Summary
It is well known that many of the actions of 17β-estradiol (E2) in the central nervous system are mediated via intracellular receptor/transcription factors that interact with steroid response elements on target genes. However, there is compelling evidence for membrane steroid receptors for estrogen in hypothalamic and other brain neurons. But it is not well understood how estrogen signals via membrane receptors, and how these signals impact not only membrane excitability but also gene transcription in neurons. Indeed, it has been known for sometime that E2 can rapidly alter neuronal activity within seconds, indicating that some cellular effects can occur via membrane delimited events. In addition, E2 can affect second messenger systems including calcium mobilization and a plethora of kinases to alter cell signaling. Therefore, this review will consider our current knowledge of rapid membrane-initiated and intracellular signaling by E2 in the hypothalamus, the nature of receptors involved and how they contribute to homeostatic functions.
Keywords: ERα, ERβ, GPCR (G protein-coupled receptor), mER (membrane estrogen receptor that is a GPCR)
Electrophysiological studies in the 1960’s and 1970’s suggested that gonadal steroids could directly modulate the electrical activity of hypothalamic neurons (Barraclough and Cross, 1963, Lincoln, 1967, Lincoln and Cross, 1967, Moss and Law, 1971, Cross and Dyer, 1970, Dyer et al., 1972, Yagi and Sawaki, 1971, Bueno and Pfaff, 1976, Yagi, 1970, Kubo et al., 1975, Yagi, 1973, Whitehead and Ruf, 1974, Dufy et al., 1976). However, it was microelectrophoresis studies that showed that 17β-estradiol (E2) could rapidly alter neuronal firing activity of hypothalamic neurons within seconds (Kelly et al., 1976, Kelly et al., 1977a, Kelly et al., 1977b, Kelly et al., 1978b, Kelly et al., 1978a). The E2 -induced inhibition of spontaneous activity was stereospecific (17α-estradiol had no effect) and occurred at low nanomolar concentrations. Extracellular recording of action potentials with application of steroids via microelectrophoresis could not pinpoint the specific target cell of steroid actions to alter electrical activity, but it clearly established that steroids could have rapid actions (within seconds) to alter electrophysiological activity similar to classical neurotransmitters (Kelly et al., 1977a). Using intracellular recording techniques, it was found that fast perfusion of estrogen (100 pM) hyperpolarized arcuate neurons within seconds in a hypothalamic slice preparation and that this effect was reversed within minutes of washing out estrogen (Kelly et al., 1980). Similar actions of E2 were also demonstrated in hypothalamic ventromedial nucleus (VMH) and amygdala neurons, even in the presence of protein synthesis inhibitors (Nabekura et al., 1986, Minami et al., 1990). Later, it was found that GnRH neurons, identified post hoc using a combination of intracellular dye labeling and immunocytochemical staining, were a target of estrogen actions (Kelly et al., 1984). In these hypothalamic neurons, estrogen rapidly induced hyperpolarization via the opening of K+ channels (Kelly et al., 1984).
The rapid electrophysiological effects of E2 suggested that there may be a membrane binding site for E2 and indeed about the same time (Pietras and Szego, 1977, Pietras and Szego, 1979) identified binding sites for E2 on endometrial cells. Several years later relatively high affinity, specific binding of [3H]-17β-estradiol was demonstrated in synaptosomal membranes prepared from the adult rat brain (Towle and Sze, 1983). These findings were later corroborated using the membrane impermeant 17β-estradiol-6-[125I]-BSA (Zheng and Ramirez, 1997). Furthermore, competition-binding assays of synaptosomal membranes showed that the hypothalamus exhibited a relatively high affinity (3 nM) binding site for E2 and somewhat lower affinity binding sites in the olfactory bulb and cerebellum (Ramirez et al., 1996, Ramirez and Zheng, 1996). The stereospecificity of the binding was demonstrated by displacement of the radiolabeled E2 with cold E2 or E2-BSA, but not by 17α-estradiol or 17α-estradiol-BSA even at micromolar concentrations (Ramirez et al., 1996). These biochemical data complemented the electrophysiological findings that gonadal steroid signaling could be initiated at the membrane.
Nuclear-initiated signaling of E2
Estrogen receptors regulate cellular function through at least two signaling pathways previously broadly classified as “genomic” versus “nongenomic” (McEwen and Alves, 1999, Björnström and Sjöberg, 2005). However, recently the FASEB steroid signaling work group suggested that “membrane-initiated steroid signaling” and “nuclear-initiated steroid signaling” are more appropriate terminologies (Hammes and Levin, 2007). The nuclear-effects on a variety of tissues that involves gene stimulation as well as gene repression (Herbison, 1998, Couse and Korach, 1999, Nilsson et al., 2001, Etgen et al., 2001, Stossi et al., 2006, Kininis et al., 2007). In general, this “classical” signaling pathway of estrogen involves steroid-dependent formation of nuclear estrogen receptor homo- or heterodimers and the subsequent binding of this complex with a unique DNA sequence known as an estrogen response element (ERE), in E2-responsive gene promoters (O’Malley and Tsai, 1992, Muramatsu and Inoue, 2000, Gruber et al., 2004). The inactive ER exists in a complex of several proteins that disassociate upon ligand binding, which transforms the receptor to an active state (Couse and Korach, 1999, Gruber et al., 2004). More specifically, recruitment of other nuclear co-activator and co-regulatory proteins and interactions with the transcription machinery results in transactivation of genes that contain EREs (Muramatsu and Inoue, 2000, Gruber et al., 2004).
Several genes in the brain that are clearly estrogen-responsive do not appear to contain ERE sequences (Malyala et al., 2004, Gruber et al., 2004). There is compelling evidence that ERα and ERβ can regulate transcription of some of these “estrogen-responsive” genes by interacting with other DNA-bound transcription factors, such as specificity protein-1 (SP-1) and activator protein 1 (AP-1), rather than binding directly to DNA (Paech et al., 1997, Jacobson et al., 2003, Gruber et al., 2004). For example, the ligand-induced responses with ERβ, in contrast to ERα, at an AP-1 site illustrates the negative transcriptional regulation by estrogen and strong positive regulation by antiestrogens like ICI 164,384 (Paech et al., 1997). This provides a mechanism for differential regulation of gene expression by estrogen.
Membrane-initiated signaling of E2
It has been known for a number of years that estrogen has acute, membrane-initiated signaling actions in the brain (for review see (Kelly and Rønnekleiv, 2002, Rønnekleiv and Kelly, 2005, Bryant et al., 2006)). The nature and significance of these actions have been a matter of dispute. However, it is now widely accepted that some of the actions of estrogen are quite rapid and cannot be attributed to the classical nuclear-initiated steroid signaling of ERα or ERβ. One view is that both nuclear and plasma membrane-associated ERs might be products of the same genes (Razandi et al., 1999, Boulware et al., 2005, Pedram et al., 2006, Szegõ et al., 2006, Dewing et al., 2007). This belief stems primarily from the fact that many of the rapid effects of E2 can be induced by selective ERα or ERβ ligands, antagonized by the ER antagonist, ICI 182,780, or are lost in animals bearing mutations in ERα and/or ERβ genes (Couse and Korach, 1999, Singer et al., 1999, Dubal et al., 2001, Wade et al., 2001, Abraham et al., 2003, Boulware et al., 2005, Boulware et al., 2007). Another view is that estrogen activates a unique membrane ER (mER) (Gu et al., 1999, Toran-Allerand, 2004, Toran-Allerand, 2005, Qiu et al., 2003, Qiu et al., 2006b).
In hippocampal slices E2 enhances N-methyl-D-aspartate (NMDA)-mediated excitatory postsynaptic potentials (EPSPs) and long-term potentiation (LTP) following Schaffer (collateral) fiber stimulation (Foy et al., 1999). Also, E2 potentiates non-NMDA (kainate)-mediated excitation of hippocampal CA1 pyramidal neurons via activation of a cAMP/PKA pathway (Gu and Moss, 1996, Gu and Moss, 1998). Based on the findings from the hippocampal slice, it was hypothesized that E2 activates a Gs-coupled receptor on the extracellular surface of hippocampal neurons, which operates in concert with an internal action of E2 on cAMP-dependent phosphorylation (Gu and Moss, 1998). Importantly, these rapid actions of E2 on kainate-induced currents in hippocampal neurons are suggesting a novel mechanism (receptor) for the rapid actions of E2 in the hippocampus (Gu et al., 1999). In addition E2 and E2-BSA, when applied acutely to the hippocampus in ovariectomized animals, produce a sustained reduction of the slow IAHP in CA1 pyramidal neurons (Carrer et al., 2003). The slow IAHP is mediated by a Ca2+ - activated K+ conductance. This provides further evidence for the involvement of a membrane ER, although, the mechanism by which E2 regulates Ca2+ influx into CA1 neurons is currently unknown. More recent studies in hippocampal CA3-CA1 neuronal cultures have revealed that E2 rapidly stimulates MAPK-dependent cAMP-responsive element binding protein (CREB) phosphorylation. In addition, E2 also decreases L-type calcium channel-mediated CREB phosphorylation, and therefore, has both positive and negative influences on CREB activity (Boulware et al., 2005). Both effects are mimicked by the membrane-impermeable E2-BSA and are inhibited by the antiestrogen ICI 182, 780, which collectively suggest that a membrane estrogen receptor is involved with some of the characteristics described by Qiu and coworkers (Qiu et al., 2003). The positive modulation of CREB activity occurs via ER interactions with metabotropic glutamate receptors 1(mGluR 1) and the negative modulation via mGluR 2/3 (Figure 1A). The type of interaction, however, is currently unknown, but potentially could be similar to that described for mER and 5HT2C receptors which have diverse but convergent signaling pathways in arcuate neurons (Qiu et al., 2007). A similar scenario has been hypothesized for the rapid E2-induced activation of μ-opioid receptors in the medial preoptic area associated with female sex behavior (Dewing et al., 2007). Based on responses to the ER agonists, propylpyrazoletriol (PPT) and diarylpropionitrile (DPN), which are selective for ERα and ERβ, respectively, and transfection studies with mutant ERα (Harrington et al., 2003, Lund et al., 2006, Boulware et al., 2007), the membrane-localized receptors in the hippocampus are hypothesized to be ERβ and ERβ (Boulware et al., 2005, Boulware et al., 2007). However further studies, such as in ERα and/or ERβ knockout mice, are needed to more clearly define these hippocampal (and hypothalamic) ERs (mERs), and whether they are indeed ERα and/or ERβ associated with the plasma membrane.
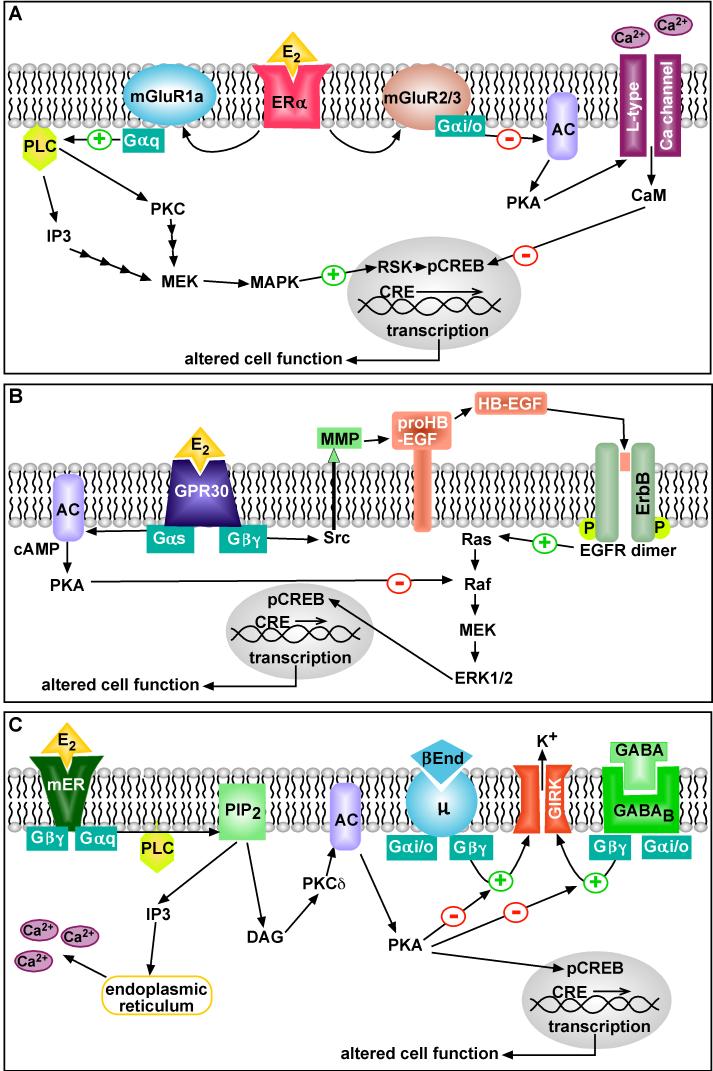
Figure 1. Cellular models of membrane-initiated signaling pathways of E2.
(A) E2 interacts with ERα locali zed at the neuronal (plasma) membrane and activates group 1 and group 2 metabotropic glutamate receptor (mGluR1a, mGluR2/3) signaling. Activation of mGluR1a causes Gαq stimulation of phospholipase C (PLC) which leads to MAPK-induced CREB phosphorylation. Activation of mGluR2/3 causes Gαi/o inhibition of adenylyl cyclase (AC) and PKA which reduces the activity of L-type calcium channels and leads to attenuated CREB phosphorylation.
(B) E2 acts via GPR30 to promote EGF-receptor transactivation. This happens through a Gβγ-subunit pathway that promotes intracellular tyrosine kinase (Src)-mediated metalloproteinase (MMP)-dependent cleavage of proheparin-binding EGF (proHB-EGF) and release of HB-EGF from the cell surface. EGF redeptor transactivation leads to Ras-dependent ERK1/2 activation and presumably CREB phosphorylation. E2 via GPR30 can also stimulate adenylyl cyclase (AC) activity, which leads to PKA-mediated suppression of EGF-induced ERK1/2 activity.
(C) E2 activates a membrane-associated ER (mER) that is Gαq -coupled to activation of phospholipase C that catalyzes the hydrolysis of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) to inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). Calcium is released from intracellular stores (endoplasmic reticulum) by IP3, and DAG activates protein kinase Cδ (PKCδ). Through phosphorylation, adenylyl cyclase (AC) activity is upregulated by PKCδ. The generation of cAMP activates PKA, which can rapidly uncouple GABAB and μ-opioid (μ) receptors from their effector system through phosphorylation of a downstream effector molecule (e.g., G protein-coupled, inwardly rectifying K+ (GIRK) channel). The mER-mediated modulation of kinase pathways reduces the capacity of neuromodulators such as GABA and β-endorphin (β-End) to inhibit POMC neuronal excitability. mER-mediated activation of PKA can lead to phosphorylation of cAMP-responsive element binding protein (pCREB), which can then alter gene transcription through its interaction with CREs on genes.
Potential candidates for novel membrane ERs include ER-X and the G-protein-coupled receptors, Gq-mER and GPR30 (Toran-Allerand, 2005, Qiu et al., 2003, Revankar et al., 2005, Qiu et al., 2006b, Filardo and Thomas, 2005, Funakoshi et al., 2006). ER-X is a plasma membrane-associated, putative ER that is enriched in caveolar-like microdomains of postnatal, but not adult, cortical membranes (Toran-Allerand et al., 1999, Toran-Allerand et al., 2002). In organotypic explants of the developing cerebral cortex, E2 induces tyrosine phosphorylation of both ERK1 (extracellular signal-regulated protein kinase 1) and ERK2, an action very similar to a number of growth factors including nerve growth factor (NGF) (Toran-Allerand, 2005). Interestingly, this E2-induced activation is not antagonized by the antiestrogen, ICI 182,780, and occurs in cortical explants from mice lacking ERα. In addition, this putative mER is particularly responsive to 17α-estradiol as compared to 17β-estradiol (Singh et al., 1999, Singh et al., 2000, Toran-Allerand et al., 2002). These and other findings have led to the hypothesis that ER-X is a novel ER that is ICI-insensitive (for review see (Toran-Allerand, 2004, Toran-Allerand, 2005)).
An orphan G-protein-coupled receptor, GPR 30, has also received considerable attention because it binds estrogen, albeit in cancer cells, and exhibits binding and signaling characteristics of a membrane ER (Revankar et al., 2005, Thomas et al., 2005). In breast cancer cells that are transfected with GPR30, estrogen activates the mitogen-activated protein kinases (MAPK), ERK1 and ERK2, and these actions are independent of ERα or ERβ (Filardo, 2002, Filardo and Thomas, 2005). According to this model (Figure 1B), E2 activates Gβγ-subunits that promote the release and activation of an epidermal growth factor precursor (proHB-EGF). The active HB-EGF binds to the EGF receptor (ErbB) to facilitate receptor dimerization and downstream activation of ERK (Filardo et al., 2000, Filardo et al., 2002, Filardo, 2002). In addition, E2 can also stimulate adenylyl cyclase activity, which leads to PKA-mediated suppression of EGF-induced ERK1/2 activity. Interestingly, the selective estrogen receptor modulator (SERM) tamoxifen and the antiestrogen ICI 182,780 both promote GPR30-dependent transactivation of the EGF receptor and subsequent MAPK activation. Therefore, the pharmacology of GPR30 as defined in cancer cells is different from that of Gq-mER in neurons (Lagrange et al., 1997, Qiu et al., 2003, Qiu et al., 2006b), suggesting that these are different receptors. In fact, the mER-mediated response to estrogen in arcuate neurons is still present in GPR30 KO mice (Qiu et al., 2008). Although GPR30 has been localized in the brain and estrogen binds to this receptor (Funakoshi et al., 2006, Bologa et al., 2006, Brailoiu et al., 2007, Prossnitz et al., 2007), further studies on GPR30 are necessary to delineate its function in the brain.
Substantial evidence has been generated in the support of a G protein-coupled membrane ER. Intracellular and whole cell recording from guinea pig and mouse hypothalamic slices have been used to characterize the mER (Lagrange et al., 1997, Qiu et al., 2003, Qiu et al., 2006b). These studies show that E2 acts stereospecifically with physiologically-relevant concentration dependence (EC50 = 8 nM) to cause a significant reduction in the potency of μ-opioid and GABAB agonists to activate an inwardly rectifying K+ conductance (Lagrange et al., 1997, Qiu et al., 2003). Estrogenic modulation of μ-opioid and GABAB agonists potency is mimicked either by stimulation of adenylyl cyclase with forskolin or by direct PKA activation with Sp-cAMP, in a concentration-dependent manner (Lagrange et al., 1997, Qiu et al., 2003). Furthermore, the selective protein kinase A (PKA) antagonists KT5720 and Rp-cAMP block the effects of E2. More recent data indicate that PKA is downstream in a signaling cascade that is initiated by a Gαq-coupled membrane ER that is linked to activation of phospholipase C-protein kinase C-protein kinase A (Figure 1C)(Qiu et al., 2003, Qiu et al., 2006b). E2, however, does not compete for the μ-opioid (or GABAB) receptor or alter the affinity for the receptor. Importantly, the antiestrogens ICI 164,384 or ICI 182,780 block the actions of E2 with subnanomolar affinity that is similar to ICI’s affinity (Ki) for ERα (Weatherill et al., 1988, Lagrange et al., 1997). These pharmacological findings clearly define a G-protein-coupled membrane receptor for estrogen.
The mER is not ERα or ERβ, since it can be activated with a diphenylacrylamide compound, STX, that does not bind ERα or ERβ (Qiu et al., 2003, Qiu et al., 2006b). STX (and E2) selectively target a Gαq-coupled phospholipase C-protein kinase C-protein kinase A pathway in mediating μ-opioid and GABAB desensitization of hypothalamic neurons in normal animals (Figure 1C). Importantly, STX (and E2) can also activate the Gq signaling pathway in mice lacking both ERα and ERβ (Qiu et al., 2006b). As with ER-X, definitive characterization of this Gq-mER awaits cloning of the gene. However, it is evident that estrogen can rapidly alter cell function through ERα, ERβ and/or novel ERs.
It has become clear that there are “indirect” genomic actions of estrogen in the brain that do not require nuclear targeting of estrogen receptors and therefore are classified as “membrane-initiated” signaling of estrogen (for review (Kelly and Levin, 2001, Bryant et al., 2006, Hammes and Levin, 2007)). Such signals that are initiated by E2 at the plasma membrane can trigger intracellular signaling events that result in gene transcription. New gene transcription can result from E2 activation of multiple intracellular kinase cascades including MAPK, phosphoinositide 3-kinase (PI3K), cAMP-protein kinase (PKA) and protein kinase C (PKC) pathways (Watters et al., 1997, Bi et al., 2001, Cato et al., 2002, Yang et al., 2003, Deisseroth et al., 2003). These events are often termed rapid signaling cascades because they are initiated at the plasma membrane or in the cytoplasm and occur much faster than ERE-driven events (Bryant et al., 2005, Björnström and Sjöberg, 2005). For example, E2 binding to its known receptors or a novel membrane ER can upregulate cAMP in hypothalamic neurons by increasing adenylyl cyclase activity (Lagrange et al., 1997). Cyclic-AMP activates PKA, which in turn phosphorylates cAMP-responsive element binding protein (CREB) and elicits new gene transcription (Zhou et al., 1996, Gu et al., 1996, Watters and Dorsa, 1998, Abraham et al., 2004). Therefore, genes with CRE binding sites can be activated within a relatively short time course in neurons independent of estrogen receptors interacting with EREs. These actions of estrogen via pCREB result in activation of genes encoding neurotransmitters such as dopamine, enkephalin, dynorphin and neurotensin, which are all critical for hypothalamic function (Gu et al., 1996, Watters and Dorsa, 1998). Moreover, in hypothalamic (mouse) GnRH neurons the rapid phosphorylation of CREB following E2 treatment is observed in ERα but not in ERβ knockout mice indicating a role for ERβ in the acute activation by pCREB in GnRH neurons (Abraham et al., 2003).
17β-estradiol, growth factors and reproduction
Systemic administration of E2 in ovariectomized rats activates IGF-I receptors and induces the association between IGF-I receptors and ERα in the hypothalamus (Quesada and Etgen, 2001, Cardona-Gómez et al., 2002, Mendez et al., 2003). Similar to the effects in cortical neurons, there is an interaction (complex formation) between the p85 subunit of PI3K and ERα within 1-3 h, which leads to activation of Akt (Cardona-Gómez et al., 2002, Mendez et al., 2003). Also, the E2-induced activation of IGF-I receptors augments α1-adrenergic receptor signaling, which is important for reproductive functions (Quesada and Etgen, 2001). On the other hand, blockade of IGF-I receptors during E2 priming prevents E2-induced increases in α1-adrenergic receptor binding density as well as IGF-I enhancement of noradrenergic receptor signaling (Quesada and Etgen, 2002). Collectively, these findings support functional interactions between E2 and IGF-I. Therefore, these actions of E2 on the IGF-I receptor signaling pathway may be a key mechanism by which estrogen affects synaptic remodeling and neuronal plasticity during the estrous cycle.
Of particular interest are the findings that intracerebroventricular (i.c.v.) infusion of JB-1, a selective competitive antagonist of IGF-1 autophosphorylation, inhibits the estrogen-induced LH surge and sexual behavior in ovariectomized rats (Quesada and Etgen, 2002). In addition, co-administration (i.c.v.) of blockers of PI3 kinase (wortmannin) and MAPK (PD98059) inhibit the long-term (48 h) effects of E2 to induce the LH surge and facilitate lordosis behavior (Etgen and Acosta-Martinez, 2003). Therefore, facilitation of female sexual behavior by E2 appears to involve activation of both PI3 kinase and MAPK signal transduction pathways. The importance of growth factors for female sexual behavior is further illustrated by observations that epidermal growth factor (EGF) and also IGF-I can, in the absence of estrogen and progesterone (within 1-4 h of i.c.v. administration), induce mating behavior in rats and mice, in part, through an ERα-dependent mechanism (Apostolakis et al., 2000). This relatively rapid, ligand-independent ER action is in striking contrast to the well established finding that estrogen priming over a period of at least 24 h is needed for progesterone induction of female reproductive behavior (Etgen et al., 2001). The cross-talk between estrogen signaling and membrane-initiated growth factor signaling in the hypothalamus is particularly interesting, although it is currently not well understood. The ability of both IGF-I and estradiol to induce female sexual behavior may involve complex interactions between ERα, the IGF-1 receptor and the PI-3 kinase p85 subunit.
17β-estradiol and GnRH neurosecretion
Despite having been studied extensively for over 25 years, the mechanism(s) by which estrogen regulates gonadotropin releasing hormone (GnRH) neurons is not well understood. It has been obvious for a number of years that GnRH neurons are modulated by estrogen in a complex manner. For example, loss of estrogen by ovariectomy disrupts GnRH regulation of pituitary LH secretion and results in elevated levels of plasma LH. This effect is due to the loss of negative feedback actions of E2. However, both negative and positive (induction of the LH surge) feedback regulation of LH (GnRH) secretion can be restored by replacement with E2. The positive feedback is believed to be by an action of E2 in the anteroventral periventricular (AVPV) nucleus (Herbison, 1998, Han et al., 2005, Smith et al., 2006, Wintermantel et al., 2006). Neurons in the AVPV express kisspeptin, a neuropeptide encoded by the Kiss gene, GABA and opioid peptides, all of which are important for regulation of GnRH neurosecretion (Simerly et al., 1988, Wagner et al., 2001a, Jackson and Kuehl, 2002, DeFazio et al., 2002, Smith et al., 2006, Christian and Moenter, 2007). The AVPV area expresses high levels of ERα and also ERβ, and the actions of the gonadal steroids are mediated, in part, via the nuclear-initiated signaling (genomic) mechanism (Shughrue et al., 1997, Wintermantel et al., 2006). However, the AVPV is also sensitive to the rapid actions of gonadal steroids. For example, E2 within 30 min increases the expression of the pCREB in the AVPV (Gu et al., 1996). At the cellular level, individual AVPV neurons including GABA neurons, respond to both β-adrenergic and α-adrenergic input by a reduction in the median afterhyperpolarization current (mIAHP), which increases the action potential firing in these neurons (Wagner et al., 2001b). Moreover, the α1-adrenergic, but not β-adrenergic inhibition of the mIAHP is potentiated after acute (15-20 min) exposure to estrogen, which further increases neuronal excitability (Wagner et al., 2001b). The estrogen-induced enhancement of the coupling of the α1-adrenergic receptors to their effector calcium-activated K+ (SK) channels (underlying the mIAHP) is initiated within 15 min in vitro and lasts for at least 24 h following systemic steroid administration, suggesting both rapid and sustained effects (Wagner et al., 2001b). Since SK channels are critical for modulating neuronal firing rate and pattern (Stocker et al., 1999, Sah and Davies, 2000), estrogen-induced modulation of these channels would have significant functional consequences for AVPV neurons and their targets.
Because estrogen receptor α (ERα), the first cloned receptor/transcription factor for E2, has not been localized to native GnRH neurons, the predominant view has been that estrogen affects GnRH neurons through pre-synaptic mechanisms. However, GnRH neurons are rapidly hyperpolarized by estrogen, an effect that inhibits the activity of GnRH neurons in the presence of tetrodotoxin (TTX) which blocks fast Na+ channel activity and thus prevents synaptic inputs (Kelly et al., 1984, Condon et al., 1989, Lagrange et al., 1995). These findings, therefore, suggest a direct hyperpolarizing action of estrogen on GnRH neurons via a Gαi,o-coupled receptor. Indeed, in GT1-7 cells, an immortalized GnRH neuronal cell line, estrogen inhibits adenylyl cyclase activity (cAMP production) via a pertussis toxin (Gαi,o coupling) mechanism (Navarro et al., 2003). The electrophysiological effects are much too rapid to involve transcription through classical ERs, but an estrogen - Gαi,o -coupled receptor has not been identified. Interestingly, E2 increases the firing in primate nasal placode GnRH neuronal cultures within 10 min (Abe and Terasawa, 2005). Therefore, E2 may have both direct inhibitory and indirect excitatory effects on GnRH neurons, since the latter experiments were done in the absence of blockade of synaptic input.
An important milestone for understanding estrogen action in GnRH neurons was the discovery of a second ER, ERβ, in 1996 and the documentation that this receptor was expressed in GnRH neurons (Kuiper et al., 1996, Hrabovszky et al., 2000, Hrabovszky et al., 2001, Kallo et al., 2001, Herbison and Pape, 2001). The latter findings combined with recent technological advances, such as the development of ER mutants and transgenic animals expressing green fluorescent protein (GFP) in GnRH neurons, have greatly facilitated studies to understand the cellular mechanisms by which GnRH neurons are regulated by estrogen (Spergel et al., 1999, Suter et al., 2000, Kato et al., 2003, Han et al., 2005, Abraham et al., 2003, Smith et al., 2006, Wintermantel et al., 2006, Zhang et al., 2007). A series of recent publications show that in mouse hypothalamic neuronal explants and in primate nasal placode cultures, E2 augments synchronous intracellular Ca2+ oscillations in GnRH neurons (Temple et al., 2004, Temple and Wray, 2005, Abe et al., 2007). This relatively rapid effect is observed within 10-30 min of E2 application. The calcium oscillations in the presence of TTX are most likely the result of estrogen action directly on GnRH neurons. Furthermore, E2 conjugated to BSA at the C-17 position (E2-17 BSA) or to a large nondegradable poly(amido)amine-dendrimer macromolecule (Harrington et al., 2006) mimics the effects of E2 on intracellular calcium oscillations (Temple et al., 2004, Temple and Wray, 2005, Abe et al., 2007). However in the mouse explant experiments (Temple et al., 2004, Temple and Wray, 2005), the E2 -induced calcium oscillations are blocked by ICI 182,780 suggesting that an ER is involved. In contrast, E2-induced calcium oscillations in the primate placode cultures are not blocked by ICI 182,780, and the antagonist ICI 182,780 alone has no effect suggesting that an ER may not be involved (Abe et al., 2007). The actions of E2-BSA in mouse GnRH neurons are abrogated by pertussis toxin treatment and not by inhibition of gene transcription, supporting a role for a G protein-coupled membrane receptor (Temple et al., 2004, Temple and Wray, 2005). However, in vivo studies have provided evidence for ERβ in the E2-induced rapid (15 min) induction of pCREB in mouse GnRH neurons (Abraham et al., 2003). Collectively, these findings indicate that there are multiple acute actions of E2 in GnRH neurons, some of which involve ERβ.
Interestingly, mice lacking ERβ appear to exhibit normal sexual behavior and reproduce successfully with the exception that their litter size is reduced, presumably due to a decline in ovarian function (Krege et al., 1998, Dupont et al., 2000). In contrast, ERα, which is not expressed in GnRH neurons, is essential for both sexual behavior and fertility (Ogawa et al., 1998, Dupont et al., 2000). Therefore, the functional significance of ERβ expression in GnRH neurons remains unclear. In fact, how the rapid actions of the steroids are related to the longer term genomic actions are currently not well understood. For example, recent findings have revealed that GnRH neurons express K-ATP channels and the agonist-induced outward (K-ATP channel) current is increased by approximately two-fold in E2-treated animals (Zhang et al., 2007). The increased activity, which occurs in the presence of TTX and GABA and glutamate blockade, is not the result of increased expression of the channel based on quantitative real-time PCR results. Therefore, it appears that there is an E2-induced change in signaling molecules (kinases) that impinge on the channel, part of which may be nuclear-initiated steroid signaling and/or acute phosphorylation events. Clearly, further studies are needed to elucidate the regulation of GnRH neurons by E2.
Studies are forthcoming on the cross-talk between rapid membrane-initiated and long-term nuclear-initiated steroid actions (Lagrange et al., 1994, Lagrange et al., 1997, Wagner et al., 2001b, Vasudevan et al., 2001, Kow and Pfaff, 2004, Malyala et al., 2005, Qiu et al., 2006b, Qiu et al., 2006a, Roepke et al., 2007). For example, it has been found that both acute effects of E2 and the transcriptional changes alter excitability of hypothalamic neurons (Kelly et al., 2003, Malyala et al., 2005, Qiu et al., 2006a). In addition, the estrogen-induced membrane actions in the ventromedial nucleus (VMH) of the hypothalamus can potentiate its genomic effects on lordosis behavior (Kow and Pfaff, 2004). Moreover, this membrane effect in the VMH appears to be mediated by signaling pathways involving PKC and PKA (Kow and Pfaff, 2004). These findings are particularly intriguing in view of other findings that estrogen activates a novel membrane ER, other than ERα or ERβ that is Gαq-coupled to phospholipase C, PKCδ and PKA to alter cell firing (Figure 1C) (Qiu et al., 2003, Qiu et al., 2006b).
Effects of 17β -estradiol on VMH and Arcuate Neurons: Role in Regulation of Feeding
In addition to its role in the control of reproduction, estrogen is involved in the regulation of appetite, energy expenditure, body weight, adipose tissue deposition and distribution in females (Milewicz et al., 2000, Geary, 2001, Poehlman, 2002). Ovariectomy induces an increase in food intake and decreases ambulatory and wheel running activities in rodents, all of which are reversed with estrogen replacement (Ahdieh and Wade, 1982, Colvin and Sawyer, 1969, Shimomura et al., 1990, Asarian and Geary, 2002). In fact, hypo-estrogenic states are associated with decreased activity and an increase in body weight in (Czaja and Goy, 1975, Butera and Czaja, 1984, Czaja, 1984, McCaffrey and Czaja, 1989, Jones et al., 2000, Asarian and Geary, 2002, Qiu et al., 2006b, Clegg et al., 2006, Clegg et al., 2007). The anorectic effects of estrogen are thought to be mediated through CNS actions based on the findings that direct injections of E2 into the paraventricular nucleus of the hypothalamus (PVH) or arcuate/ventromedial nucleus are effective to reduce food intake, body weight and increase wheel running activity in females (Colvin and Sawyer, 1969, Ahdieh and Wade, 1982, Butera and Czaja, 1984). It is evident that neurons in these hypothalamic nuclei regulate energy homeostasis and are affected by E2. For example, E2 up-regulates the expression of the peptide β-endorphin in proopiomelanocortin (POMC) neurons in ovariectomized female guinea pigs (Thornton et al., 1994, Bethea et al., 1995). Furthermore, there is a decrease in hypothalamic β-endorphin levels in the hypothalamus of postmenopausal women who do not take hormone replacement that correlates with weight gain (Leal et al., 1998). In contrast, E2 and mER agonist STX reverses the ovariectomy-induced increase in arcuate neuropeptide Y (NPY) mRNA expression in rodents (Shimizu et al., 1996, Roepke et al., unpublished observations). Therefore, it appears that the arcuate nucleus and specifically POMC and NPY neurons are a major target for the anorectic actions of estrogen, which underscores their importance in the control of energy homeostasis. Indeed, POMC neurons are critical for the regulation of feeding and are also involved in the rewarding aspects of food intake (Hayward et al., 2002, Appleyard et al., 2003).
Important inhibitory regulators of POMC neuronal activity are the G-protein activated, inwardly-rectifying potassium (GIRK) channels. Both μ-opioid receptor (e.g., by β-endorphin or by selective μ-opioid agonists) or GABAB receptor activation of these GIRK channels directly hyperpolarize and thereby inhibit hypothalamic neurons (Loose et al., 1990, Kelly et al., 1992, Lagrange et al., 1994, Lagrange et al., 1995, Lagrange et al., 1996). Brief (<20 min) application of E2 or BSA-E2 in vitro causes a four-fold decrease in the potency of μ-opioid receptor agonists and GABAB receptor agonists to inhibit POMC neurons (Lagrange et al., 1994, Lagrange et al., 1996, Qiu et al., 2003), indicating that a membrane ER (mER) is involved. As stated previously, this mER is distinct from ERα or ERβ (Qiu et al., 2003, Qiu et al., 2006b). Therefore, E2 via a mER-pathway rapidly decreases the potency of μ-opioid and GABAB ligands at their receptors (desensitization), thus increasing POMC neuronal firing and the release of the POMC products, β-endorphin and melanocyte stimulating hormone (MSH).
Interestingly, inwardly rectifying K+ channels are also a point of convergence for the effects of leptin and insulin in POMC neurons (Plum et al., 2006). Both leptin and insulin activate PI3Kinase (via insulin receptor substrate) that leads to the metabolism of phosphatidylinositol (4.5) biphosphate (PIP2) to phoshatidylinositol (3,4,5)-triphosphate (PIP3) in POMC neurons (Xu et al., 2005). Activation of GIRK channels requires permissive levels of membrane PIP2 and increased channel activity results from Gβγ-mediated stabilization of PIP2-GIRK binding (Huang et al., 1998, Zhang et al., 1999). In fact, the regulation of channel activities by Gβγ, PIP2, or phosphorylation occurs on the time scale of a few seconds which can be resolved by electrophysiological techniques (Suh and Hille, 2002). In recent studies from our lab we have found that PI3K plays a critical role in facilitating the rapid membrane response to estrogen, as elucidated by the use of PI3K inhibitors (Malyala et al., 2008). Indeed, we found that PI3K inhibitors, which block the phosphorylation of PIP2 and thereby increase the levels of PIP2 in the membrane, augmented GIRK channel activity and attenuated the effects of E2 on the mER signaling pathway.
As proof of principle of the importance of the membrane-initiated estrogen signaling pathway in the control of energy homeostasis, we used STX to selectively target the mER (i.e., in vivo treatment), and found that STX, similar to E2, attenuated the weight gain following ovariectomy (Qiu et al., 2006b). Moreover, the fact that both E2 and STX are fully efficacious in activating this signaling pathway in double-estrogen receptor knock-out mice is further proof for the existence of a novel mER that is involved in critical physiological processes such as the control of energy homeostasis.
Cross talk between membrane actions and genome activation
The gonadal steroid E2 participates in numerous functions including reproduction, feeding, neuroprotection and cognition. It has been known for some time that the main actions of E2 is to regulate gene transcription through binding to and activating nuclear receptors that can stimulate or inhibit gene transcription at specific DNA binding sites. However, recently it has become clear that E2 can exert its action through multiple signaling mechanisms including membrane-initiated, cytoplasmic as well as nuclear-initiated steroid signaling (Figure 1). For example, there is evidence that E2 increases the activity of hypothalamic POMC and dopamine neurons through binding to a membrane-localized receptor distinct from ERα or ERβ (Qiu et al., 2003, Qiu et al., 2006b). In addition, E2 conjugated to macromolecules can activate calcium pulses in GnRH neurons by a mechanism that appears to be independent of ERβ (Temple and Wray, 2005, Abe et al., 2007). There are many examples in the hypothalamus and other brain regions for an E2-induced upregulation of PKC, PKA, PI3K and MAP kinase activity leading to altered neuronal activity, as well as altered behavioral activities. Although the exact identity of the mER is still not known, we are making substantial progress towards a better understanding of the full complement of E2 actions in the brain. Also, we are beginning to understand the interaction between rapid signaling events and changes in gene expression. However, many challenges lie ahead to fully identify the nature of membrane steroid receptors, functional properties and integration (cross-talk) with nuclear steroid receptors.
ACKNOWLEDGMENTS
The authors thank members of their laboratories who contributed to the work described herein, especially Drs. Jian Qiu, Chunguang Zhang, Troy A. Roepke, Anna Malyala and Ms. Martha A. Bosch. Also, special thanks to Ms. Martha A. Bosch for her skilled assistance with the illustrations and manuscript preparation. The work from the authors’ laboratories was supported by PHS grants NS 43330, NS 38809 and DK 68098.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1 neurons. Endocrinology. 2007;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–4320. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J. Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Ahdieh HB, Wade GN. Effects of hysterectomy on sexual receptivity, food intake, running wheel activity, and hypothalamic estrogen and progestin receptors in rats. J. Comp. Physiol. Psychol. 1982;96:886–892. [PubMed] [Google Scholar]
- Apostolakis EM, Garai J, Lohmann JE, Clark JH, O’Malley BW. Epidermal growth factor activates reproductive behavior independent of ovarian steroids in female rodents. Mol. Endocrinology. 2000;14:1086–1098. doi: 10.1210/mend.14.7.0490. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Barraclough CA, Cross BA. Unit activity in the hypothalamus of the cyclic female rat: effect of genital stimuli and progesterone. J. Endocrinology. 1963;26:339–359. doi: 10.1677/joe.0.0260339. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Hess DL, Widmann AA, Henningfeld JM. Effects of progesterone on prolactin, hypothalamic beta-endorphin, hypothalamic substance P, and midbrain serotonin in guinea pigs. Neuroendo. 1995;61:695–703. doi: 10.1159/000126897. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc. Natl. Acad. Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnström L, Sjöberg M. Mechanisms of estogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinology. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Bryant DN, Bosch MA, Rønnekleiv OK, Dorsa DM. 17β-estradiol rapidly enhances extracellular signal-regulated kinase 2 phosphorylation in the rat brain. Neurosci. 2005;133:343–352. doi: 10.1016/j.neuroscience.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29:199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- Bueno J, Pfaff DW. Single unit recordings in the hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain Res. 1976;101:67–78. doi: 10.1016/0006-8993(76)90988-4. [DOI] [PubMed] [Google Scholar]
- Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- Cardona-Gómez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Mol. Brain Res. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Carrer HF, Araque A, Buño W. Estradiol regulates the slow Ca2+- activated K+ current in hippocampal pyramidal neurons. J. Neurosci. 2003;23:6338–6344. doi: 10.1523/JNEUROSCI.23-15-06338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato ACB, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Science’s STKE. 2002;2002:re9–re21. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J. Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Colvin GB, Sawyer CH. Induction of running activity by intracerebral implants of estrogen in overiectomized rats. Neuroendo. 1969;4:309–320. doi: 10.1159/000121762. [DOI] [PubMed] [Google Scholar]
- Condon TP, Rønnekleiv OK, Kelly MJ. Estrogen modulation of the α1-adrenergic response of hypothalamic neurons. Neuroendocrinology. 1989;50:51–58. doi: 10.1159/000125201. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Cross BA, Dyer RG. Characterization of unit activity in hypothalamic islands with special regerence to hormone effects. In: Martini L, Motta M, Fraschini F, editors. The Hypothalamus. Academic Press; New York: 1970. pp. 115–122. [Google Scholar]
- Czaja JA. Sex differences in the activational effects of gonadal hormones on food intake and body weight. Physiol. Behav. 1984;33:553–558. doi: 10.1016/0031-9384(84)90370-6. [DOI] [PubMed] [Google Scholar]
- Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm. Behav. 1975;6:329–349. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric receptors excites gonadotropin-releasing hormone neurons. Mol. Endocrinology. 2002;16:2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr. Opinion Neurobiol. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MB, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receoptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J. Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. USA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufy B, Partouche C, Poulain D, Dufy-Barbe L, Vincent JD. Effects of estrogen on the electrical activity of identified hypothalamic units. Neuroendo. 1976;22:38–47. doi: 10.1159/000122610. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Dyer RG, Pritchett CJ, Cross BA. Unit activity in the diencephalon of female rats during the oestrus cycle. J. Endocrinology. 1972;53:151–160. doi: 10.1677/joe.0.0530151. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology. 2003;144:3828–3835. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: Implications for female reproductive physiology. Horm. Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J. Steroid Biochem. Mol. Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton ARJ. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr., Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-Mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol. Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17 β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J. Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem. Biophys. Res. Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- Geary N. Estradiol, CCK and satiation. Peptides. 2001;22:1251–1263. doi: 10.1016/s0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC. Anatomy of the estrogen response element. Trends Endocrinol. Metab. 2004;15:73–78. doi: 10.1016/j.tem.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J. Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Korach KS, Moss RL. Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J. Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. Novel mechanism for non-genomic action of 17β-estradiol on kainate-induced currents in isolated rat CA1 hippocampal neurones. J. Physiol. (Lond.) 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extra-nuclear steroid receptors: nature and actions. Endocr. Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol. Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha-and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol. Cell. Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking β-endorphin and enkephalin. J. Neurosci. 2002;22:8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr. Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front. Neuroendo. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Huang C-L, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Jackson GL, Kuehl D. Gamma-aminobutyric acid (GABA) regulation of GnRH secretion in sheep. Reproduction. 2002;59:15–24. [PubMed] [Google Scholar]
- Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochem. Biophys. Res Commun. 2003;303:660–668. doi: 10.1016/s0006-291x(03)00408-x. [DOI] [PubMed] [Google Scholar]
- Jones MEE, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao SG, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallo I, Butler JA, Barkovics-Kallo M, Goubillon ML, Coen CW. Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J. Neuroendocrinol. 2001;13:741–748. doi: 10.1046/j.1365-2826.2001.00708.x. [DOI] [PubMed] [Google Scholar]
- Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology. 2003;144:5118–5125. doi: 10.1210/en.2003-0213. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Kuhnt U, Wuttke W. Hyperpolarization of hypothalamic parvocellular neurons by 17β-estradiol and their identification through intracellular staining with procion yellow. Exp. Brain Res. 1980;40:440–447. doi: 10.1007/BF00236152. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol. Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Rønnekleiv OK. Estrogen suppresses μ-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J. Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The effects of microelecrophoretically applied estrogen, cortisol, and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of the female rat. Exp. Brain Res. 1977a;30:53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The effects of ovarectomy on preoptic-septic area neurons to microelectrophoresed estrogen. Neuroendocriol. 1978a;25:204–211. doi: 10.1159/000122742. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The stereospecific changes in the unit activity of preoptic-septal neurons to microelectrophoresed estrogen. In: Ryall RW, Kelly JS, editors. Iontophoresis and Transmitter Mechanisms in the Mammalian Central Nervous System. Elsevier/North-Holland Biomedical Press; New York: 1978b. pp. 113–116. [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA, Fawcett CP. The specificity of the response of preoptic-septal area neurons to estrogen: 17α-estradiol versus 17β-estradiol and the response of extrahypothalamic neurons. Exp. Brain Res. 1977b;30:43–52. doi: 10.1007/BF00237857. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Wagner EJ, Rønnekleiv OK. Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS) J. Steroid Biochem. Mol. Biol. 2003;83:187–193. doi: 10.1016/s0960-0760(02)00249-2. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, editor. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 361–380. [Google Scholar]
- Kelly MJ, Rønnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res. Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- Kininis M, Chen BS, Diehl AG, Issacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol. Cell. Biol. 2007;27:5090–5104. doi: 10.1128/MCB.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc. Natl. Acad. Sci. USA. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JÅ, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Gorski RA, Kawakami M. Effects of estrogen on neuronal excitability in the hippocampal-septal-hypothalamic system. Neuroendocrinology. 1975;18:176–191. doi: 10.1159/000122397. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JÅ. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. The potency of μ-opioid hyperpolarization of hypothalamic arcuate neurons is rapidly attenuated by 17β-estradiol. J. Neurosci. 1994;14:6196–6204. doi: 10.1523/JNEUROSCI.14-10-06196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: A cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol. Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Wagner EJ, Rønnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology. 1996;64:114–123. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- Leal S, Andrade JP, Paula-Barbosa MM, Madeira MD. Arcuate nucleus of the hypothalamus: Effects of age and sex. J. Comp. Neurol. 1998;401:65–88. doi: 10.1002/(sici)1096-9861(19981109)401:1<65::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Lincoln DW. Unit activity in the hypothalamus, septum and preoptic area of the rat: Characteristics of spontaneous activity and the effect of oestrogen. J. Endocrinol. 1967;37:177–189. doi: 10.1677/joe.0.0370177. [DOI] [PubMed] [Google Scholar]
- Lincoln DW, Cross BA. Effect of oestrogen on the responsiveness of neurons in the hypothalamus, septum and preoptic area of rats with light-induced persistent oestrus. J. Endocrinol. 1967;37:191–203. doi: 10.1677/joe.0.0370191. [DOI] [PubMed] [Google Scholar]
- Loose MD, Rønnekleiv OK, Kelly MJ. Membrane properties and response to opioids of identified dopamine neurons in the guinea pig hypothalamus. J. Neurosci. 1990;10:3627–3634. doi: 10.1523/JNEUROSCI.10-11-03627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17 β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J. Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyala A, Kelly MJ, Rønnekleiv OK. Estrogen modulation of hypothalamic neurons: Activation of multiple signaling pathways and gene expression changes. Steroids. 2005;70:397–406. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Malyala A, Pattee P, Nagalla SR, Kelly MJ, Rønnekleiv OK. Suppression subtractive hybridization and microarray identification of estrogen regulated hypothalamic genes. Neurochem. Res. 2004;29:1189–1200. doi: 10.1023/b:nere.0000023606.13670.1d. [DOI] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant D, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J. Comp. Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Czaja JA. Diverse effects of estradiol-17 beta: concurrent suppression of appetite, blood pressure and vascular reactivity in conscious, unrestrained animals. Physiol. Behav. 1989;45:649–657. doi: 10.1016/0031-9384(89)90086-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phospatidylinositol 3-kinase in the adult rat brain. Mol. Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Milewicz A, Bidzinska B, Mikulski E, Demissie M, Tworowska U. Influence of obesity and menopausal status on serum leptin, cholecystokinin, galanin and neuropeptide Y levels. Gynecol. Endocrinol. 2000;14:196–203. doi: 10.3109/09513590009167682. [DOI] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Nabekura J, Fukuda A. 17β-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- Moss RL, Law OT. The estrous cycle: its influence on single unit activity in the forebrain. Brain Res. 1971;30:435–438. doi: 10.1016/0006-8993(71)90097-7. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Inoue S. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem. Biophys. Res. Commun. 2000;270:1–10. doi: 10.1006/bbrc.2000.2214. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. Mechanism of the rapid effect of 17β-estradiol on medial amygdala neurons. Science. 1986;233:226–228. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol. Endocrinol. 2003;17:1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JÅ. Mechanisms of estrogen action. Physiol. Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Tsai M-J. Molecular pathways of steroid receptor action. Biol. Reprod. 1992;46:163–167. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson JÅ, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol. Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Estrogen receptors in uterine plasma membrane. J Steroid Biochem. 1979;11:1471–1483. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- Plum L, et al. Enhanced PIP3 signaling in POMC neurons causes Katp channel activation and leads to diet-sensitive obesity. J. Clin. Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlman ET. Menopause, energy expenditure, and body composition. Acta Obstet. Gynecol. Scand. 2002;81:603–611. doi: 10.1034/j.1600-0412.2002.810705.x. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol. Cell. Endocrinol. 2007;265-266:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Jamali K, Xue C, Kelly MJ, Rønnekleiv OK. Estrogen upregulates T-type calcium channels in the hypothalamus and pituitary. J. Neurosci. 2006a;26:11072–11082. doi: 10.1523/JNEUROSCI.3229-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J. Neurosci. 2006b;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids. 2008 doi: 10.1016/j.steroids.2007.11.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Mol. Pharm. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Insulin-like growth factor-1 regulation of α1-adrenergic receptor signaling is estradiol dependent in the preoptic area and hypothalamus of female rats. Endocrinology. 2001;142:599–607. doi: 10.1210/endo.142.2.7946. [DOI] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of α1B-Adrenoceptors and female reproductive function. J. Neurosci. 2002;22:2401–2408. doi: 10.1523/JNEUROSCI.22-06-02401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J. Membrane sex-steroid receptors in the brain. Front. Neuroendo. 1996;17:402–439. doi: 10.1006/frne.1996.0011. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Zheng JB, Siddique KM. Membrane receptors for estrogen, progesterone, and testosterone in the rat brain: Fantasy or reality. Cell. Mol. Neurobiol. 1996;16:175–198. doi: 10.1007/BF02088175. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- Rønnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front. Neuroendo. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Sah P, Davies P. Calcium-activated potassium currents in mammalian neurons. Clin. Exp. Pharmacol. Physiol. 2000;27:657–663. doi: 10.1046/j.1440-1681.2000.03317.x. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M. Estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neurosci. Lett. 1996;204:81–84. doi: 10.1016/0304-3940(96)12322-3. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Shimizu H, Takahashi M, Sato N, Uehara Y, Fukatsu A, Negishi M, Kobayashi I, Kobayashi S. The significance of decreased ambulatory activity during the generation by long-term observation of obesity in ovariectomized rats. Physiol. Behav. 1990;47:155–159. doi: 10.1016/0031-9384(90)90055-9. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, McCall LD, Watson SJ. Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J. Comp. Neurol. 1988;276:442–459. doi: 10.1002/cne.902760309. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J. Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo GJ, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J. Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr., Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: Convergence of estrogen and neurotrophin signaling pathways. J. Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J. Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA-and glutamate activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J. Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyrmadial neurons. Proc. Natl. Acad. Sci. USA. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossi F, Likhite VS, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cylcin G2 promoter. J. Biol. Chem. 2006;281:16272–16278. doi: 10.1074/jbc.M513405200. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin J-P, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorscent protein to gonadotropin-releasing hormone neurons: Characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- Szegõ ÉM, Barabás K, Balog J, Szilágyi N, Korach KS, Juhász G, Abrahám IM. Estrogen induces estrogen receptor α-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons In Vivo. J. Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J. Neurosci. 2004;24:6326–6333. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Wray S. BSA-estrogen compounds differentially alter gonadotropin-releasing hormone-1 neuronal activity. Endocrinology. 2005;146:558–563. doi: 10.1210/en.2004-1117. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G-protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J. Comp. Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain: beyond ER-α, ER-β and 17β-estradiol. Ann. NY Acad. Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo GJ. Novel mechanisms of estrogen action in the brain: New players in an old story. Front. Neuroendo. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Towle AC, Sze PY. Steroid binding to synaptic plasma membrane: Differential binding of glucocorticoids and gonadal steroids. J. Steroid Biochem. 1983;18:135–143. doi: 10.1016/0022-4731(83)90079-1. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc. Natl. Acad. Sci. USA. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ. Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J. Neurosci. 2001a;21:2085–2093. doi: 10.1523/JNEUROSCI.21-06-02085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Kelly MJ. The noradrenergic inhibition of an apamine-sensitive small conductance Ca2+ -activated K+ channel in hypothalamic γ-aminobutyric acid neurons: Pharmacology, estrogen sensitivity and relevance to the control of the reproductive axis. J. Pharmacol. Exp. Ther. 2001b;299:21–30. [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Dorsa DM. Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J. Neurosci. 1998;18:6672–6680. doi: 10.1523/JNEUROSCI.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill PJ, Wilson APM, Nicholson RI, Davies P, Wakeling AE. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J. Ster. Biochem. Mol. Biol. 1988;30:263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Whitehead SA, Ruf KB. Responses of antidromically identified preoptic neurons in the rat to neurotransmitters and to estrogen. Brain Res. 1974;79:185–198. doi: 10.1016/0006-8993(74)90410-7. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne H-J, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to ganadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K. Effects of estrogen on the unit activity of the rat hypothalamus. Nippon Seirigaku Zasshi. 1970;32:629–693. [PubMed] [Google Scholar]
- Yagi K. Changes in firing rates of single preoptic and hypothalamic units following an intravenous administration of estrogen in the castrated female rat. Brain Res. 1973;53:343–352. doi: 10.1016/0006-8993(73)90219-9. [DOI] [PubMed] [Google Scholar]
- Yagi K, Sawaki Y. Changes in the electrical activity of the hypothalamus during sexual cycle and the effect of castration on it in the female rat. J. Physiol. Japan. 1971;33:546–547. [Google Scholar]
- Yang S-H, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP Channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J. Neurosci. 2007;27:10153–10164. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, He C, Yan X, Mirshashi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nature Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD. Demonstration of membrane estrogen binding proteins in rat brain by ligand blotting using a 17β-estradiol-[125I]bovine serum albumin conjugate. J. Ster. Biochem. Mol. Biol. 1997;62:327–336. doi: 10.1016/s0960-0760(97)00037-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]