal self-report was ≥ the 70th percentile for the group. The remainders were classified as lighter exposure. Control subjects were negative for all of the above measures. Both groups included women who used alcohol, marijuana, or tobacco during pregnancy.
To recruit the birth cohort, a nurse recruiter approached 647 women just prior to or after delivery, of whom 54 were excluded (20 cocaine positive, 34 cocaine negative). Reasons for exclusion included no meconium (54), Down Syndrome (2), maternal psychiatric history (16), primary heroin user (2), HIV positive (5), maternal low IQ (1), fetal alcohol syndrome (1), maternal age <19 years (2), maternal chronic illness (4), infant illness (3), and other (3). One hundred fifty-five mothers refused to participate (49 cocaine positive, 106 cocaine negative), and another 23 (9 cocaine positive, 14 cocaine negative) agreed but did not come to the enrollment visit. Mothers who refused to participate were more likely to be younger (p = 0.04) and not to be cocaine users (p < 0.001). Four infants died before the first visit, leaving 415 mothers and their infants enrolled in the study (218 cocaine positive, 197 cocaine negative). Of the 415 subjects, 10 additional subjects died prior to the 6-month visit. Eleven subjects (six cocaine, five non-cocaine) dropped out prior to the 6-month visit. Five additional subjects were excluded from the analyses because of prenatal heroin exposure. The final study sample consisted of 389 (200 cocaine, 189 non-cocaine) infants, of whom 352 were evaluated at 6.5 or 12 months on visual recognition memory, representing 90% of the 389 subjects available for assessment.
At 6.5 months of age, 283 infants (144 cocaine-exposed, 139 non-exposed) were seen. Forty-two infants (20 cocaine-exposed, 22 non-exposed) did not come to the visit; 64 (36 cocaine-exposed, 28 non-exposed) were untestable (too sleepy or fussy to be administered the test) or did not complete the assessment.
At 12 months, 297 infants (147 cocaine-exposed, 150 non-exposed) were seen. Eighteen (11 cocaine-exposed, 7 non-exposed) did not come to the visit; 70 (39 cocaine-exposed, 31 non-exposed) were untestable, or completed less than five test items.
Cocaine-exposed infants tested were more likely to be African-American (p < 0.0001), with lower 1-min Apgar scores (p < 0.05). Their mothers were older (p < 0.01), had more education (p < 0.01) and were less likely to smoke during the pregnancy (p < 0.05) than mothers of infants not seen. Their current caregivers used more marijuana (p < 0.05) at the 6.5-month visit and more alcohol, marijuana, and cocaine at the 12-month visit (p’s < 0.001).
Non-exposed infants seen had lower 1-min Apgar scores (p < 0.05) and higher Hobel risk scores (p < 0.001). Their mothers drank more alcohol in the 2nd trimester (p < 0.05), and used more marijuana during the pregnancy (p < 0.05). Their current caregivers had higher psychological distress at the 6.5-month visit (p < 0.001) and reported drinking more alcohol (p < 0.01) at both visits than those infants not assessed.
3. Technical information
Infants and their caregivers were seen as soon as possible after birth, at which time the caregiver was interviewed regarding drug use. For infants in non-maternal care, biological mothers were interviewed separately. An adaptation of the Maternal Post-Partum Questionnaire (Singer, Arendt, et al., 2002; Singer, Salvator, et al., 2002) quantified maternal drug use for the month prior to and for each trimester of pregnancy. Mothers were asked to recall both frequency and quantity of drug use for each of these time periods. For tobacco, the number of cigarettes smoked per day was indicated; for marijuana, the number of marijuana cigarettes smoked per day; for alcohol, the number of drinks of beer, wine, or hard alcohol per day, with each drink equivalent to 0.5 mL of absolute alcohol. For each drug, frequency of use was recorded using a Likert-type scale ranging from 0 (not at all) to 7 (daily use), which was converted to reflect the average number of days a drug was used each week. Frequency of use was multiplied by the amount used per day to compute a severity of use score for the month before pregnancy and for each trimester. These scores were then averaged for a total score for the prenatal exposure for each drug.
Demographic and medical characteristics at time of infant birth were taken from hospital birth records. These included maternal age, race, parity, number of prenatal care visits, type of medical insurance, infant gestational age, birth weight, length, head circumference, and Apgar scores. The Hobel Neonatal Risk Index (Hobel, Gyarinem, Okado, & Oh, 1973) was computed to obtain a measure of neonatal risk. At the initial visit, maternal socioeconomic status and education level were calculated. Mothers were given the Peabody Picture Vocabulary Test—Revised, PPVT-R (Dunn & Dunn, 1981), the block design (BD), and picture completion (PC) subscales of the Weschler Adult Intelligence Scales—Revised (WAIS-R; Wechsler, 1981), and the Brief Symptom Inventory (Derogatis, 1992) to obtain measures of maternal vocabulary, non-verbal intelligence, and psychological distress, respectively. The Brief Symptom Inventory yields a measure of overall psychological distress, the Global Severity Index (GSI).
At 6.5 and 12 months (corrected ages), the Fagan Test of Infant Intelligence (FTII) was administered. The FTII is a standardized test of intelligence using visual recognition memory for infants during the first year of life (Fagan & Detterman, 1992; Fagan & Shephard, 1989). The FTII uses a paired comparison procedure in a series of ten tasks in which one picture is presented to the infant to study during the familiarization period, and is then presented again alongside a novel picture for a trial period. The trial occurs after the infant has accumulated a predetermined amount of time looking at the familiarization stimulus. Pictures consist of black and white or colored photos of faces of men, women, and infants. The test apparatus consists of a pivoting stage that holds two pictures in front of the infant. The test administrator sits behind the stage and records the time the infant spends looking at each picture. Looking is judged by observing corneal reflections, visible through a peephole, situated midway between the two pictures (see Fagan & Detterman, 1992 for a complete technical description). A novelty score is obtained by dividing the amount of time in seconds that the infant spends looking at the novel picture by the total time spent looking at both pictures during the trial period. A mean percentage novelty score is calculated by averaging over the ten test items. At-risk performance, used to identify children at risk for developmental delay (Rose & Orlian, 2001), is defined as a mean novelty score of ≤53%.
Tests of infant visual recognition memory demonstrate good predictive and discriminant validity (Fagan & Singer, 1983) and have greater predictive validity for later cognitive performance than standard infant sensorimotor assessments (Bornstein & Sigman, 1986; Fagan & Singer, 1983; McCall & Carringer, 1993). Visual recognition memory tests also show promise in early detection of cognitive delay. Studies have demonstrated a difference in performance on visual recognition tasks between term healthy infants and infants at risk for developmental delays, including preterm infants (Sigman & Parmalee, 1974; Rose, 1983), those with central nervous system impairments (Fagan, Singer, & Montie, 1985), Down Syndrome (Miranda & Fantz, 1974), organic and non-organic failure to thrive (Singer & Fagan, 1984), exposure to polychlorinated biphenyls (PCBs; Jacobson, Fein, Jacobson, Schwartz, & Dowler, 1985) and nutritional insult (Rose, Feldman, & Jankowski, 2004).
Measures of visual fixation duration, i.e. the amounts of time an infant spends looking at each stimulus, were also taken during the assessment. Fixation duration or looking time, is calculated by dividing total accumulated looking time by the number of individual looks at a stimulus, and was calculated separately for the familiarization and the trial periods.
Looking time is one component of attention, and evaluates the speed or efficiency with which the infant gathers information presented (Colombo, 1993). Look duration has a robust developmental course, decreasing as infants mature and, in some studies, is negatively correlated with tests of concurrent visual recognition memory (Rose & Orlian, 2001) and later IQ (Colombo, 2002). The direction of this association may be reversed for very complex stimuli or may be unrelated to memory performance (Colombo, 2002).
Examiners were blinded to the drug status of each infant, and trained to reliability on the Fagan test as per the manual (Fagan & Shephard, 1989). All children and mothers received transportation and a $35 stipend for participation. The study was approved by the Institutional Review Board of the participating hospitals and informed written consent obtained from caregivers. All mothers were protected by a Writ of Confidentiality (DA-98-91) issued by the Department of Health and Human Services, which prevented the principal investigator from being forced to release any research data in which subjects are identified, even under court order or subpoena.
3.1. Statistical analyses
Prior to analysis, drug self report measures, the Global Severity Index, and meconium and fixation duration variables, all positively skewed, were normalized by log (x + 1) transformation. Mean and standard deviations are reported in terms of the original distribution, while transformations were used in analyses.
Cocaine negative and cocaine positive mothers and infants were compared on demographic variables using t tests for continuous variables and Pearson’s χ2 tests for categorical variables. Continuous outcomes over time were compared using a mixed linear model with restricted maximum likelihood estimating as implemented in SAS Proc Mixed (SAS v. 8.2; SAS Institute Inc, Cary, NC). The covariance structure of the multivariate response was modeled with an unstructured matrix to account for the correlation. The unstructured covariance matrix allows for the estimation of separate variances at each time point and allows for a general correlation between responses within a subject. Binary outcomes over time were modeled using generalized estimating equations (GEEs) with the logit link and an unstructured covariance matrix. Each model initially considered only cocaine status, time and the interaction between cocaine status and time, and then controlled for appropriate confounders when significant. The adjusted least squares means and standard errors were calculated from the models. In addition to testing for the statistical independence of any cocaine exposure effects, the independence of the relations between the outcome variables and other exposures was explored. Confounders were entered into the model if they were related to outcome at p < 0.1 and significantly (p < 0.05) related to these other substance exposures. Statistical tests were one-tailed for those comparisons for which there were a priori hypotheses.
The order of entry was designed to account for demographic, environmental, and medical factors prior to other drug exposure factors, consistent with a teratologic model, and to reduce the number of correlated variables in the models. Demographic and prenatal factors were considered first, followed by infant caregiving and environmental variables and drug exposure variables in the following order: maternal age, parity, number of prenatal care visits, maternal years of education, marital status, socioeconomic status, biologic and current caregiver (time dependent) PPVT-R, WAIS-R BD and PC scores, non-maternal care status, biologic maternal and current caregiver (time dependent) psychological distress and prenatal as well as current caregiver measures of cigarette, alcohol, and marijuana exposure. Since many of the confounding variables were correlated, they were entered stepwise into the model separately and retained only if they added significantly (p < 0.10) to the prediction of outcome. GSI was tested last because maternal psychological distress can be both a precipitant of and an effect of cocaine use.
Because infant birth parameters or medical condition can be affected by cocaine exposure, birth outcomes which differed between groups were assessed as possible mediating variables by entering them into the model after all other variables, if there was a significant group effect. Infant race and gender, which did not differ between exposure groups, were considered moderator variables with their effects tested through interaction terms. Examiner effects were also tested.
Because we were also interested in the relationship between novelty scores and length of looking time, a mixed model repeated measures analysis was used regressing novelty score on looking time and cocaine exposure status.
4. Results
4.1. Sample characteristics
Characteristics of mothers of infants who received the assessment at least at one time point are listed in Table 1. Cocaine using mothers were older, had more children, and were less likely to be married or employed. They had fewer prenatal care visits. There was a trend for cocaine using mothers to have lower WAIS-R block design and picture completion scores. Cocaine using mothers had less advanced vocabulary, and higher psychological distress. They used more cigarettes, alcohol, and marijuana during pregnancy. There was also a higher incidence of benzodiazepine and PCP use among cocaine using women during pregnancy. The current caregivers for cocaine-exposed infants used more cigarettes, alcohol, and cocaine than those of non-exposed infants (Table 2). Cocaine-exposed infants had a lower gestational age, birth weight, length, and head circumference than the non-exposed infants (Table 1). There were no differences on any medical or demographic variable when the cocaine group was dichotomized into heavier or lighter categories.
Table 1.
Subject characteristics
| Cocaine (N = 177)
|
Non-cocaine (N = 175)
|
t/χ2 |
p |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | N (%) | Mean | S.D. | N (%) | |||
| Maternal demographics | ||||||||
| Age (years) | 30.1 | 5 | 25.7 | 5 | −8.4 | 0.0001 | ||
| Parity | 3.5 | 2 | 2.8 | 2 | −3.6 | 0.0004 | ||
| Number of prenatal visits | 5.3 | 4 | 8.7 | 5 | 6.9 | 0.0001 | ||
| Education (years) | 11.7 | 2 | 11.9 | 1 | 1.3 | 0.18 | ||
| PPVT-R score | 73.5 | 15 | 78.1 | 15 | 2.9 | 0.00 | ||
| WAIS-R block design score | 8.0 | 2 | 7.2 | 2 | 1.7 | 0.08 | ||
| WAIS-R picture completion score | 6.5 | 2 | 7.0 | 2 | 1.9 | 0.06 | ||
| Global Severity Index | 0.8 | 0.7 | 0.5 | 0.5 | −4.9 | 0.0001 | ||
| African-American | 153 (87%) | 141 (81%) | 2.2 | 0.14 | ||||
| Married | 14 (8%) | 31 (18%) | 7.6 | 0.006 | ||||
| Maternal employment | 10 (6%) | 38 (22%) | 19.3 | 0.0001 | ||||
| Low socioeconomic status | 173 (99%) | 172 (98%) | 0.1 | 0.99 | ||||
| Prenatal drug exposure | ||||||||
| Cigarettes/day | 10.6 | 9 | 4.3 | 8 | −9.5 | 0.0001 | ||
| Alcohol drinks/week | 9.5 | 18 | 1.4 | 4.5 | −9.9 | 0.0001 | ||
| Marijuana joints/week | 1.3 | 3 | 0.6 | 4 | −3.8 | 0.001 | ||
| Cocaine rocks/week | 23.3 | 46 | ||||||
| Cocainea,* | 0 (0–3112) | |||||||
| Cocaethylenea,* | 0 (0–419) | |||||||
| Benzoylecgoninea,* | 27 (0–9998) | |||||||
| Metahydroxy-benzoylecgoninea,* | 0 (0–9998) | |||||||
| Alcohol | 144 (85%) | 112 (66%) | 16.8 | 0.0001 | ||||
| Marijuana | 83 (49%) | 24 (14%) | 47.9 | 0.0001 | ||||
| Tobacco | 148 (87%) | 71 (42%) | 76.9 | 0.0001 | ||||
| Amphetamine** | 3 (2%) | 2 (1%) | 0.2 | 0.68 | ||||
| Barbiturate** | 1 (1%) | 1 (1%) | 0.0 | 0.99 | ||||
| Benzodiazepine** | 18 (14%) | 0 (0%) | 20.0 | 0.0001 | ||||
| Phencyclidine** | 9 (5%) | 0 (0%) | 9.3 | 0.002 | ||||
| Infant characteristics | ||||||||
| Corrected age at test (weeks) | ||||||||
| 6.5 months | 29.02 | 2.93 | 29.11 | 3.34 | .24 | 0.81 | ||
| 12 months | 54.26 | 5.23 | 53.54 | 3.34 | −1.38 | 0.17 | ||
| Birth length (cm) | 47.4 | 4 | 49.0 | 3.4 | 4.1 | 0.0001 | ||
| Gestational age (week) | 37.8 | 3 | 38.5 | 3 | 2.1 | 0.03 | ||
| Birthweight (g) | 2738 | 649 | 3090 | 696 | 4.9 | 0.0001 | ||
| Head circumference (cm) | 32.3 | 2 | 33.5 | 2 | 4.7 | 0.0001 | ||
| Hobel neonatal risk score | 6.7 | 16 | 6.1 | 16 | −0.4 | 0.73 | ||
| Gender (male) | 77 (44%) | 87 (50%) | 1.4 | 0.24 | ||||
Nanograms/gram in meconium.
Medians (minimum and maximum).
Fisher’s exact test.
Table 2.
Current caregiver characteristics for 6 and 12 months
| 6 months
|
12 months
|
|||
|---|---|---|---|---|
| Cocaine (N = 144) M (S.D.) | Non-cocaine (N = 139) M (S.D.) | Cocaine (N = 147) M (S.D.) | Non-cocaine (N = 150) M (S.D.) | |
| PPVT-R score | 77.5 (16) | 78.5 (15) | 78.1 (16) | 78.6 (15) |
| WAIS-R BD scorea | 6.8 (2) | 7.2 (2) | 6.7 (2) | 7.1 (2) |
| WAIS-R PC score | 6.8 (2) | 7.1 (2) | 6.6 (2) | 7.1 (2) |
| Global Severity Index | 0.4 (0.5) | 0.4 (0.5) | 0.4 (0.6) | 0.4 (0.5) |
| Cigarettes/day** | 7.3 (8) | 4.1 (8) | 7.4 (8) | 5.0 (9) |
| Alcohol drinks/week* | 2.9 (5) | 1.3 (3) | 3.4 (7) | 1.5 (3) |
| Marijuana joints/week | 0.98 (5) | 2.4 (17) | 0.5 (2) | 0.8 (5) |
| Cocaine rocks/week | 1.6 (8) | – | 1.1 (4) | – |
p < 0.10 at both 6 and 12 months.
p < 0.05 at both 6 and 12 months.
p < 0.0001 at both 6 and 12 months.
4.2. Test outcomes
Novelty scores over the two ages are plotted by cocaine status in Fig. 1. Cocaine-exposed infants had, on average, lower novelty scores at both 6.5 and 12 months but a non-significant overall group effect for a main effect of cocaine at 6 months. The joint test of cocaine indicated a significant effect (p < 0.05). The difference in novelty scores between groups at 6 months was not statistically significant (p = 0.14); however, at 12 months the cocaine group achieved significantly lower novelty scores (mean±S.E.: 57.20±0.55 versus 58.74±0.55, p < 0.025). None of the confounding variables assessed predicted novelty score.
Fig. 1.
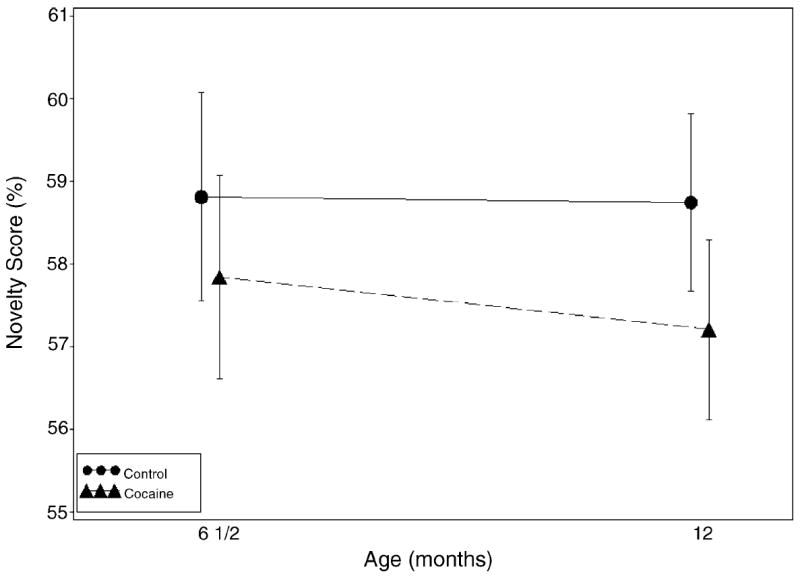
Novelty score over time by cocaine status. Bars represent 95% confidence intervals. The joint test of cocaine (d.f.: 2, 296, F = 2.36, p < 0.05) was significant, indicating that at 12 months cocaine-exposed infants had lower scores (Beta (S.E.) = −1.54 (0.78), d.f.: 1, 296, t = −1.98, p < 0.024).
|
| ||||
| Regression model results | ||||
|
| ||||
| Effect | Beta (S.E.) | d.f. | F | p |
|
| ||||
| Intercept | 58.814 (0.639) | |||
| Cocaine | −0.972 (0.895) | (2, 296) | 2.36 | <0.05 |
| Time | −0.069 (0.793) | (1, 294) | 0.01 | 0.9306 |
| Cocaine × Time | −0.568 (1.124) | (1, 296) | 0.26 | 0.6135 |
|
| ||||
When the percentage of infants performing in the risk range was calculated, there was a non-significant trend for a difference suggesting greater risk for exposed versus non-exposed groups at 6.5 months [42 (29%) exposed versus 30 (22%) non-exposed, p < 0.09]; and by 12 months, cocaine-exposed infants were more likely to be classified as at-risk for developmental delay [39 (27%) exposed versus 25 (17%) non-exposed, p < 0.025]. When groups were compared with the cocaine-exposed infants dichotomized into heavier and lighter groups, there was a significant effect of heavier exposure on risk status at 6.5 months, with 35% of the more heavily exposed infants classified at-risk, compared to 24% of lighter-exposed and 22% of non-exposed (Mantel–Haenszel χ2 = 3.9, p < 0.05).
When the average looking time for the familiarization phase of the test was considered, no effect of prenatal cocaine exposure was found; however, the average number of cigarettes smoked during the third trimester predicted shorter looking times (see Fig. 2). When the mean looking time during the trial phase of the novelty problem was the outcome (Fig. 3), a significant negative effect for third trimester severity of marijuana exposure was found.
Fig. 2.
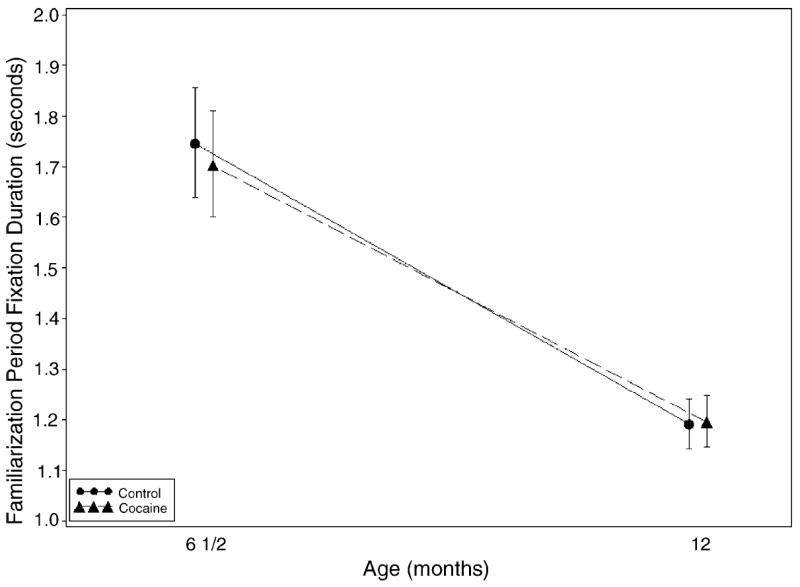
Familiarization fixation over time by cocaine status. Bars represent 95% confidence intervals. Tests of cocaine effect at 6 and 12 months were not significant (p < 0.5788 and <0.9082, respectively).
|
| ||||
| Regression model results: | ||||
|
| ||||
| Effect | Beta (S.E.) | d.f. | F | p |
|
| ||||
| Intercept | 0.583 (0.032) | |||
| Cocaine | −0.025 (0.045) | (1, 300) | 0.31 | 0.5788 |
| Time | −0.382 (0.037) | (1, 302) | 106.28 | <0.0001 |
| Cocaine × Time | 0.029 (0.052) | (1, 304) | 0.30 | 0.5847 |
| Log (3rd trimester cigarette use) | −0.022 (0.011) | (1, 319) | 4.25 | 0.0400 |
|
| ||||
Fig. 3.
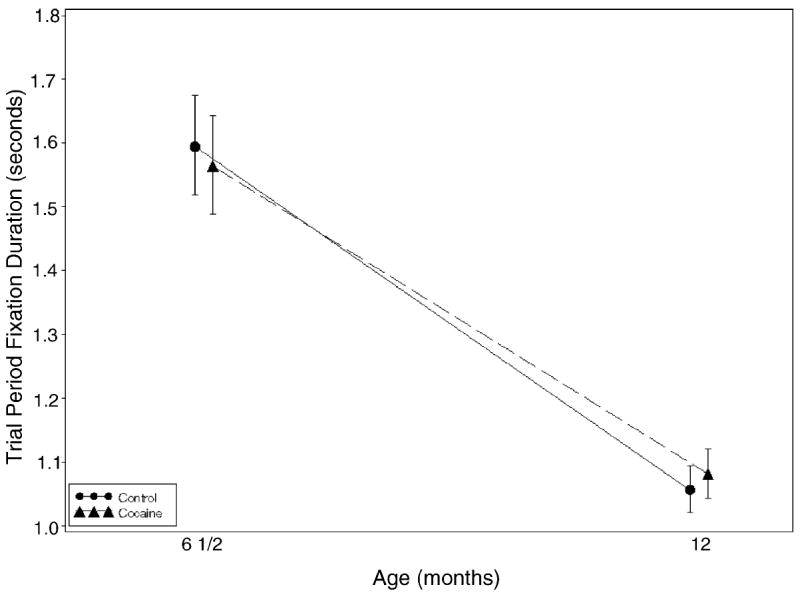
Trial Period fixation over time by cocaine status. Bars represent 95% confidence intervals. Tests of cocaine effect at 6 and 12 months were not significant (p = 0.5842 and 0.3743, respectively).
|
| ||||
| Regression model results | ||||
|
| ||||
| Effect | Beta (S.E.) | d.f. | F | p |
|
| ||||
| Intercept | 0.473 (0.025) | |||
| Cocaine | −0.019 (0.035) | (1, 279) | 0.30 | 0.5842 |
| Time | −0.412 (0.029) | (1, 296) | 204.03 | <0.0001 |
| Cocaine × Time | 0.042 (0.041) | (1, 299) | 1.06 | 0.3049 |
| Log (3rd trimester marijuana use) | −0.053 (0.023) | (1, 326) | 5.30 | 0.0220 |
|
| ||||
Average looking time during both familiarization and trial phases decreased over the 6–12 months time period for both groups indicating a developmental effect.
4.3. Relationship of looking time to visual recognition memory
A mixed model repeated measures analysis revealed a significant interaction between look duration average during familiarization trials and cocaine exposure on novelty scores (interaction term, F = 6.26, p < 0.02). There was no relationship between look duration and novelty scores for either group at 6.5 months. At 12 months, longer look duration was related to poorer visual recognition memory (r = −0.24, p < 0.01) for the cocaine-exposed infants.
5. Discussion
To assess teratologic effects of prenatal cocaine exposure on infant cognition, we longitudinally examined visual recognition memory and attention, using measures of visual recognition memory and information processing speed, in cocaine-exposed and non-exposed infants at 6.5 and 12 months of age. After control for relevant confounds, cocaine exposure predicted poorer recognition memory at 12 months, and predicted a greater likelihood of an infant’s classification in the risk range. At 6.5 months, heavily cocaine-exposed infants were also more likely to be classified as at-risk for developmental delay. Prior studies have indicated that novelty scores in the risk range were more likely to be found in populations at high risk for later developmental delay, such as failure to thrive or Down Syndrome infants (Rose & Orlian, 2001). These findings corroborate our previous studies of this sample, in which we found that heavily cocaine-exposed infants had poorer visual recognition memory in the neonatal period (Singer et al., 1999), and that heavily cocaine/polydrug exposed infants were more likely to be rated as having attentional abnormalities on a neurobehavioral exam (Singer et al., 2000). At 4 years, in this sample, cocaine-exposed children had more errors indicating less selective attention on a task vigilance (Noland et al., 2005). Our current findings are consistent with previous findings of similar deficits in polydrug-exposed infants and in heavily cocaine-exposed infants at 6.5 and 12 months (Jacobson et al., 1996).
Animal studies have found differences in responsivity to environmental cues based on cocaine exposure (Dow-Edwards, Freed, & Fico, 1990). Using rat models, researchers have identified selective attention as one of the cognitive functions that appears to be most adversely affected by prenatal cocaine exposure (Garavan et al., 2000). In redundant learning tasks, cocaine-exposed animals were deficient on tasks, which required attention under conditions of salient, irrelevant environmental cues (Strupp, Morgan, Garavan, Mactutus, & Booze, 1998). Similarly, impairment was noted on visual attention tasks, which tapped sustained attention and inhibitory control (Morgan et al., 1997), both functions thought to be critical to visual recognition memory tasks in humans.
We also measured looking time as an indicator of information processing speed, which has been conceptualized as a significant component of infant cognition measures. As in other studies (Colombo, 1993) longer look durations were characteristic of younger infants, confirming developmental changes in this function over the first year of life. Tobacco and marijuana exposure were related to shorter looking times, but were unrelated to visual recognition memory performance, indicating that speed of processing and memory performance were separate components of information processing. In prior studies, prenatal tobacco and marijuana exposure have been associated with faster reaction times, impulsivity on some tasks (Fried, Watkinson, & Gray, 1992), and more errors on attentional tasks (Noland et al., 2005; Fried et al., 1992; Leech et al., 1999).
There was a relationship between length of look and cognitive performance only for cocaine-exposed infants at 12 months when cocaine-exposed infants with slower processing speed performed more poorly on visual recognition memory tasks. Given the robust developmental change to faster processing speed demonstrated by the overall sample, cocaine-exposed infants may be experiencing a developmental lag in the shift towards sustained attention which requires disengagement from a stimulus (McCall, 1994).
Some limitations of the present study should be considered. First, although examiners were blinded to infant cocaine exposure status, all infants were tested with the caregiver present, and thus it may have been possible to identify exposed infants based on maternal or caregiver characteristics and behaviors. Second, drug assessments were done retrospectively, and therefore, maternal self-report may not have been consistently reliable. As is typical in other such studies of infant perception, not all infants were able to be tested at both ages, but infants tested were comparable to those not tested, suggesting generalizability of the findings.
The methodology used in the present study has several advantages over previous investigations. Maternal drug status was determined through both biologic (meconium and urine screen) and self-report measures, enhancing reliability of classification. Further, severity of nicotine, alcohol, and marijuana exposure was quantified, reducing the likelihood that the effects of other drugs were under controlled and that the observed effects are due to other drugs. Thus, our findings go beyond the previous work to suggest that effects were not attributable to group differences in maternal psychological distress or severity of exposure to other substances.
Our findings parallel prior studies that indicate significant adverse effects of prenatal cocaine exposure on important early dimensions of infant attention and cognitive functions that are not attributable to environmental or caregiving influences. Use of newer assessments of infant intelligence, which measure discrete aspects of attention and cognitive functioning, may aid in earlier detection of children at risk for later developmental delays and lead to the design of appropriate early interventions.
Acknowledgments
Thanks are extended to the participating families, to the Project Newborn staff, to Terri Lotz-Ganley for manuscript preparation, and to H. Lester Kirchner, Ph.D. for statistical consultation.
References
- Arendt R, Singer LT, Minnes S, Salvator A. Accuracy in detecting prenatal drug exposure. Journal of Drug Issues. 1999;29(2):390–403. doi: 10.1177/002204269902900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Sigman MD. Continuity in mental development from infancy. Child Development. 1986;57:251–274. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Colombo J. Infant cognition: Predicting later intellectual functioning. Newbury Park, CA: Sage; 1993. [Google Scholar]
- Colombo J. Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective. Current Directions in Psychological Science. 2002;11(6):196–200. [Google Scholar]
- Derogatis L. The brief symptom inventory: Administration, scoring and procedures manual. 2. Baltimore, MD: Clinical Psychometric Research, Inc; 1992. [Google Scholar]
- Dow-Edwards DL, Freed LA, Fico TA. Structural and functional effects of prenatal cocaine exposure in adult rat brain. Brain Research. 1990;57(2):263–268. doi: 10.1016/0165-3806(90)90052-z. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test—Revised. Circle Pines, MN: American Guidance Service; 1981. [Google Scholar]
- Fagan JF, Detterman D. Fagan Test of Infant Intelligence: A technical report. Journal of Applied Developmental Psychology. 1992;13:153–157. [Google Scholar]
- Fagan JF, Shephard PA. Fagan Test of Infant Intelligence: Training manual. Cleveland, OH: Infantest Corporation; 1989. [Google Scholar]
- Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. In: Lipsitt LP, editor. Advances in infancy research. Vol. 2. Norwood, NJ: Ablex; 1983. pp. 31–78. [Google Scholar]
- Fagan JF, Singer LT, Montie JM. An experimental selective screening device for the early detection of intellectual deficit in at-risk infants. In: Frankenburg WK, Emde RN, Sullivan J, editors. Early identification of children at risk: An international perspective. New York: Plenum Press; 1985. pp. 257–265. [Google Scholar]
- Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicology and Teratology. 1992;14(5):299–311. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- Garavan H, Morgan RE, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ. Prenatal cocaine impairs selective attention: Evidence from serial reversal and extradimensional shift tasks. Behavioral Neuroscience. 2000;114(4):725–738. [PubMed] [Google Scholar]
- Hobel C, Gyarinem M, Okado D, Oh W. Prenatal and intrapartum high-risk screening. American Journal of Obstetrics and Gynecology. 1973;114:1–9. doi: 10.1016/0002-9378(73)90720-5. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. The effect of intrauterine PCB exposure on visual recognition memory. Child Development. 1985;56(4):853–860. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. New evidence of neurobehavioral effects of in utero cocaine exposure. Journal of Pediatrics. 1996;129:581–588. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Wilkins AS. A mouse model of transplacental cocaine exposure: Clinical implications for exposed infants and children. Annals of the New York Acadamy of Sciences. 1998;846:248–261. [PubMed] [Google Scholar]
- Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: Effects on attention and impulsivity of 6-year-olds. Neurotoxicology and Teratology. 1999;21(2):109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Mactutus CF. Prenatal intravenous cocaine adversely affects attentional processing in preweanling rats. Neurotoxicology and Teratology. 1999;21(5):539–550. doi: 10.1016/s0892-0362(99)00024-0. [DOI] [PubMed] [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: Effects on neural ontogeny. Developmental Psychopathology. 1999;11:85–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- McCall RB. What process mediates predictions of childhood IQ from infant habituation and recognition memory? Speculations on the roles of inhibition and rate of information processing. Intelligence. 1994;18:107–125. [Google Scholar]
- McCall RB, Carringer MS. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Development. 1993;64:57–79. [PubMed] [Google Scholar]
- Miranda SB, Fantz RL. Recognition memory in Down’s syndrome and normal infants. Child Development. 1974;45:651–660. [PubMed] [Google Scholar]
- Morgan RE, Garavan H, Mactutus CF, Booze RM, Strupp BJ. Prenatal cocaine exposure: Enduring effects on sustained and selective attention. Neurotoxicology and Teratology. 1997;19:249. [Google Scholar]
- National Institute on Drug Abuse. National pregnancy and health survey: Drug abuse among women delivering live births. Rockville, MD: National Institutes of Health, Publication; 1992. pp. 96–3819. [Google Scholar]
- Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicology and Teratology. 2005 doi: 10.1016/j.ntt.2005.02.001. in press. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: Effects on the development of school-age children. Neurotoxicology and Teratology. 1996;18(6):627–634. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- Rose SA. Differential rates of visual information processing in full term and preterm infants. Child Development. 1983;54:1189–1198. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Developmental Review. 2004;24:74–100. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rose SA, Orlian EK. Visual information processing. In: Singer LT, Zeskind PS, editors. Biobehavioral assessment of the Infant. New York: Guilford Press; 2001. pp. 274–292. [Google Scholar]
- Sigman M, Parmalee AH. Visual preferences of four month old premature and full term infants. Child Development. 1974;45:959–965. [PubMed] [Google Scholar]
- Singer LT, Arendt R, Fagan J, Minnes S, Salvator A, Bolek T, et al. Neonatal visual information processing in cocaine-exposed and non-exposed infants. Infant Behavior and Development. 1999;22(1):1–15. doi: 10.1016/S0163-6383(99)80002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kirchner HL, et al. Cognitive and motor outcomes of cocaine-exposed infants. Journal of the American Medical Association. 2002;287:1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Arendt RA, Minnes S, Farkas K, Guo S, Salvator A. Neurobehavioral outcomes of cocaine-exposed infants. Neurotoxicology and Teratology. 2000;22:1–14. doi: 10.1016/s0892-0362(00)00092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Fagan JF. Cognitive development in the failure-to-thrive infant: A three year longitudinal study. Journal of Pediatric Psychology. 1984;9:363–383. doi: 10.1093/jpepsy/9.3.363. [DOI] [PubMed] [Google Scholar]
- Singer LT, Minnes S, Arendt RE, Farkas K, Short E, Lewis B, et al. Cognitive outcomes of preschool children with prenatal cocaine exposure. Journal of the American Medical Association. 2004;291(20):2448–2456. doi: 10.1001/jama.291.20.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Salvator A, Arendt R, Minnes S, Farkas K, Kliegman R. Effects of cocaine/polydrug exposure and maternal psychological distress on infant birth outcomes. Neurotoxicology and Teratology. 2002;24(2):127–135. doi: 10.1016/s0892-0362(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Shumsky JS, Levitt P. Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience. 2001;106(1):5–14. doi: 10.1016/s0306-4522(01)00256-1. [DOI] [PubMed] [Google Scholar]
- Strupp BJ, Morgan RE, Garavan H, Mactutus CF, Booze RM. Prenatal cocaine exposure: An emerging cognitive profile. Neurotoxicology and Teratology. 1998;20:355. [Google Scholar]
- Volpe J. Effects of cocaine on the fetus. New England Journal of Medicine. 1992;327:135–142. doi: 10.1056/NEJM199208063270607. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]


