Summary
Although karyotypic differences between species have long been recognized, the question of whether these mutations play a causal role in speciation remains unanswered. This is because most models of chromosomal speciation focus on underdominance, which presents a theoretical paradox in that the strength of an underdominant barrier is inversely proportional to its fixation probability. To counter this problem, a new model has been proposed that focuses on the modification of effective recombination rates, whereby rearrangements facilitate the build up of linkage disequilibrium in the presence of gene flow. This model is discussed, along with new supporting data from the Solanaceae.
Keywords: chromosomal rearrangements, karyotype, karyotypic evolution, recombination, reproductive isolation, speciation
A brief history of chromosomal evolution
We now know a great deal about the nature and distribution of karyotypic changes in plants. Taken together, the tremendous number of cytogenetic analyses published since the discovery of chromosomes has revealed an array of karyotypic diversity in basic number, physical size, and the organization of homologous regions between related taxa (Levin, 2002). The causes of some of these differences are now generally accepted. For example, much of the quantitative difference in genome content can be attributed to either the proliferation of mobile genetic elements (SanMiguel et al., 1996) or duplications of genes or chromosomal segments (Initiative, 2000). Allo- and autopolyploidy are additional widespread mechanisms of karyotypic change that have been intensely studied (Grant, 1981; Soltis, 2004). While the proliferation of transposons and retroelements is viewed predominantly as neutral change within a species, polyploidy (Grant, 1981) and gene duplication (Lynch & Conery, 2000; Lynch & Force, 2000a; Lynch & Force, 2000b) can be causative factors in adaptive divergence and reproductive isolation. Chromosomal rearrangements, in contrast, still lack a generally accepted explanation of how they become established and whether they contribute to speciation.
The majority of what we know regarding the prevalence of chromosomal rearrangements has come through cytogenetic studies of population samples and interspecific hybrids. Work done by Carr and Carr studying races in the annual herb Calycadenia can serve as a general example of both the methods and findings. In Calycadenia, individual chromosomes are indistinguishable except for those carrying the nucleolar organizing region (Carr, 1975), so meiotic analyses of interracial F1 individuals were used to determine the karyotypic differences. In the first study, two distinct groups in what is now part of the Calycadenia fremontii –C. pauciflora alliance were identified: race Pauciflora with 2N = 2X = 10 and a 2N = 2X = 12 group which can be further subdivided into races Elegans, Healdsburg, Ramulosa, Tehama, and Wurlitzer. Apart from the apparent aneuploid reduction (Carr, 1975), these races differ by a series of reciprocal translocations and a pericentric inversion (Carr, 1975; Carr & Carr, 2000). Study of C. ciliosa populations and races (now C. fremontii; 2N = 2X = 12) revealed a similar pattern of translocations and possibly an inversion both between races and segregating within populations (Carr & Carr, 1983).
At a broader level in the genus, both morphologically distinct species that lacked chromosomal differences and morphologically cryptic species with chromosomal differences can be found (Carr, 1977). The Calycadenia work thus illustrates that chromosomal rearrangements, including both translocations and inversions, can be found within and between species. Moreover, the amount of phenotypic evolution is not a good predictor of the amount of karyotypic evolution. Generalizations made from similar studies across additional species and life history groups show that chromosomal differences are most common in annual herbs, and decreasingly present between related perennial herbs and woody plants, at least in the north-temperate regions (Grant, 1981).
More recently, comparative genetic mapping has provided another means of detecting karyotype differences. Comparative mapping further extends our understanding because it can overcome some important limitations in cytological studies, such as genic disruption of meiotic pairing or indistinguishable chromosomes. The precise localization of known orthologous sequences through mapping allows comparisons to be made between species, genera, and even families, providing a more detailed picture of the historical changes in the karyotype. For example, comparative maps now cover eight grass species across the family (Gale & Devos, 1998) and nine species in the Solanaceae (Tanksley et al., 1992; Livingstone et al., 1999; Perez et al., 1999; Doganlar et al., 2002; Pertuzé et al., 2002). These studies reveal that reciprocal and nonreciprocal translocations and paracentric and pericentric inversions are the gross structural changes differentiating these taxa. The combination of these deeper comparisons with phylogenetic data again suggests that the accumulation of rearrangements does not appear to be a constant function of evolutionary time (Devos & Gale, 2000; J. M. Burke, pers. comm.; but see Lagercrantz, 1998).
The next step in our understanding of karyotypic evolution will most likely come about as a result of the genome sequencing efforts now underway and the continuing refinement of cytogenetic techniques. Comparisons of large lengths of sequence data combined with the ability to fine-map across the genome should eventually lead to a molecular characterization of rearrangement events and a clearer picture of the amount of micro vs macrocollinearity between species. In addition, techniques such as fluorescent in situ hybridization may make feasible larger studies of intraspecific karyotypic variation. The availability of more precise data from a greater number of species should finally clarify the apparent relationship between intra- and interspecific variation. At the time Grant wrote Plant Speciation the general belief was that the types of differences segregating within species were qualitatively different from those found between species (pp. 159, 175). In Drosophila, for example, there are thousands of within species polymorphisms for paracentric inversions, which are not underdominant, but almost no polymorphisms for pericentric inversions, which often are underdominant (Patterson & Stone, 1952). So far, evolutionary geneticists have failed to document a similar pattern in plants.
Chromosomal rearrangements as species barriers
Cytogenetic and genetic mapping studies have also defined the primary consequences of karyotypic rearrangements in heterozygotes. In both translocation and inversion heterozygotes, crossing over in certain areas of rearranged chromosomes creates chromatids with duplications and deficiencies in gene content. The patterns of disjunction at the first meiotic division and the segregation of meiotic products into the gametes can cause varying degrees of sterility depending on the fitness consequences of duplication/deficiency gametes in the gametophytic and sporophytic phases. Because viable recombinants from inversion heterozygotes are extremely rare, inversions have been classically defined as recombination suppressors. Translocations can have similar, albeit more complicated effects on fertility and linkage relationships over parts of their component chromosomes (Livingstone et al., 2000). The presence of such conspicuous variation with dramatic effects on fertility has for many years suggested that chromosomal rearrangements are an important factor in the speciation process (Spirito, 1998; Rieseberg, 2001). These models almost exclusively focus on the underdominant fitness effects seen in heterozygotes, postulating that the fixation of different chromosomal arrangements in different lineages has created reproductive barriers, and hence biological species. The Achilles heel of these models, however, is that ‘the problem of establishment of a new segmental arrangement is inseparable from the problem of its functional role’ (Grant, 1981). Newly arisen chromosomal rearrangements will exist in the population almost exclusively as heterozygotes. If the fitness effects of the rearrangement at the time it originates are similar to the contemporary reductions seen in most heterokaryotypes, the new arrangements are likely to be eliminated before they can become a crossing barrier.
Theoretical population genetic studies over the past 60 yr (Wright, 1941; Hedrick, 1981; Walsh, 1982; Lande, 1984; Barton & Rouhani, 1991; Spirito, 1992) have formalized the paradox of chromosomal rearrangements as causative factors in speciation. The strength of a rearrangement as a reproductive barrier is proportional to the amount of underdominance, but the rate of fixation of a rearrangement is inversely proportional to its level of underdominance, making the strongest barriers the least likely to be fixed. While some models are not as susceptible to this paradox because of special conditions, in general population genetic theory has had a discouraging effect on the formulation of a widely applicable model of chromosomal speciation.
Rise of the r theory
Despite theory, empirical data suggesting that chromosomal rearrangements play a causative role in the speciation process continues to accumulate, most notably in annual sunflowers and Drosophila. In sunflower, Rieseberg et al. (1999 and unpublished) have shown that the lengths of chromosomal segments around isolating genes that are prevented from introgressing across hybrid zones are longer in rearranged vs collinear chromosomes. In Drosophila, genes causing hybrid sterility and conditioning female species preferences between Drosophila pseudoobscura and D. persimilis map predominantly to inversions that differentiate the species (Noor et al., 2001).
These observations led Rieseberg (2001) and Noor et al. (2001) to elaborate a new theory of how chromosomal rearrangements could participate in speciation through their effects on recombination. These models propose that chromosomal rearrangements provide large regions of the genome protected from gene flow where isolating genes may accumulate until complete reproductive barriers exist. While the importance of recombination in speciation has long been recognized (Ortíz-Barrientos et al., 2003), this new model differs in emphasizing the gradual build up of isolation as opposed to the protection or establishment of linkage disequilibrium among preexisting segregating variants. The model envisions one or more genes that contribute to reproductive isolation becoming associated with a chromosomal rearrangement. In interspecific hybridizations, the isolating effects of the gene are then extended across the entire rearrangement through the modulation of recombination. Subsequent evolution of the genes within the rearrangement increases the overall isolating effects of the region to the point of genome-wide reproductive isolation. In the model, the reduction in recombination can be either effective or real. A true reduction in recombination would be manifest as a karyotypic heterozygote with complete fertility that segregated only parental types (Snow, 1960; Wedberg et al., 1968; Coyne et al., 1991; Coyne et al., 1993). In this case the selective advantage of the genes within the rearrangement would only need to be slight. In cases where fertility is affected and the reduction in recombination is effective, the selective advantage of the included genes would need to be stronger to drive the rearrangement/gene combination to fixation.
In contrast to models of chromosomal evolution based on underdominance, population genetic theory supports a role for rearrangements in models of speciation with persistent gene flow. The effects of chromosomal rearrangements on the build up of one category of isolation genes, Dobzhansky–Muller incompatibilities, have been modeled recently by Navarro & Barton (2003a). They found that divergence in parapatry occurs much more readily in the presence of rearrangements than with Dobzhansky–Muller incompatibilities alone. If rearrangements are critical to parapatric speciation, we would expect them to be more common where gene flow was present during speciation. This is exactly the pattern found by Noor et al. (2001): inversions are more common between Drosophila species that are sympatric compared to allopatric pairs.
Most importantly, however, this new model makes testable predictions regarding levels of molecular divergence between taxa where speciation was facilitated by rearrangements. During the process of parapatric speciation, effective rates of gene flow will be reduced in rearranged, but not collinear chromosomes. As a consequence, greater interspecific sequence divergence is predicted in rearranged than collinear chromosomes, and this prediction should hold for both neutral and selected differences. Results from comparisons between human and chimpanzee genes bear out this prediction in that more amino acid substitutions were found between genes in rearranged (mean KA/KS = 0.84) compared to collinear chromosomes (mean KA/KS = 0.37) (Navarro & Barton, 2003b). Interestingly, this same pattern is seen in comparisons between rearranged and collinear regions within D. pseudoobscura (Schaeffer et al., 2003), which may represent the initial stages of speciation between chromosomal races.
Chromosomal evolution in the Solanaceae
The Solanaceae present an attractive plant system for the study of historical chromosomal rearrangements. As the first comparative maps showed, tomato (Solanum lycopersicum) and potato (Solanum tuberosum) are differentiated by a series of whole arm paracentric inversions of chromosomes 5, 9, 10, 11, and 12 (Bonierbale et al., 1988; Tanksley et al., 1992). Later work in pepper (Capsicum spp.) suggested that the chromosome 10 inversion arose within the tomato lineage after the split from the common ancestor with potato (Livingstone et al., 1999; Fig. 1). It has been shown recently that chromosome 10 in the sister taxa of tomato, Solanum sitiens and S. lycopersicoides, is collinear with S. tuberosum (Pertuzé et al., 2002), confirming the chromosome 10 inversion was fixed in the common ancestor of the tomato lineage. Confirmation of the placement of this rearrangement event in a more recent split in the Solanum phylogeny, coupled with our knowledge that the approximately seven named tomato species are parapatric, provides an opportunity to test for the predicted patterns of diversity where the signal should still be strong. Given the availability of mapped sequences in tomato and the tremendous genetic resources available for tomato and potato, an investigation was initiated to ascertain whether genes on tomato chromosome 10 have diverged more rapidly than genes from collinear chromosomes, as predicted by the model.
Fig. 1.
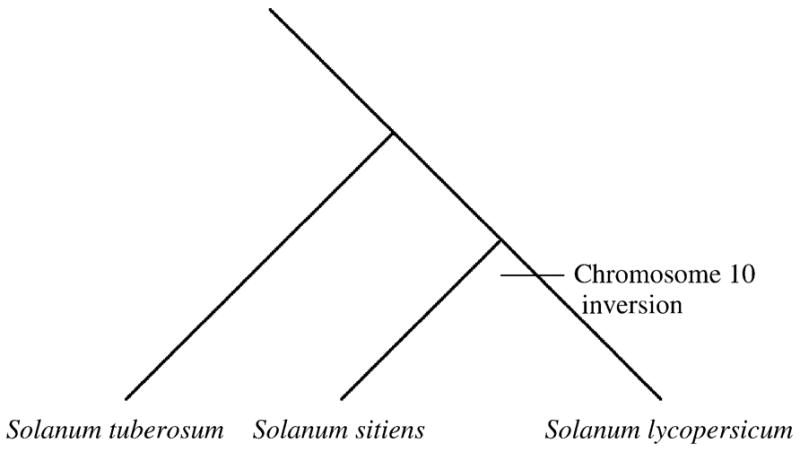
Fixation of chromosome 10 inversion in tomato, Solanum lycopersicum.
Materials and methods
The tomato (LGI build 051903) and potato (STGI build 051603) gene indices were obtained from The Institute for Genomic Research (http://www.tigr.org/tdb/tgi/plant.shtml). Tomato EST sequences mapped either through PCR amplification of microsatellite sequences or used as probes in RFLP studies were identified from the Solanaceae Genomics Network database (http://www.sgn.cornell.edu/). The corresponding unigene for each EST was then used as the query sequence in a BLAST search (Altschul et al., 1990) of the potato unigene set to identify a potato homolog. Orthologous sequence pairs were determined for the few tomato sequences with multiple high-scoring potato homologs by the reciprocal best hits rule. The putative tomato-potato orthologous pairs were then aligned using ClustalW (Thompson et al., 1994) and a percent identity calculated by dividing the number of identical residues with the total number of aligned residues (indels were treated as noninformative data). Linear modeling in JMP (SAS Institute, Inc., Cary, NC, USA) was used to assess the relationship between chromosomal rearrangements and degree of sequence divergence.
Results and discussion
A total of 570 putative tomato-potato ortholog pairs with inferred map position were produced for the analysis. The mean length of the tomato genes was 1162 bp and the mean length of the potato genes was 1026 bp. The distribution of sequence identity showed a long tail (Fig. 2), and a lower bound of 80% identity was arbitrarily chosen for inclusion in the model, leaving 534 pairs in the analysis (presumably pairs with lower identities are not true orthologs). When all 12 chromosomes were considered, the rearranged chromosomes (5, 9, 10, 11, and 12; N = 364 genes) showed a marginally significant (P = 0.064) increase in the predicted percent divergence of 0.23% (mean of rearranged = 5.2%, mean of collinear = 4.8%). When the analysis was restricted to only a comparison between the genes on chromosome 10 (N = 42) and the collinear chromosomes (N = 364), the effect of the chromosome 10 rearrangement was significant (P = 0.027), increasing the predicted percent divergence from 3.42% to 4.33%.
Fig. 2.
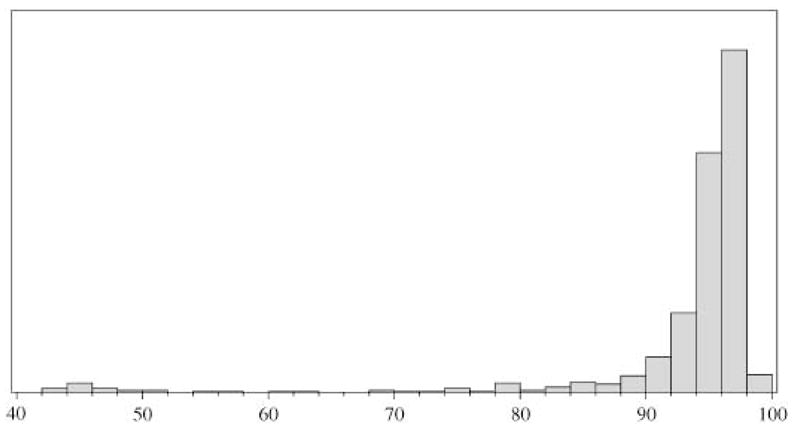
Distribution of sequence identities between orthologous tomato and potato unigene sequences (N = 570)
These results, while preliminary, support the hypothesis that the rearrangement of chromosome 10 in the tomato lineage may have facilitated speciation. Contemporary wild tomato species are sympatric with S. lycopersicoides and S. sitiens, consequently there is a strong possibility that the speciation event that initiated the tomato lineage occurred in parapatry. Future sequencing of whole alleles from S. lycopersicum and related wild tomatoes, along with sequencing of alleles from S. sitiens and S. lycopersicoides, will provide a more complete, rigorous test of this hypothesis. Moreover, integration of positional information from chromosome 10 will allow testing for subpatterns in diversity within the rearranged and nonrearranged chromosomal arms. Analyses of these sequences will further reveal the extent of divergence in coding vs noncoding regions of the genes, and could be used to detect genes that show a history of positive selection that may have driven the fixation of this rearrangement.
Conclusions
We need a chromosomal speciation theory based on the primary consequences of karyotypic rearrangements that explains extant karyotype evolution and the distribution of karyotypic diversity within and between species. The model must also include a gene’s eye perspective to encompass genic divergence and reproductive isolation. The new recombination-based model largely fulfills these requirements. As Grant (1981) suggested, ‘chromosomal sterility … can be regarded as a condition superimposed on a more widespread and perhaps more basic condition of genic sterility’ (p. 116). Rearrangements that alter recombination rates without substantial overall fitness effects could extend the amount of the genome protected from gene flow in the face of hybridization, and therefore could allow more genes to become differentiated, promoting speciation.
Acknowledgments
Funding for this research came from the U. S. Department of Agriculture (NRI 2001-35301-09971 to K.L.) and the U.S. National Institutes of Health (GM059065 to L.H. Rieseberg).
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Barton NH, Rouhani S. The probability of fixation of a new karyotype in a continuous population. Evolution. 1991;45:499–517. doi: 10.1111/j.1558-5646.1991.tb04326.x. [DOI] [PubMed] [Google Scholar]
- Bonierbale MW, Plaisted RL, Tanksley SD. RFLP maps based on a common set of clones reveal modes of chromosomal evolution in potato and tomato. Genetics. 1988;120:1095–1103. doi: 10.1093/genetics/120.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GD. Chromosome evolution and aneuploid reduction in Calycadenia pauciflora (Asteraceae) Evolution. 1975;29:681–699. doi: 10.1111/j.1558-5646.1975.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Carr GD. A cytological conspectus of the genus Calycadenia (Asteraceae): an example of contrasting modes of evolution. American Journal of Botany. 1977;64:694–703. [Google Scholar]
- Carr RL, Carr GD. Chromosome races and structural heterozygosity in Calycadenia ciliosa Greene (Asteraceae) American Journal of Botany. 1983;70:744–755. [Google Scholar]
- Carr GD, Carr RL. A new chromosome race of Calycadenia pauciflora (Asteraceae: Heliantheae–Madiinae) from Butte County, California. American Journal of Botany. 2000;87:1459–1465. [PubMed] [Google Scholar]
- Coyne JA, Aulard S, Berry A. Lack of underdominance in a naturally occurring pericentric inversion in Drosophila melanogaster and its implications for chromosome evolution. Genetics. 1991;129:791–802. doi: 10.1093/genetics/129.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Meyers W, Crittenden AP, Sniegowski P. The fertility effects of pericentric inversions in Drosophila melanogaster. Genetics. 1993;134:487–496. doi: 10.1093/genetics/134.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos K, Gale MD. Genome relationships: the grass model in current research. Plant Cell. 2000;12:637–646. doi: 10.1105/tpc.12.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doganlar S, Frary A, Daunay M-C, Lester RN, Tanksley SD. A comparative genetic linkage map for eggplant (Solanum melongena) and its implications for genome evolution in the Solanaceae. Genetics. 2002;161:1697–1711. doi: 10.1093/genetics/161.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale MD, Devos KM. Plant comparative genetics after 10 years. Science. 1998;282:656–658. doi: 10.1126/science.282.5389.656. [DOI] [PubMed] [Google Scholar]
- Grant V. Plant speciation. New York, USA: Columbia University Press; 1981. [Google Scholar]
- Hedrick PW. The establishment of chromosomal variants. Evolution. 1981;35:322–332. doi: 10.1111/j.1558-5646.1981.tb04890.x. [DOI] [PubMed] [Google Scholar]
- Initiative TAG. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Lagercrantz U. Comparative mapping between Arabidopsis thaliana and Brassica nigra indicates that Brassica genomes have evolved through extensive genome replication accompanied by chromosome fusions and frequent rearrangements. Genetics. 1998;150:1217–1228. doi: 10.1093/genetics/150.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R. The expected fixation rate of chromosomal inversions. Evolution. 1984;38:743–752. doi: 10.1111/j.1558-5646.1984.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Levin DA. The role of chromosomal change in plant evolution. New York, USA: Oxford University Press; 2002. [Google Scholar]
- Livingstone K, Churchill G, Jahn MK. Linkage mapping in populations with karyotypic rearrangements. Journal of Heredity. 2000;91:423–428. doi: 10.1093/jhered/91.6.423. [DOI] [PubMed] [Google Scholar]
- Livingstone KD, Lackney VK, Blauth JR, Van Wijk R, Jahn MK. Genome mapping in Capsicum and the evolution of genome structure in the Solanaceae. Genetics. 1999;152:1183–1202. doi: 10.1093/genetics/152.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000a;154:459–473. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Force AG. The origin of interspecific genomic incompatibility via gene duplication. American Naturalist. 2000b;156:590–605. doi: 10.1086/316992. [DOI] [PubMed] [Google Scholar]
- Navarro A, Barton NH. Accumulating postzygotic isolation genes in parapatry: a new twist on chromosomal speciation. Evolution. 2003a;57:447–459. doi: 10.1111/j.0014-3820.2003.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Navarro A, Barton NH. Chromosomal speciation and molecular divergence – accelerated evolution in rearranged chromosomes. Science. 2003b;300:321–324. doi: 10.1126/science.1080600. [DOI] [PubMed] [Google Scholar]
- Noor MAF, Grams KL, Bertucci LA, Reiland J. Chromosomal inversions and the reproductive isolation of species. Proceedings of the National Academy of Sciences, USA. 2001;98:12084–12088. doi: 10.1073/pnas.221274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortíz-Barrientos D, Reiland J, Hey J, Noor MAF. Recombination and the divergence of hybridizing species. Genetica. 2003;116:167–178. [PubMed] [Google Scholar]
- Paterson JT, Stone WS. Evolution in the genus Drosophila. New York, USA: Macmillan; 1952. [Google Scholar]
- Perez F, Menedez A, Dehal P, Quiros CF. Genomic structural differentiation in Solanum: comparative mapping of the A- and E-genomes. Theoretical and Applied Genetics. 1999;98:1183–1193. [Google Scholar]
- Pertuzé RA, Ji Y, Chetelat RT. Comparative linkage map of the Solanum lycopersicoides and S. sitiens genomes and their differentiation from tomato. Genome. 2002;45:1003–1012. doi: 10.1139/g02-066. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH. Chromosomal rearrangements and speciation. Trends in Ecology and Evolution. 2001;16:351–358. doi: 10.1016/s0169-5347(01)02187-5. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Whitton J, Gardner K. Hybrid zones and the genetic architecture of a barrier to gene flow between two sunflower species. Genetics. 1999;152:713–727. doi: 10.1093/genetics/152.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanMiguel P, Tikhonov A, Jin Y-K, Molchalskia N, Zakharov D, Melake-Bernham A, Springer PS, Edwards KJ, Lee M, Auramova Z, Bennetzen JL. Nested retrotransposons in the intergenic regions of the maize genome. Science. 1996;274:765–768. doi: 10.1126/science.274.5288.765. [DOI] [PubMed] [Google Scholar]
- Schaeffer SW, Goetting-Minesky MP, Kovacevic M, Peoples JR, Graybill JL, Miller JM, Kim K, Nelson JG, Anderson WA. Evolutionary genomics of inversions in Drosophila pseudoobscura: evidence for epistasis. Proceedings of the National Academy of Sciences, USA. 2003;100:8319–8324. doi: 10.1073/pnas.1432900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. Chromosomal differentiation in Clarkia dudleyana. American Journal of Botany. 1960;47:302–309. [Google Scholar]
- Soltis DE, Soltis PS, Tate JA. Advances in the study of polyploidy since plant speciation. New Phytologist. 2004;161 In press. [Google Scholar]
- Spirito F. The exact values of the probability of fixation of underdominant chromosomal rearrangements. Theoretical Population Biology. 1992;41:111–120. doi: 10.1016/0040-5809(92)90039-v. [DOI] [PubMed] [Google Scholar]
- Spirito F. The role of chromosomal change in speciation. In: Howard DJ, Berlocher SH, editors. Endless forms: species and speciation. New York, USA: Oxford University Press; 1998. pp. 320–329. [Google Scholar]
- Tanksley SD, Ganal MW, Prince JP, et al. High density molecular linkage maps of the tomato and potato genomes. Genetics. 1992;132:1141–1160. doi: 10.1093/genetics/132.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JB. Rate of accumulation of reproductive isolation by chromosome rearrangements. American Naturalist. 1982;120:510–532. [Google Scholar]
- Wedberg HL, Lewis H, Venkatesh CS. Translocation heterozygotes and supernumerary chromosomes in wild populations of Clarkia williamsonii. Evolution. 1968;22:93–107. doi: 10.1111/j.1558-5646.1968.tb03453.x. [DOI] [PubMed] [Google Scholar]
- Wright S. On the probability of fixation of reciprocal translocations. American Naturalist. 1941;75:513–522. [Google Scholar]


