Abstract
Vagus nerve stimulation (VNS) is used as an adjunctive therapy for treatment-resistant depression (TRD). Its mechanism of action is not fully understood. Longitudinal measurement of changes in brain metabolism associated with VNS can provide insights into this new treatment modality. Eight severely depressed outpatients who were highly treatment-resistant underwent electrical stimulation of the left vagus nerve for approximately one year. The main outcome measures were resting regional brain glucose uptake measured with positron emission tomography (PET) and the 24-item Hamilton Depression Scale. The most significant and extensive change over one year of chronic VNS localized to the ventromedial prefrontal cortex extending from the subgenual cingulate to the frontal pole. This region continued to decline in metabolism even toward the end of the study. Clinically, this cohort showed a trend for improvement. No correlations surfaced between change in glucose uptake and depression scores. However, the sample size was small; none remitted; and the range of depression scores was limited. Chronic VNS as adjunctive therapy in patients with severe TRD produces protracted and robust declines in resting brain activity within the ventromedial prefrontal cortex, a network with dense connectivity to the amygdala and structures monitoring the internal milieu.
Keywords: computed tomography, emission (PET), glucose metabolism, treatment-resistant depression, emotion, default-mode, ventromedial prefrontal cortex (VMPFC), subgenual anterior cingulate, amygdala, nucleus accumbens, pleasure, electroconvulsive therapy (ECT), deep brain stimulation (DBS), default-mode, neuromodulation
Introduction
Depression is a clinical syndrome defined as a decline in function associated with at least two weeks of sustained depressed mood or loss of interest or pleasure (First et al., 2004). Criteria include persistent evidence of at least five of a number of symptoms including diminished interest, feelings of sadness, and physical effects. These symptoms must not result from a medical condition (e.g., hypothyroidism, anemia); or a medication (e.g., reserpine, interferon); or uncomplicated bereavement.
Different patients show considerable variability in response to treatments for depression. Depression is frequently considered to have remitted or responded fully when the level of symptoms has decreased to a low level, a threshold usually defined a priori. Patients are considered to have responded to treatment, or improved clinically, when there is 50% or greater reduction in symptoms as assessed with a depression rating scale.
The definition of treatment-resistant depression (TRD) varies across studies depending on the number of failed medication trials, psychotherapies, or electroconvulsive treatments. Conventional antidepressant therapies are unsuccessful in achieving a full response (i.e., remission) in about 36% of patients (Fava and Davidson, 1996). However, TRD should be distinguished from treatment-nonresponse. Treatment-nonresponse is simply a failed adequate trial of a treatment, whereby another drug or modality could readily induce a response. On the other hand, TRD is a failure to respond to multiple treatment modalities and drugs despite adequate trials, i.e., appropriate doses and length of treatment. Treatment-intolerance occurs when the patient can not conclude a trial because of side effects. Non-compliance with taking the prescribed medication can often appear as treatment-nonresponse and requires checking serum drug levels to ensure that the medication was taken or that the patient did not have extremely rapid metabolism of the drug because of their P450 genotype.
TRD has high morbidity and mortality and consumes a major share of both direct and indirect healthcare costs. When TRD patients are tracked over two years with treatment according to the current standard of care (i.e., “treatment as usual” such as medications with augmentation strategies, psychotherapy, electroconvulsive therapy), over 65% do not respond at anytime, while 81% do not remit (Dunner et al, 2006). These figures are particularly worrisome when considering that men with severe impairment from depression have a suicide rate of approximately 4.9% (Hirschfeld, 2000). Overall, depression is the leading cause of suicide.
VNS is an adjunctive therapy for TRD in adults. The treatment employs a device similar to an externally programmable pacemaker that sends electrical impulses up the left vagus nerve at the neck to the nucleus of the solitary tract (NTS) in the dorsal medulla. The vagus nerve, through the NTS, provides widespread neuromodulatory control of subcortical and cortical structures (George et al., 2005; Rush et al., 2005a; Henry, 2002).
The NTS projects directly to the amygdala (Rogers and Fryman, 1988) which plays an essential role in affective processing, particularly through interactions with the ventromedial prefrontal cortex (VMPFC). Functionally, amygdala activity tracks with activity in the VMPFC during tasks using interroceptive and exteroceptive feedback (Hurliman et al., 2005); during the resting default-mode of brain function (Kilpatrick et al., 2006); during script-driven imagery in remitted PTSD patients (unpublished observation); and during treatments for depression (Pardo et al., 2007b). However, negative correlations can occur depending on the task and on the direction of influence between the amygdala and VMPFC (Ochsner et al., 2004; Stein et al., 2007; van Reekum et al., 2007). The NTS also projects directly to the locus coeruleus (van Bockstaele et al., 1999), the principal source of cortical noradrenergic innervation. It indirectly projects to raphé nuclei that provide widespread cortical serotonergic innervation. Furthermore, the NTS projects densely to the parabrachial nucleus (PBN) that receives afferents from the amygdala, hypothalamus, and cortex (insula, infralimbic and lateral prefrontal regions; Moga et al., 1990).
The parabrachial nucleus is a complex, heterogeneous, autonomic integration network, particularly involved in processing visceral afferent information. It projects to the hypothalamus, periaqueductal grey, amygdala, thalamus (including intralaminar neurons which in turn project densely to the VMPFC; Hsu and Price, 2007), bed nucleus of the stria terminalis, NTS, and zona incerta (Bianchi et al., 1998). The PBN, through its connections to the hypothalamus, innervates orexin neurons that project throughout the cortex and brain stem (Mieda and Yanagisawa, 2002). In particular, orexin neurons in the lateral hypothalamus, through actions upon the ventral tegmental area (and plausibly other sites with orexinergic innervation such as the extended amygdala and the mesocortical limbic system), have been implicated in reward processing: reward-seeking, food and drug preferences, drug relapse, and addiction (Harris and Aston-Jones, 2006; Harris et al., 2007). Considerable evidence indicates that depression involves dysfunctional reward processing which may result from dopaminergic deficits (Dunlop and Nemeroff, 2007).
Consistent with this anatomy, chronic VNS has been shown to increase locus ceoeruleus and dorsal raphé firing in animals (Groves et al., 2005; Dorr and Debonnel, 2006). Elements of the circuitry reviewed above have been implicated during chronic VNS in diverse effects. For example, VNS over 2 years induced weight loss in TRD patients that was linearly dependent on the initial weight and was not correlated with changes in depression symptoms (Pardo et al., 2007a). VNS for epilepsy improved mood irrespective of the degree of seizure control (Elger et al., 2000; Harden et al., 2000). Also, chronic VNS can worsen respiration during sleep (Marzec et al., 2003).
Nevertheless, VNS’s mode of action remains incompletely understood, especially because the antidepressant effects continue to accrue over at least one year, a phenomenon not seen with other traditional antidepressant treatments (Rush et al., 2005b; Nahas et al., 2005; Sackeim et al., 2007). A recent publication has reviewed plausible mechanisms of VNS in TRD (Nemeroff et al., 2006).
Functional neuroimaging provides insights into the mode of action of treatments for depression, including some changes that correlate with clinical improvement (Drevets et al., 2002; Drevets 2003; Seminowicz et al., 2004; Goldapple et al., 2004). However, few studies specifically target TRD (Pardo et al., 2007b). Some argue that evidence may be insufficient to consider TRD as a specific subtype of depression (Fagiolini and Kupfer, 2003). To our knowledge, there is no PET study identifying the resting metabolic abnormalities (i.e., biomarkers) associated with unmedicated TRD. Some studies have examined metabolic correlates of those without TRD for whom a particular treatment was unsuccessful (e.g., Little et al., 2005) or of those with TRD during experimental treatments such as deep brain stimulation (e.g., Mayberg et al., 2005; Schlaepfer et al., 2007). Recently, several groups have reported on neuroimaging studies of TRD patients under treatment with VNS.
Zobel et al. (2005) measured blood flow using SPECT with 99mTc–hexamethyl-propylene amine oxime immediately after a sequence of VNS stimulations in 12 TRD patients. All patients were taking mirtazipine or citalopram for at least 6 weeks before entering into the imaging study. The patients were studied once before implantation and after 4 weeks of VNS treatment while resting with eyes closed in the supine state. The tracer was injected about 20–30 minutes immediately following a stimulation sequence. They reported a decline of flow in an extensive network including amygdala; hippocampus; thalamus; putamen; caudate; brainstem; subgenual, ventral anterior, posterior, and dorsal anterior cingulate cortex; and orbital, ventrolateral, and dorsolateral prefrontal cortex. The only focus of increased flow arose in the middle frontal gyrus.
Subsequently, Conway et al. (2006) used 15O-water PET to study four TRD women treated with VNS for 3 weeks. The patients were on complex regimens of psychoactive medications including antidepressants, atypical neuroleptics, hormones, benzodiazepines, and dopaminergic agents. The medications were not changed during the 3 week trial. All were studied in the supine state. Before imaging, the device was turned off for 30 minutes. The patients were scanned during the four subsequent blood flow scans in an “off-on-off-on” design. During the “on” scans, 90 s of VNS stimulation occurred immediately before tracer injection. During active stimulation, blood flow increased in the orbitofrontal cortex (BA 11, 47); dorsal (BA 24, 32) and ventral anterior cingulate; superior and inferior frontal gyri; cerebellum; and putamen. Blood flow decreased in temporal cortex (BA 20, 21), parietal cortex (BA 7, 40); and pre- and post-central gyri.
In a case study of a TRD patient who began VNS treatment seven months earlier, Critchley et al (2007) found that during a mnemonic encoding period, VNS while turned on, as contrasted with while turned off, selectively disrupted the memory for negative words. They posited that disruption of encoding of negative information may contribute to the antidepressant effects of VNS. Functional MRI during encoding, i.e., semantic, abstract, self-referential judgments of positive, negative, and neutral words presented visually, identified a main effect of VNS with increased activity in the dorsolateral and ventromedial prefrontal cortex. There was also a word valence (negative, but not positive or neutral) by VNS interaction that localized increased activity to lateral orbital and ventromedial prefrontal cortex; frontal pole; ventral pons; and insula.
Nahas et al. (2007) conducted a 1.5 T fMRI activation study on nine subjects treated with chronic VNS for up to 20 months. They examined the direct effects of VNS stimulation by comparing the blood oxygen level dependent (BOLD) signal during active VNS (i.e., device on) to a resting state (i.e., device off). They found activation in bilateral superior temporal and left somatosensory cortex and deactivation in left middle frontal gyrus, left fusiform gyrus, left VMPFC, right cerebellum, and midbrain.
Overall, most studies reviewed show limbic and paralimbic changes; however, the direction of change varied, which likely reflects differences across studies. First, the effects of VNS were studied either while VNS was turned on or following discontinuation of stimulation with differing stimulation parameters and imaging delays. Second, methods have differed, and variously include 99mTc–hexamethyl-propylene amine oxime SPECT; 15O-water PET, and 1.5 T fMRI. The behavioral state has also differed across studies, e.g., eyes closed rest; eyes open rest; watching words with varying affective valence while making self-referential judgments; and getting runs of repeated stimulation. Additionally, subjects had varying concurrent medications. Nevertheless, a change with VNS in the VMPFC has occurred across all studies.
Decreased activity within the VMPFC occurs frequently during therapy for TRD (Pardo et al., 2007b). We reasoned that if VNS follows this pattern, a decrease in metabolism during the resting state would occur in this area. Additionally, altered activity in other brain regions such as the amygdala, hippocampus, anterior cingulate, and insula could provide clues about treatment-specific changes, thereby furthering our understanding of the mode of action of VNS.
The present study examines brain metabolic changes in TRD patients undergoing chronic VNS according to standard protocols. To date, there are no published neuroimaging studies using FDG PET on TRD patients in the resting state with chronic VNS extending up to 1 year. Chronic stimulation becomes an important issue given that the clinical effects of VNS occur over a protracted time course (Rush et al., 2005b; Nahas et al., 2005; Nahas et al., 2007; Sackeim, et al., 2007). The resting state was selected because it is considered the “default-mode” of brain function (Raichle et al., 2001). PET was chosen as the modality of choice for several reasons.
Although each neuroimaging method has potential advantages and disadvantages, FDG PET was used in this study for three reasons. First, measurement of glucose metabolism (more precisely, normalized glucose uptake) provides a more direct assessment of neuronal activity than measurement of cerebral blood flow, particularly when patients are on medications, the situation in most TRD patients receiving adjunctive VNS. Indeed, polypharmacy has uncertain effects upon flow/metabolism coupling, potentially confounding any changes observed in flow with neuronal activity. Second, FDG PET has an overall good signal-to-noise ratio, and specifically does not demonstrate signal loss in the ventral frontal regions (including VMPFC) frequently observed with high-field fMRI. Third, temporal averaging during the FDG uptake period has the potential theoretical advantage of decreased sensitivity to transient changes in brain state.
Our objective was to compare basal, resting brain activity across different time points during the course of chronic treatment. Since the antidepressant effects of VNS, unlike pharmacotherapies or electroconvulsive therapy, appear to have a lengthy time course evolving over many months to a year and beyond (Rush et al., 2005b; Nahas et al., 2005; Nahas et al., 2007), we anticipated that the neural correlates might differ from those related to acute or subacute stimulation. In particular, given the consistency of changes in VMPFC during antidepressant treatments, we expected decreased metabolism in the VMPFC. Ethical considerations precluded a prolonged trial of sham VNS for comparison given the severity of illness and suicide risk.
Methods
Patients
Eight outpatients were recruited from those who participated in a trial of VNS for adjunctive treatment of TRD at the University of Minnesota site (Rush et al., 2005a). All patients signed informed consent as approved by the institutional review boards of the University of Minnesota and the Minneapolis Veterans Affairs Medical Center. The demographic data appear in Table 1. Three were male, and five were female. The average age was 44 years (range 32–55). All but one were right-handed. Patients were typically on multiple medications including antidepressants, neuroleptics, and anticonvulsant mood stabilizers. Any addition of a new medication between the scans at “Chronic 6 Months” and “Chronic 1 Year” are listed (see Figure 1 and section below, “PET Scan Dataset”). One of the four patients from these time points began lamotrigine. Patients also had psychiatric comorbidities. Substance abuse or dependence was not active for at least six months before enrollment. Patients were randomized initially to have their device either turned on or off. The study was double blind during this phase, so in recruiting eight subjects, the investigators did not know the duration of active treatment until the blind was broken at 3 months at which time all enrollees could elect turning the device on. All patients underwent physical examination, electrocardiogram, laboratory evaluation, and clinical MRI to exclude those with anatomical brain lesions or diagnoses other than TRD. Every effort was made to avoid medication changes during the acute phase of the VNS trial (i.e., first 3 months of the trial). However, medication changes were permitted after the initial 3 month double-blind period. Mood was assessed with the 24 item Hamilton Depression Scale (HmD24; Hamilton, 1967). All women of childbearing potential underwent a urine pregnancy test before each scan to minimize the possibility of unknown pregnancy.
Table 1.
Clinical data for study participants
| Age | Gender | Handed | Depression | Current mA | PW µs | Psychiatric Comorbidity | Initial HmD | Chronic 1 Year HmD | Medications | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F | R | unipolar | 0.75 | 500 | h/o bulimia, etoh: FR | 29 | 14 | disulfiram, fish oil, fluoxetine, modafinil |
| 2 | 55 | M | R | unipolar | 1.50 | 250 | h/o etoh, polysubstanc e abuse: FR | 29 | 32 | acetylsalicylic acid, bupropion, diphenhydramine, fish oil, pravastatin, sertraline, terazosin, triamcinolone acetonide, |
| 3 | 46 | F | R | unipolar | 1.50 | 500 | None | 18 | 16 | acetylsalicylic acid, bupropion, caffeine, venlafaxine; |
| 4 | 47 | F | R | unipolar | 1.50 | 500 | Panic disorder | 32 | 13 | calcium, conjugated estrogens, fexofenadine, trazodone, clonazepam, fluoxetine, modafinil, omeprazole, quetiapine |
| 5 | 32 | M | L | bipolar | 1.00 | 500 | h/o etoh, cocaine dep (FR); dysthymia; sexual paraphilia | 27 | 20 | clonazepam, fluoxetine, modafinil,omeprazole, quetiapine |
| 6 | 52 | F | R | unipolar | 0.75 | 250 | None | 24 | 25 | calcium, dyazide, fluvoxamine, mirtazapine, naproxen, oxybutynin, conjugated estrogens, medroxyprogesterone acetate, glucosamine |
| 7 | 27 | M | R | unipolar | 0.75 | 500 | OCD, panic disorder | 23 | 26 | Sertraline |
| 8 | 54 | F | R | unipolar | 0.50 | 250 | h/o etoh & PTSD | 37 | 27 | acetylsalicylic Acid |
Pt, patient number; Hand, handedness; M, male; F, female; R, right-handed; L, left-handed; mA, milliamp; PW, pulse width; µs, microsecond; etoh, alcohol dependence; FR, full remission; OCD, obsessive compulsive disorder; PTSD, post-traumatic stress disorder; new medication (lamotrigine) added for patient 6, 6 months following implant & discontinued at 9 months.
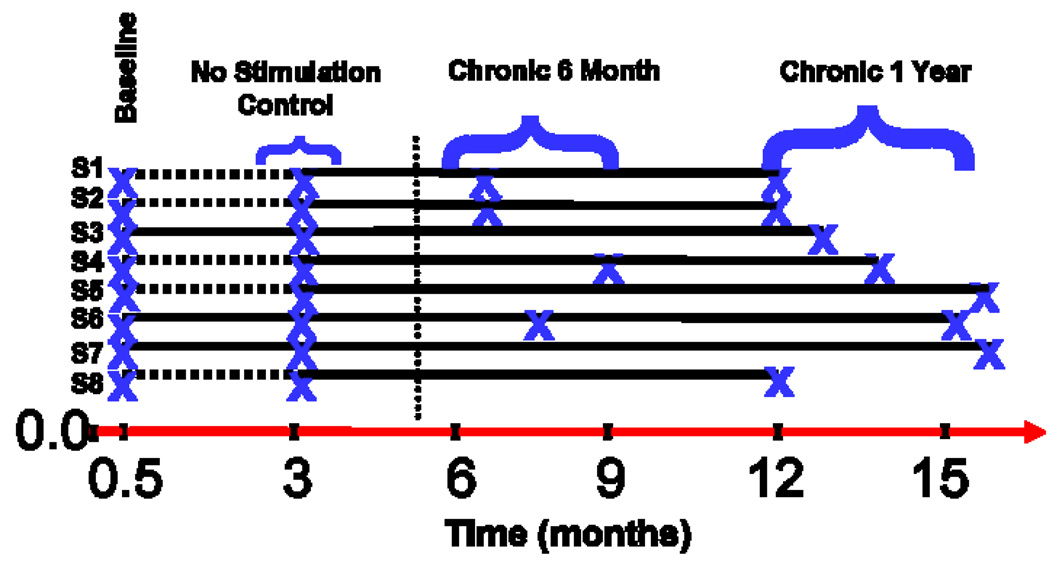
Figure 1.
Summary diagram with timeline indicating time of scanning and grouping of PET scan data. The subjects are labeled S1–S8; “x” denotes PET scan acquisition. The timeline starts at 0, when the VNS device was implanted. At 0.5 months, the VNS device was turned on in a random, blind manner. At 3 months into the trial, the blind was broken. The horizontal dashed lines (-----) indicate those subjects whose device was not turned on; they subsequently had the device turned on at three months. The solid horizontal line indicates a time period when the device was on. The vertical dotted line indicates time of a change in our institution’s PET camera. The subjects were grouped as follows: Baseline (all eight subjects scanned about 2 weeks after implantation and before any device was turned on); No Stimulation Control (5 subjects whose device was never turned on at 3 months into the trial); Chronic 6 months (4 subjects who were stimulated between 3 and 7 months); and Chronic 1 year (8 subjects who were stimulated between 9 and 14 months).
VNS Intervention
All patients followed the trial protocol as described elsewhere (Rush et al., 2005b). Stimulation parameters at 1 year were the following: signal frequency, 20 Hz; signal on time, 30 s; signal off time, 5 min; current and pulse width as listed in Table 1. The average output current was 1.0 (SD 0.4; median 0.75) mA with an average pulse width of 406 (SD 129; median 500) µs. The device was turned off for 2 hours before PET scanning to avoid visualizing any direct effects of acute VNS on the eyes closed resting state. The principal interest was to visualize the long-term effects of chronic neuromodulation rather than the immediate, direct effect of VNS.
PET Imaging
Patients fasted overnight, and their blood glucose and urine drug screen were checked immediately before scanning to ensure that instructions were followed. The VNS device was turned off approximately 2 hours before the 18 F-fluorodexoyglucose was injected intravenously at a dose of 5 mCi/kg. Subjects rested with their eyes closed in a quiet, darkened room. They were monitored to ensure that they did not fall asleep while resting. The data for “No Stimulation Control” and “Baseline” scans were obtained on an ECAT 953 B (Siemens, Knoxville, TN); see vertical dotted line in Figure 1 and description of PET scan datasets in next section) in two-dimensional mode using two interlaced scans (each lasting 10 minutes) providing whole brain coverage. These scans were normalized during post-processing based upon the counts in the area of overlap. The data for the “Chronic 6 Months” and “Chronic 1 Year” scans were collected on an ECAT 47 (Siemens, Knoxville, TN) in two-dimensional mode providing whole brain coverage in one emission scan lasting 10 minutes (See Figure 1 and next section, “PET Scan Datasets”). Attenuation correction was measured along with correction of the emission scans for scatter, decay, electronic deadtime, and random coincidences. Images were reconstructed using filtered back-projection with a Hann filter of 9 mm FWHM. Final image resolution was approximately 12 mm FWHM. Emission images contained 10–20 million counts.
PET Scan Datasets
The usable dataset consisted of 25 PET scans (Figure 1). Upon initiation of the trial and approximately 2 weeks after implantation, all 8 patients were scanned before the device was turned on for the first time (i.e., 2 weeks or 0.5 months into the study); these data are hereafter referred to as “Baseline” (N = 8). At three months after study initiation (when the blind was broken), 5 of 8 subjects had no stimulation: the data from these 5 patients are hereafter referred to as “No Stimulation Control” (N = 5). About half-way into the study, 4 subjects were scanned, and these had between 3–7 months of stimulation; this comparison group is hereafter referred to as “Chronic 6 Months” (N = 4). Upon the final scanning session, the 8 patients had between 9–14 months of stimulation; these data are hereafter referred to as “Chronic 1 Year” (N = 8).
PET Data Analysis
PET data were coregistered, normalized to a whole brain mean activity of 1,000 counts, and warped nonlinearly to the Talairach stereotactic atlas (Talairach and Tournoux, 1988). Voxel-wise paired t-tests were calculated using Neurostat software (Statistical Image Analysis for Paired Data v. 8.1) provided by Dr. Satoshi Minoshima (University of Washington, Seattle, WA; Minoshima et al., 1992; Minoshima et al., 1993, 1994; Minoshima et al., 1995). T-tests were converted to Z-scores for final display. The threshold for significance was set at Z = 3.29 (P < 0.001 uncorrected) based upon bootstrapping (Zald et al., 1998). Structures were identified by the Talairach coordinates and the corresponding atlas as well as with the Talairach Daemon (http://ric.uthscsa.edu/projects/talairachdaemon.html). For display purposes, a threshold of p < 0.05 (uncorrected) was used.
Results
Clinical Data
This imaging study of VNS at the University of Minnesota (one of 21 sites recruiting for this study) was powered to achieve statistically significant metabolic changes and was not designed as an efficacy trial (but see Rush et al., 2005a). The presentation of the clinical data indicates only what changes in symptoms occurred in the group undergoing imaging. Table 1 displays the depression scores at Baseline and at Chronic 1 Year. A mixed model regression (unconditional growth model) using all available HmD24 scores (8 subjects × 4 visits = 32 scores; clinic visits at baseline, 3 months, 6 months, and 12 months) as a function of time had an intercept of 27.5 (S.E. = 1.58; 95% C.I. = 24.09–30.86), the estimated group HmD24 at implant. The HmD24 point change per month after the implant was −0.48 (S.E. = 0.23; 95% confidence interval −0.98 to 0.02) with p = 0.06. Of note, degrees of freedom in mixed model regressions are not typically reported as they are difficult to interpret and are frequently fractions. Matched-pair t-tests between baseline score and scores at 3 months; 6 months; and 12 months were p = 0.71; p = 0.48; and p = 0.09, respectively.
PET Results
Our institution’s scanner changed between 3 and 6 months into this study (marked by vertical dotted bar in Figure 1). Pixel-wise repeated measures ANOVA was not used because of confounding between time and scanner type. Also, several patients did not complete all planned PET scans, resulting in missing data cells. The PET scan of one patient at one time point was discarded because of technical problems and was not included in the final dataset. Therefore, we present individual contrasts from scans pooled as in Figure 1 and present only the data corroborated by contrasts from the same scanner.
Here, we focus upon decreases in VMPFC metabolism with VNS therapy for two reasons. An extensive literature highlights the importance of the circuit involving the VMPFC and amygdala to affective processing. Second, the VMPFC was the most significant and extensive change observed in this study. The decrease in metabolism in the VMPFC could be confirmed from data using the same scanner (i.e., Chronic 6 Months and Chronic 1 Year). This region of decreased metabolism extended from the subgenual cingulate cortex (BA 25) to the frontal pole (BA 10p). Table 2 shows the changes in metabolism between Chronic 1 Year and Baseline. A complete catalog of all changes from specific contrasts is available (see Supplementary Result, Table S1). However, caution in interpreting these changes is warranted because of the difficulty in validating changes across time and scanners as well as varying number of subjects for any given time.
Table 2.
VNS Peaks in Talairach (88) coordinates for contrast: Chronic 1 Year vs. Baseline
| Location | X | Y | Z | Z-score | Cluster size (mm3) |
|---|---|---|---|---|---|
| Increase with VNS treatment | |||||
| Left Lingual Gyrus, BA19 | −26 | −73 | −4 | 5.3 | 4339 |
| Left Lingual Gyrus, BA17 | −19 | −91 | −4 | 4.0 | |
| Left Parietal, Sub-Gyral | −35 | −40 | 34 | 5.1 | 5148 |
| Left Parietal, Post-central gyrus, BA3 | −21 | −37 | 67 | 4.8 | 12723 |
| Left Frontal Lobe, Paracentral Lobule, BA5 | −12 | −31 | 49 | 3.8 | |
| Left Precuneus, BA7 | −12 | −42 | 54 | 3.7 | |
| Right Post-central gyrus, BA2 | 21 | −37 | 67 | 3.6 | |
| Left Superior Frontal Gyrus, BA6 | −21 | −4 | 67 | 3.5 | |
| Right Precuneus, BA7 | 19 | −55 | 45 | 4.6 | 2437 |
| Left Precuneus, BA7 | −19 | −64 | 34 | 4.0 | 467 |
| Right Lingual Gyrus, BA19 | 28 | −73 | −4 | 3.9 | 1139 |
| Right Middle Occipital Gyrus, BA18 | 19 | −87 | 4 | 3.6 | |
| Left Cingulate Gyrus, BA24 | −10 | −10 | 34 | 3.6 | 159 |
| Right Lingual Gyrus, BA17 | 3 | −94 | −2 | 3.4 | 45 |
| Decrease with VNS treatment | |||||
| Left Precentral Gyrus, BA6 | −42 | 3 | 34 | −5.3 | 112 |
| Right Thalamus – Medial dorsal nucleus | 10 | −17 | 7 | −5.0 | 445 |
| Left Thalamus – Medial dorsal nucleus | −8 | −19 | 7 | −4.2 | |
| Right Medial Frontal Gyrus, BA25 | 6 | 26 | −16 | −4.8 | 612 |
| Left Anterior Cingulate, BA32 | −1 | 35 | −7 | −4.5 | |
| Right Middle Frontal Gyrus, BA10 | 35 | 46 | 16 | −3.8 | 57 |
| Right Middle Frontal Gyrus, BA9 / BA44 | 37 | 8 | 31 | −3.7 | 18 |
| Left Transverse Temporal Gyrus, BA41 | −44 | −28 | 11 | −3.6 | 24 |
| Left Fusiform Gyrus, BA20 | −53 | −31 | −20 | −3.6 | 9 |
Note: The name of regions that are shown indented without a corresponding cluster size localize to local maxima within a cluster whose global maximum is un-indented with a listed cluster size. Note that the order in the list is based upon Z-score.
Figure 2 shows the progressive decrease in resting VMPFC metabolism during chronic VNS. Figure 2A shows that no VMPFC changes occurred between No Stimulation Control and Baseline (N = 5; same scanner). In fact, there was a non-significant increase in activity in the subgenual cingulate cortex. Only two weak, marginally significant foci (right insula and putamen) surfaced in the entire brain. None of the other contrasts examined showed these two foci. Figure 2B shows a significant decrease in VMPFC metabolism arising for the first time in the contrast between Chronic 6 Months and Baseline (N = 4; different scanners). Figure 2C (N = 4; same scanner; Chronic 1 Year and Chronic 6 Months) shows even further reduction of resting VMPFC metabolism during this latest phase of the study when there is ongoing symptomatic improvement (see Clinical Data). Figure 2D shows the contrast between Chronic 1 Year and Baseline (N = 8; different scanners) and reveals up to an 11% decrease in VMPFC activity from baseline. There were no significant correlations between change in VMPFC activity and clinical response. However, the range of the data was limited (HmD24 13–32), and none of the eight patients achieved full remission. Figure 3 displays graphically the changes in metabolism in an ROI within the VMPFC at peak coordinates (6, 26, −16).
Figure 2.

Midline sagittal sections through the prefrontal cortex showing the progressive reduction in VMPFC metabolism with chronic VNS in TRD patients. A. Contrast between No Stimulation Control and Baseline scans (see text) showing no significant changes in the VMPFC before the device was activated. B. Contrast between Chronic 6 Months and Baseline showing the start of reduction in metabolism in VMPFC and related cingulate areas, specifically perigenual and supragenual anterior cingulate. C. Contrast between Chronic 1 Year and Chronic 6 Months showing progressive reduction of metabolism in the VMPFC during this late phase of the study along with a trend for further delayed clinical improvement. D. Contrast between Chronic 1 Year and Baseline showing robust deactivation in VMPFC extending from subgenual cingulate to the frontal pole.
Figure 3.

This figure depicts the normalized mean counts within a VMPFC region of interest defined by the contiguous region found using the Chronic 1 Year vs. Baseline contrast at a threshold of p < 0.05. (peak coordinates 6, 26, −16). Error bars show +/− Standard Error of the Mean. Note that at ~3 months, N = 7; at ~6 months, N = 4; and at baseline and ~1 year, N = 8.
Discussion
Summary
The goals of this study were to measure the longitudinal changes in regional cerebral glucose metabolism associated with chronic VNS in TRD patients. The study found that there was a large and extensive decrease in metabolism in the VMPFC along with a trend-level change in depression scores. These changes evolved over one year. No significant correlations arose between regional metabolism and depression score. However, the sample was small; the range of depression scores was limited; and no patient achieved remission, Nevertheless, these data are consistent with observations showing gradual clinical improvement with VNS up to one year and beyond, as well as previous findings of VMPFC deactivation with a variety of antidepressant treatments.
Clinical Response
This add-on imaging study to a 10 week clinical trial of VNS in TRD (Rush et al., 2005a) was not designed to demonstrate clinical efficacy, but rather to identify brain metabolic changes associated with chronic VNS. Outright, it is important to emphasize that associations with metabolic change do not prove that the observed changes mediate any therapeutic effect. Despite studying only 8 patients, reliable statistically significant changes in brain metabolism were identified in the VMPFC. The clinical response of these 8 patients approached significance over the follow-up interval of approximately 1 year; however, no one remitted. By comparison, Rush et al. (2005a) have reported the results of the 10 week main clinical trial of VNS in TRD.
In this trial, with 222 evaluated participants, the primary response measure did not reach significance after 10 weeks of stimulation. Subsequent observations suggested that the 10 week interval for VNS may not have been an optimal duration of treatment for the testing of efficacy. There is evidence from non-randomized, longitudinal studies that the antidepressant response to VNS evolves slowly over months to years (Rush et al., 2005b; Nahas et al., 2005; Nahas et al., 2007; Sackeim et al., 2007). This time course of response differs markedly from other antidepressant therapies such as antidepressant medication, transcranial magnetic stimulation, and electroconvulsive therapy, all of which show clinical improvement occuring over several weeks. The comparatively slow evolution of response to VNS also differs from experimental antidepressant therapies with acute and sub acute effects: deep brain stimulation (DBS) of the subgenual cingulate (Mayberg et al, 2005); nucleus accumbens (Schlaepfer et al., 2007); or inferior thalamic peduncle (Jimenez et al., 2007). None of the 8 patients in this study achieved a full remission after one year of VNS therapy. At a purely descriptive level, the mixed model regression indicated a consistent trend toward improvement. However, this imaging study was not blinded after the initial 3 month period; so these data do not, nor were they meant to, assess efficacy.
The present imaging data indicate that the metabolic changes associated with VNS occur slowly and progressively over months to years. Few metabolic or clinical changes were observed during the first three months. Significant reductions in VMPFC metabolism were still evolving between 6 months and 1 year along with trend-level reductions in HmD24.
No significant correlation surfaced between change in metabolism and depression score. However, the sample size was small, and the range of change scores was limited. Also, the patients who participated in this study were extremely treatment-resistant. Existing data suggest that the greater the refractoriness of the illness, the less the efficacy for VNS (Sackeim et al., 2001). Nevertheless, these data provide no direct support for a relationship between the decrease in VMPFC metabolism and depression score. Further, an association between decreased VMPFC metabolism and clinical improvement does not imply causality.
Neuroimaging
This research makes two significant contributions: 1) functional imaging of TRD, a population that is difficult to study and that warrants further research because of key clinical importance; and 2) mapping brain activity associated with chronic VNS extending to one year with FDG PET, which is insensitive to the effects of flow/metabolism coupling. To our knowledge, no FDG PET study of TRD patients in the resting state undergoing chronic VNS has been published. The present experiment shows that treatment of severe TRD with chronic cervical VNS is associated with a profound decrease (11%) in resting metabolism in the VMPFC extending from the subgenual cingulate (BA 25) to the frontal pole (BA 10p). This magnitude of change approaches the large changes seen with sensory stimulation such as visual or tactile stimuli. The decline in activity is ongoing even after six months of chronic stimulation and is consistent with the observation of evolving improvement in mood with VNS over 1 to 2 years (Rush et al., 2005b; Nahas et al., 2005; Nahas et al., 2007; Sackheim et al., 2007).
These large and extensive VMPFC changes are unlikely to represent a placebo response for several reasons: (1) The placebo response is low in TRD (0–10%; Thase and Rush, 1995). (2) When the placebo response occurs, it usually appears early in treatment and does not remain sustained (Quitkin et al., 1987). (3) In the randomized, placebo-controlled, 10 week trial of VNS, the placebo rate was low (10%) (Rush et al., 2005a). (4) The placebo response to antidepressant treatments visualized with neuroimaging does not show either the magnitude or extent of metabolic change seen here (Mayberg et al., 2002). However, the placebo response to VNS has not been studied directly using neuroimaging. (5) TRD patients undergoing sham DBS of the subgenual cingulate show no placebo effects at least acutely (Mayberg et al, 2005). (6) The contrast between the “No Stimulation Control” and “Baseline” conditions, when placebo effects should appear most robust because of higher expectancy shortly after implantation, does not show a decline in VMPFC metabolism. The two foci seen elsewhere barely passed the significance threshold. (6) The patients in this study had severe TRD and had failed multiple courses of antidepressant trials. Repeated treatment failures induce a negative expectancy that can reduce a placebo response or can even be associated with a ‘nocebo’ response (reviewed in Swartzman and Burkell, 1998).
Decreased resting metabolism in the VMPFC following chronic VNS is supported by the existing literature. Henry et al. (2004) showed in epileptic patients that when compared to acute VNS, chronic VNS diminishes VMPFC activation in response to the same stimulation. Conway et al. (2006) found robust activation to acute, direct VNS in the same region (left VMPFC) that was found here to decrease in metabolism after chronic stimulation with the device turned off before scanning.
The finding of decreased resting VMPFC metabolism may either directly explain the antidepressant effects (i.e., response-specific), or it may reflect a phenomenon unrelated to antidepressant action, perhaps a treatment-specific effect. The small patient enrollment and partial responses precluded a separate analysis of responders vs. nonresponders. Whether decreased resting activity in the VMPFC is response-specific remains uncertain and will require further research. Despite an extensive literature, however, decreased resting VMPFC activity during VNS treatment for epilepsy has rarely been reported. The only study of epilepsy showing decreased VMPFC activity during VNS, similar to that observed here, examined the effects of direct, acute VNS for 60 s (Barnes et al., 2003). Therefore, we conclude that decreased VMPFC activity with chronic VNS is only observed in TRD, and is not a non-specific effect of chronic VNS.
VMPFC activity is highest in healthy controls when the brain is idle during rest, considered to reflect a “default-mode” state (Raichle et al., 2001). Of interest, increased activity in the subgenual anterior cingulate (BA 25), a component of the VMPFC, is correlated positively with negative affect in healthy control subjects when studied in this default-mode condition (Zald et al., 2002). Patients at rest with active depression without medications show no significant changes in the activity of the subgenual relative to healthy controls (Mayberg et al., 1997), while remitted depressed patients on medications show a significant decrease (Liotti et al., 2002). TRD patients at rest show elevated subgenual activity and show a decrease in VMPFC activity while undergoing DBS of the subgenual cingulate cortex (Mayberg et al., 2005). Mechanistically, although perhaps oversimplified, the decline in activity associated with chronic VNS may reduce default-mode hyperactivity, thereby leading to decreased negative affect.
The subgenual cingulate, perigenual cingulate, and the amygdala constitute a key affective circuit. Recent research has begun to characterize network interactions within this circuit (Margulies et al., 2007). For example, the short allele of the length polymorphism of the promoter for the serotonin transporter (5-HTTLPR), a known risk factor for anxiety and depression, decreases the functional connectivity in this circuit (Pezawas et al., 2005). In contrast, the functional connectivity of the “default-mode” network is increased among depressed patients, and includes a circuit involving the VMPFC (subgenual cingulate, BA 11); thalamus; and the cuneus/precuneus (Greicius et al., 2007). Further, reduction in VMPFC metabolism may be a final common network participating in the resolution of depression as described in the existing literature examining patients with or without TRD under several treatment modalities.
Each of the following antidepressant treatments is associated with decreased VMPFC activity: SSRI antidepressants (i.e., fluoxetine, Mayberg et al, 2000; paroxetine, Brody et al., 2001; sertraline, Drevets et al., 2002); sleep deprivation (Wu et al., 1999); successful placebo response (Mayberg et al., 2002); and during cognitive behavioral therapy (Goldapple et al, 2004). However, different treatments recruit somewhat different subregions within this network as well as other associated brain structures.
Reduced VMPFC activity has also been reported following electrical stimulation of the brain. PET studies of brain metabolism before and after ECT at bifrontotemporal regions have shown decreased metabolism in the VMPFC, and more specifically, in the subgenual cingulate cortex (Nobler et al., 2001). This study of inpatients did not list TRD inclusion criteria, although standard practice suggests such a sample had failed less invasive treatments such as psychotherapy and pharmacotherapy. The effects of ECT were measured 5 days after completing a course of bilateral treatments. Presumably the changes did not reflect direct stimulation effects and represented more chronic than acute effects.
Bilateral DBS of the subgenual cingulate cortex, an experimental treatment for TRD, also induces progressive decline in metabolism of the VMPFC with extension from the subgenual cingulate toward the frontal pole through six months of stimulation (Mayberg et al., 2005). This pattern is strikingly reminiscent of our present findings. Of interest, the antidepressant effects of DBS were time-locked to stimulation and produced antidepressant effects acutely (in 1–5 minutes) with a very different time course to that seen here with VNS that developed over 1 year.
Bilateral DBS of the nucleus accumbens, also an experimental treatment for TRD, results in decreased metabolism in the VMPFC (Schlaepfer et al., 2007). The comparison PET scans (before vs. after stimulation) were taken one week apart, reflecting sub acute treatment. There were no immediate antidepressant effects, and no patient reported pleasure upon stimulation. After one week, a significant decrease occurred in depression scores along with a metabolic decrease in BA 10p, similar to the result of Goldapple et al., (2004). To summarize, the convergence of decreased VMPFC activity across all of the discussed antidepressant modalities suggests that decreased metabolism in the VMPFC constitutes a critical node for antidepressant action
There are, however, some notable exceptions. For example, in one study, treatment with paroxetine was not associated with a reduction of VMPFC metabolism (Kennedy et al., 2001), but decreased VMPFC metabolism surfaced in another study (Brody et al., 2001). Likewise, decreased VMPFC metabolism did not arise after interpersonal psychotherapy (Brody et al., 2001). The overall change in depression scores was less than 50%, a frequent response criterion. This limited response may have precluded detecting VMPFC changes in activity. However, the subjects in our study also did not meet this criterion, yet VMPFC activity decreased robustly. Taken together, these findings suggest that reduced VMPFC activity may be necessary but not sufficient for antidepressant response.
Our principal finding regarding chronic VNS, i.e., reduced VMPFC activity in the resting state, merits placing our results in context with respect to two other recent studies of prolonged, chronic VNS. The first, the study by Nahas et al. (2007), found reduced activation (or change in BOLD signal with fMRI) over time in the VMPFC in response to direct stimulation. Of note, this result converges well with the findings of Henry et al. (2004). One explanation of both findings (i.e., reduced activation with chronic VNS) and the present results is that the basal, default-mode level of VMPFC activity may have exhibited a floor effect such that direct activation in this area became progressively reduced. The second, a case study by Critchley et al. (2007), examined BOLD signal following 7 months of VNS during the encoding of words of different affective valence with the device turned on and off. They found increased activation in the VMPFC under two conditions: 1) as a main effect of direct VNS stimulation; and 2) as an interaction effect of VNS with word valence. In other words, the latter condition showed increased activity in the VMPFC during direct VNS stimulation when encoding negative words as compared to when encoding positive or neutral words. It is difficult to integrate the findings of Nahas et al (2007) with those of Critchley et al. (2007) as the studies were designed differently--longitudinal vs. cross-sectional, respectively. However, the second finding of Critchley et al. raises the possibility that the relationship between VNS stimulation and VMPFC activity may be sensitive to task conditions. This possibility could not be addressed in the current study of the resting state.
The VMPFC is highly complex architectonically; densely interconnected with itself, and highly integrative (Ongur and Price, 2000; Price, 2005). Information from the vagus nerve reaches the VMPFC via indirect pathways through the amygdala, periaqueductal grey, hypothalamus, raphé and locus coeruleus (Henry, 2002). The VMPFC connects to a lateral orbital system that in turn receives projections from multiple sensory modalities (Carmichael and Price, 1995) and to the amygdala. Thereby, the VMPFC has access to the emotional content of external sensory information (smell, taste, hearing, sight) and to the internal milieu through vagal afferents carrying information from mechano-, chemo-, noci-, osmo-, and temperature sensors (Berthoud 2004; Berthoud and Neuhuber 2000).
The VMPFC encompasses Brodmann areas 24a, 10m, 10r, 10o, 10p, 32m, 14r, 14c, 13a, 13b, 11m, Iai, 47/12s, and 25 (Ongur et al., 2003). The latter, part of the subgenual cingulate, appears frequently in depression research (Mayberg et al., 2005, Wu et al., 1999, Drevets et al., 1997, Drevets et al., 1998, Ongur et al., 1998, Mayberg et al., 2000; Liotti et al., 2000; Little et al., 2005), but its connection to other components of the medial system merit highlighting (Ongur and Price, 2000). This medial network in turn connects to rostral superior temporal, parahippocampal, and posterior cingulate/retrosplenial cortices for further integration of information (Kondo et al., 2005).
The VMPFC probably does not have a “unitary” cognitive operation, but likely contains numerous specialized functional modules whose elementary cognitive operations are unknown. This appears supported by the anatomy. The VMPFC appears to be most active in the resting “default” mode of brain function (Raichle and Snyder, 2007). Activity in this region relates to the generation and representation of galvanic skin responses important for somatic marker theories of emotion and decision-making (Bechara et al., 1999; Critchley et al., 2000). A double dissociation of activity, or reciprocal sign change, arises between the VMPFC and lateral orbitofrontal regions during working memory for visceroappetitive/interroceptive vs. sensory/exterroceptive information, respectively (Hurliman et al., 2005). Activation of the VMPFC is a common feature of drug craving (e.g., Grant et al., 1996) which also shows this reciprocal relationship between the VMPFC and lateral orbital orbitofrontal cortex, depending upon the level of withdrawal or satiety to a drug (unpublished work from this laboratory). One interpretation of these data concerns a role for the VMPFC in the processing of afferent internal signals. Activity in the VMPFC also codes for more enduring dispositional traits such as negative affect in personality (Zald et al., 2002).
In conclusion, we show here that progressive reductions in VMPFC metabolism in the resting state are associated with chronic VNS in patients with TRD. Such changes have been reported previously after successful antidepressant treatment. (e.g., medication trials, DBS, ECT, sleep deprivation). In this study, no significant correlation between clinical score and reduction in VMPFC activity surfaced. The lack of a significant relationship is likely a consequence of the small sample size and narrow range of depression scores. Thus, rigorous investigation of whether decreased VMPFC activity is response-specific vs. treatment-specific will require further study. Nevertheless, these data do show robust metabolic changes in TRD with chronic VNS that track with a slow and modest improvement in symptoms. These results are also consistent with the hypothesis that a decline in VMPFC activity may be an important part of the final common pathway for antidepressant response.
Limitations
This study has several limitations. (1) According to the pivotal 10 week VNS trial (Rush et al., 2005a), inclusion criteria included both unipolar and bipolar depression; one subject in our series had bipolar disorder. Ideally for this imaging study, the affective disorder would have been more homogeneous. Fortuitously, omitting this subject from the data analysis did not remove the changes observed in VMPFC. (2) As VNS was adjunctive, it is possible that a medication change, as noted in Table 1, might have contributed to the progressive decrease in VMPFC metabolism between Chronic 6 Months and Chronic 1 Year scans. However, removing from the analysis the one patient that started on a new medication, lamotrigine, did not eliminate the reduction in VMPFC activity. (3) The modest sample size may limit the ability to generalize these results to other samples. However, the decreased metabolism in the VMPFC was very robust and statistically significant, even approaching the changes in brain activity seen with gross sensory stimulation. (4) Missing data in several cells could be attributed to several causes: from the randomization protocol, which was initially blinded; from variable volunteer compliance in participating in multiple PET scan sessions; and from rare technical difficulties with image acquisition. (5) Another issue is the change in scanners between 3 and 6 months into the study. Because different generation scanners have differing performance characteristics (sensitivity, resolution, axial sampling, etc.), data from different scanners can not be compared readily. This change prevented a pixel-wise, repeated-measures ANOVA, precluding a complete catalog of all metabolic changes associated with chronic VNS. However, the data using the same scanner (Chronic 6 months and Chronic 1 year) showed ongoing decreases in the VMPFC all the way to the end of the study. We, therefore, focused on the VMPFC, the largest and most extensive change.
Supplementary Material
Supplementary information is available at the NeuroImage website.
Acknowledgments
This work was supported in part by NARSAD; an Investigator-Initiated Grant from Cyberonics, Inc., Houston, TX; the Evert E. Bradbury Fund for Neuroimaging of Depression; RO1 AG120852; and the Department of Veterans Affairs. We thank Hemant Shah, M.D., and Ambreen Sattar, M.D., for assistance with data collection; Susan Siefert for proofreadng an earlier version of the manuscript; and our generous volunteer subjects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Barnes A, Duncan R, Chisholm JA, Lindsay K, Patterson J, Wyper D. Investigation into the mechanisms of vagus nerve stimulation for the treatment of intractable epilepsy using 99mTc-HMPAO SPET brain images. Eur. J. Nucl. Mol. Imaging. 2003;30:301–305. doi: 10.1007/s00259-002-1026-8. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol. Behav. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber W. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Corsetti G, Rodella L, Tredici G, Gioia M. Supraspinal connections and termination patterns of the parabrachial complex determined by the biocytin anterograde tract-tracing technique in the rat. J. Anat. 1998;193:417–430. doi: 10.1046/j.1469-7580.1998.19330417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR., Jr Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch. Gen. Psychiatry. 2001;58:631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial cortex. J. Comp. Neuro. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA. Cerebral blood flow changes during vagus nerve stimulation for depression. Psych. Res. 2006;146:179–184. doi: 10.1016/j.pscychresns.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J. Physiol. (Lond) 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Lewis PA, Orth M, Josephs O, Deichmann R, Trimble MR, Dolan RJ. Vagus nerve stimulation for treatment-resistant depression: behavioral and neural effects on encoding negative material. Psychosom. Med. 2007;69:17–22. doi: 10.1097/PSY.0b013e31802e106d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Price JL. Midline and intralaminar thalamic connections with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 2007;504:89–111. doi: 10.1002/cne.21440. [DOI] [PubMed] [Google Scholar]; Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J. Pharmacol. Exp. Ther. 2006;318:890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Reduced glucose metabolism in the subgenual cingulate in unipolar depression. Mol. Psychiatry. 1998;3:200–206. doi: 10.1038/sj.mp.4000380. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur. Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood. Ann. N. Y. Acad. Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The Role of Dopamine in the Pathophysiology of Depression. Arch. Gen. Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Dunner DL, Rush AJ, Russell JM, Burke M, Woodard S, Wingard P, Allen J. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J. Clin. Psychiatry. 2006;67:688–695. doi: 10.4088/jcp.v67n0501. [DOI] [PubMed] [Google Scholar]
- Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42:203–210. doi: 10.1016/s0920-1211(00)00181-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York: New York State Psychiatric Insititute; 2004. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with psychotic screen (SCID-I/P W/ PSY Screen), November Revision with January 2004 update. [Google Scholar]
- Fagiolini A, Kupfer DJ. Is treatment-resistant depression a unique subtype of depression? Biol. Psychiatry. 2003;53:640–648. doi: 10.1016/s0006-3223(02)01670-0. [DOI] [PubMed] [Google Scholar]
- Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr. Clin. North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM, Howland R, Kling MA, Moreno F, Rittberg B. A one year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol. Psychiatry. 2005;58:364–3673. doi: 10.1016/j.biopsych.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci. Lett. 2005;379:174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British J. Soc. Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harden CL, Pulver MC, Ravdin LD, Nikolov B, Halper JP, Labar DR. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1:93–99. doi: 10.1006/ebeh.2000.0046. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav. Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59 Supplement 4:S3–S14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- Henry TR, Bakay RA, Pennell PB, Epstein CM, Votaw JR. Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. Prolonged effects at high and low levels of stimulation. Epilepsia. 2004;45:1064–1070. doi: 10.1111/j.0013-9580.2004.03104.x. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA. Suicide and Antidepressant Treatment. Arch Gen. Psychiatry. 2000;57:325–326. doi: 10.1001/archpsyc.57.4.325. [DOI] [PubMed] [Google Scholar]
- Hurliman E, Nagode JC, Pardo JV. Double dissociation of exteroceptive and interoceptive feedback systems in the orbital and ventromedial prefrontal cortex of humans. J. Neurosci. 2005;18:4641–4648. doi: 10.1523/JNEUROSCI.2563-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Price JL. Midline and intralaminar thalamic connections with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 2007;504:89–111. doi: 10.1002/cne.21440. [DOI] [PubMed] [Google Scholar]
- Jimenez F, Velasco F, Salin-Pascual, Velasco M, Nicolini H, Velasco AL, Castro G. Neuromodulation of the inferior thalamic peduncle for major depression and obsesive compulsive disorder. Acta Neurochir. Suppl. 2007;97:393–398. doi: 10.1007/978-3-211-33081-4_44. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Krüger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am. J. Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. NeuroImage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol. Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Little JT, Ketter TA, Kimbrell TA, Dunn RT, Benson BE, Willis MW, Luckenbaugh DA, Post RM. Bupropion and venlafxine responders differ in pretreatment regional cerebral metabolism in unipolar depression. Biol. Psychiatry. 2005;57:220–228. doi: 10.1016/j.biopsych.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Marzec M, Edwards J, Sagher O, Fromes G, Malow BA. Effects of vagus nerve stimulation on sleep-related breathing in epilepsy patients. Epilepsia. 2003;44:930–935. doi: 10.1046/j.1528-1157.2003.56202.x. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;88:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol. Psychiatry. 1997;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA. The functional neuroanatomy of the placebo effect. Am. J. Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Berger KL, Lee KS. An automated method for rotational correction and centering of three-dimensional functional brain images. J. Nucl. Med. 1992;33:1579–1585. [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, Kuhl DE. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J. Nucl. Med. 1993;34:322–329. [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J. Nucl. Med. 1994;35:1528–1537. [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J. Nucl. Med. 1995;36:1238–1248. [PubMed] [Google Scholar]
- Mieda M, Yanagisawa M. Sleep, feeding, and neuropeptides: roles of orexins and orexin receptors. Curr. Opin. in Neurobiol. 2002;12:339–345. doi: 10.1016/s0959-4388(02)00331-8. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J. Comp. Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Nahas Z, Marangell LB, Husain MM, Rush AJ, Sackeim HA, Lisanby SH, Martinez JM, George MS. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J. Clin. Psychiatry. 2005;66:1097–11094. doi: 10.4088/jcp.v66n0902. [DOI] [PubMed] [Google Scholar]
- Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, Walker J, Anderson B, Koola J, Kosse S, Lomarev M, Bohning DE, George MS. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacol. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacol. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA, Mann JJ. Decreased regional brain metabolism after ECT. Am. J. Psychiatry. 2001;158:305–308. doi: 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13290–1295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cereb. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, Rittberg BR, Adson DE. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int. J. Obes. (Lond.) 2007a;31:1756–1759. doi: 10.1038/sj.ijo.0803666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Sheikh SA, Schwindt GC, Lee JT, Surerus-Johnson C, Pardo PJ, McCarten JR, Kuskowski MA, Dysken MW, Abbuzzahab FS, Rittberg BR, Adson DE. Functional imaging in treatment-resistant depression. Depression: Mind and Body. 2007b;3:47–57. [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Price JL. Free will versus survival: brain systems that underlie intrinsic constraints on behavior. J. Comp. Neurol. 2005;493:132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- Quitkin FM, Rabkin JD, Markowitz JM, Stewart JW, Mcgrath PJ, Harrison W. Use of pattern analysis to identify true drug response--a replication. Arch. Gen. Psych. 1987;44:259–264. doi: 10.1001/archpsyc.1987.01800150071009. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default-mode of brain function. Proc. Natl. cad. Sci. U.S.A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default-mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Fryman DL. Direct connections between the central nucleus of the amygdala and the nucleus of the solitary tract: an electrophysiological study in the rat. J. Auton. Nerv. Syst. 1988;22:83–87. doi: 10.1016/0165-1838(88)90157-9. Proc.Natl.Acad.Sci.U.S.A 98, 676–682. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, Howland R, Kling MA, Rittberg BR, Burke WJ, Rapaport MH, Zajecka J, Nierenberg AA, Husain MM, Ginsberg D, Cooke RG. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol. Psychiatry. 2005a;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, Lavori P, Howland R, Kling MA, Rittberg B. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol. Psychiatry. 2005b;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Rush AJ, George MS, Marangell LB, Husain M, Nahas Z, Johnson CR, Seidman S, Giller C, Haines S, Simpson RK, Goodman RR. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacol. 2001;25:713–728. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Brannan SK, Rush AJ, George MS, Marangell LB, Allen J. Durability of antidepressant response to vagus nerve stimulation (VNS) Int. J. Neuropsychopharmacol. 2007;10:817–826. doi: 10.1017/S1461145706007425. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacol. 2007;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Swartzman LC, Burkell J. Clin. Pharmacol. Ther. 64, 1=Talairach, J., Tournoux, P. 1998. Thieme, New York: Coplanar Stereotaxic Atlas of the Human Brain; 1998. Expectations and the placebo effect in clinical drug trials: why we should not turn a blind eye to unblinding, and other cautionary notes. [DOI] [PubMed] [Google Scholar]
- Thase ME, Rush AJ. Treatment-resistant Depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 1081–1097. [Google Scholar]
- van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J. Comp. Neurol. 1999;412:410–428. doi: 10.1002/(sici)1096-9861(19990927)412:3<410::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Urry HL, Johnstone T, Thurow ME, Frye CJ, Jackson CA, Schaefer HS, Alexander AL, Davidson RJ. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. J. Cogn. Neurosci. 2007;19:237–248. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, Najafi A, Klein E, Hazen K, Bunney WE, Fallon JH, Keator D. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am. J. Psychiatry. 1999;156:1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates the limbic system in humans. Brain. 1998;121:1143–1154. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel A, Joe A, Freymann N, Clusmann H, Schramm J, Reinhardt M, Biersack HJ, Maier W, Broich K. Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach. Psychiatry Res. 2005;139:165–179. doi: 10.1016/j.pscychresns.2005.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information is available at the NeuroImage website.