Abstract
We have previously used inhibitors interacting with the Qn site of the yeast cytochrome bc1 complex to obtain yeast strains with resistance-conferring mutations in cytochrome b as a means to investigate the effects of amino acid substitutions on Qn site enzymatic activity (1). Although the screening produced various interesting cytochrome b mutations, it depends on the availability of inhibitors and can only reveal a very limited number of mutations. Furthermore, mutations leading to a respiratory deficient phenotype remain undetected. We therefore devised an approach where any type of mutation can be efficiently introduced in the cytochrome b gene. In this method ARG8, a gene that is normally encoded by nuclear DNA, replaces the naturally occurring mitochondrial cytochrome b gene, resulting in ARG8 expressed from the mitochondrial genome (ARG8m). Subsequently replacing ARG8m with mutated versions of cytochrome b results in arginine auxotrophy. Respiratory competent cytochrome b mutants can be selected directly by virtue of their ability to restore growth on non-fermentable substrates. If the mutated cytochrome b is non-functional, the presence of the COX2 respiratory gene marker on the mitochondrial transforming plasmid enables screening for cytochrome b mutants with a stringent respiratory deficiency (mit-). With this system, we created eight different yeast strains containing point mutations at three different codons in cytochrome b affecting center N. In addition, we created three point mutations affecting arginine 79 in center P. This is the first time mutations have been created for three of the loci presented here, and nine of the resulting mutants have never been described before.
1. Introduction
The yeast Saccharomyces cerevisiae is very versatile for the investigation of components of the respiratory chain, because of its ability to live by fermentation. Another advantage is the accessibility of this organism to the introduction of foreign genes into their mitochondrial DNA via biolistic transformation and subsequent homologous recombination (2). Furthermore, due to the natural instability of heteroplasmy, i.e. the coexistence of different mtDNA molecules within a cell, it is easy to isolate homoplasmic strains of S. cerevisiae where all the mtDNA molecules are identical (3-5). This creates excellent conditions to investigate the consequences on mitochondrial structure and function of specific mtDNA mutations.
The ARG8 gene is located in the nucleus, and its gene product, the protein acetyl-ornithine aminotransferase, which is involved in ornithine and arginine biosynthesis, is imported from the cytosol into mitochondria. It can also be expressed in its mitochondrial version, ARG8m, within the organelle, and thus complement nuclear arg8 mutants (2). This gene has been widely used as a reporter gene (2), and recently also used as a selectable placeholder for ATP6 to investigate mutations that otherwise would lead to unstable DNA and ultimately to its loss (6).
The cytochrome bc1 complex is an essential enzyme of the respiratory chain, with its key protein, cytochrome b, encoded by the mitochondrial DNA. Many point mutations have been described for this protein, from yeast (7) to higher eukaryotes (8), with consequences ranging from drug resistance to disease. In order to better understand the basic mechanisms of the cytochrome bc1 complex, it is necessary to be able to produce point mutations at specific sites, that may alter or even result in loss of cytochrome b function, and to have efficient screening techniques to enhance the process. We describe here the creation of a strain containing ARG8m as a placeholder for the cytochrome b gene, which functions as a recipient strain for the introduction of mutated versions of cytochrome b. The utility of this procedure is demonstrated here by the successful introduction of eleven different point mutations at four different locations in cytochrome b, covering the center N (Qn) and center P (Qp) sites of the protein. Except for one location, no mutations have been reported and characterized before for these locations.
2. Materials and Methods
2.1 Yeast strains and media
The S. cerevisiae strains used and their genotypes are listed in Table I. Media used for growth of S. cerevisiae were YPD, 2% glucose (Fisher Scientific), 1% yeast extract (US-Biological), 1% bactopeptone (BD); YPDA, YPD supplemented with 40 mg/L adenine (Sigma); YP10, 10% glucose, 1% yeast extract, 1% bactopeptone, supplemented with adenine; CSM-media (complete supplement mixture lacking a certain supplement) were prepared according to the manufacturer’s instructions (Bio 101, Inc.); W0, 2% glucose, 0.67% yeast-nitrogen base without amino acids. Sorbitol plates (for bombardment) contain 5% glucose, 0,67% yeast-nitrogen base without amino acids, 0.69 g/L of CSM-leucine, 1M sorbitol and 0.1 g/L of adenine. YPEG medium contains 1% yeast extract, 2% bactopeptone, 3% glycerol and 4% ethanol. N3 medium contains 2% glycerol (LabChem Inc.), 1% yeast extract, 1% bactopeptone, 20 mg/ml adenine, 50 mM phosphate buffer, pH 6.2. For plates, 2% agar (Difco) was added.
2.2 Construction of plasmid pSCSI containing an ARG8m cassette flanked by COB gene 5’- and 3’ UTRs
Plasmid pSCSI, which contains an ARG8m cassette in lieu of the cytochrome b gene, flanked by 100 bps of COB 5’- and 3’-UTR sequences, was generated by PCR using primers containing those flanking sequences and pDS24 (9) as a template. The sense primer was COB-ARG8-F, 5’-gct cta gaT AAT TAA TAA TAT ATA TTT ATA TAT TTT TTA TTA ATT AAT ATA TAT AAA ATA TTA GTA ATA AAT AAT ATT ATT AAT ATT TTA TAA ATA AAT AAT AAT AAT atg ttc aaa aga tat tta tca tca-3’ and antisense primer COB-ARG8-R 5’-gcc tcg agT TAA AGT ATT ATT ATT ATT AAT AAT TTT ATT TTT ATT TTT ATT ATA TTA TTA ATA ATA ATA ATA TAT ATA TTA TAT CTA TGT ATT AAT TTA ATT ATA TAT aag cat ata cag ctt cga tag c-3’. The underlined basepairs were introduced for improved restriction enzyme cutting. The upper case letters depict cytochrome b 5’- and 3’-UTR sequences, and the lower case letters are ARG8m sequence capable of binding to pDS24. The letters in italics depict restriction sites, XbaI on the 5’-end, XhoI on the 3’-end, via which the DNA fragment was cloned into pBS KS, resulting in plasmid pSCS1.
2.3 Construction of the yeast recipient strain YTE31 in which cytochrome b is replaced by ARG8m
Plasmid pSCSI was transformed into the mitochondria of yeast strain DFS160 rho0 (9) as described previously (2), using a biolistic PDS1000/He particle delivery system (Biorad), together with plasmid YEP351 carrying the nuclear selectable LEU2 marker, yielding strain CAB50. CAB50 was mated with strain SAS1A (10), and an Arg+ cytoductant bearing the nucleus from CAB50, termed CAB52-A, was selected. Since the mtDNA of CAB52-A is derived from strain background D273-10B, the COX1 gene contains introns that cannot be spliced in the absence of introns normally present in COB (11). Thus, the COX1 introns were removed from this mtDNA by mating CAB52-A with a synthetic rho- strain, XPM177-1, bearing the intronless COX1 gene derived from CK520 (12), and selecting cytoductants that could be restored to respiratory competence by mating with an intronless cox1Δ∷ARG8m tester strain (XPM62a). One such cytoductant, bearing the nucleus of XPM177-1, was isolated and named YTE31. It is congenic with the well characterized and sequenced strain S288c.
2.4 Construction of plasmid pMD2 containing intronless cytochrome b and COX2
The cytochrome b gene and approximately 340 bps of UTRs flanking sequences on either side were amplified from the intron-less mitochondrial DNA of yeast strain CK520, with sense primer SS-1 5’-CCA CGG GAC CAA TGA CCA AC-3’ and anti-sense primer SS-30 5’-AAA TGA GTA CTC CTT CGG GGT TCG-3’. The PCR product was cloned into pBluescript by cutting both with HincII and ApaI. This resulted in vector pCB6. COX2, which serves as a rescue gene, was amplified from the mitochondrial DNA of strain W303-1B, with sense primer MD30 AAAA CTG CAG CCC CTC CCT AAC GGG AGG AGG ACC GAA GG and anti-sense primer MD31 ATAAGAAT GC GGC CGC CTA CCA TCT CCA TCT GTA AAT CCT ACT AAT C. The PCR product was cloned into the pGEM®-T vector from Promega according to the manufacturer’s instructions. From there it was excised with PstI, which is contained in the primer sequence, and cloned into vector pCB6 cut with PstI, resulting in vector pMD1.
When proof-sequencing the PCR products, we noticed that the cytochrome b gene contained a mutation at nucleotide position 755, leading to an exchange of glycine to aspartate at protein position 252, which renders the enzyme labile at higher temperatures (13). That mutation occurred at an exon/intron boundary at the end of exon 4, at the time the intron-less mitochondrial genome was created (14). The mutation was changed back to glycine with the site-directed mutagenesis kit from Stratagene (see below) using the primers listed for D252G in Supplementary Primer Table (see Supplemental Material). The resulting vector was named pMD2, and used as a template for all mutagenesis reactions.
2.5 Introduction of mutations in COB and amplifying and sequencing the mutated COB genes
The primers used to introduce mutations with pMD2 as a template and the Stratagene Quikchange II Site-Directed Mutagenesis Kit are listed in the Supplementary Primer Table (see Supplemental Material). The reaction was performed according to the instruction manual. Since the mitochondrial genome is high in AT content, the amount of primer was varied (i.e. doubled). The extension time/cycle was also doubled compared to the instructions.
Total DNA of the created strains was used as a template in the PCR performed to verify the presence of the intron-less mutated COB gene. Sense primer MD26 starts 78 bps upstream from ATG, 5’- TTT ATA TAT TTT TTA TTA ATT AAT ATA TAT AAA ATA TTA G-3’, and anti-sense primer MD27 starts 61 bps downstream of the stop codon, 5’- TTA TTA TAT TAT TAA TAA TAA TAA TAT ATA TAT TAT ATC TAT G -3’. The primer used for sequencing and confirming the mutations was anti-sense primer MD2 5’-CCA TAA TAT AAA CCT TTA GCC ATA TGC-3’, located in exon 1.
2.6 Biolistic transformation of yeast strain DFS160 rho0 with plasmids containing mutated versions of the cytochrome b gene
The mutated vector, pMDx (where x is a number assigned to a plasmid with a certain COB mutation) and vector YEP351 containing LEU2 for nuclear selection were shot onto yeast strain DFS160 Mat α rho0 as described above, on sorbitol medium lacking leucine.
When colonies of the bombarded strain emerged, they were replica plated on lawns of recipient strains YTE31 and NB97, respectively, spread on YPDA. After two days of mating, the YPDA plates where replica plated on the YPEG non-fermentable medium. Colonies emerging from the YTE31 cross had the mutated but functional COB DNA incorporated in their mtDNA, replacing ARG8m by homologous recombination. Two colonies were carefully picked and sub-cloned on YPDA. Approximately 5 colonies thereof were picked and tested for their nuclear background on plates after diluting them in 200 ∝l of water. The YTE31 nuclear background was selected by growth on minimal plates supplemented with uracil, adenine and arginine. Functional cytochrome b was tested for on YPEG plates.
In the event that the mutant COB DNA was non-functional in the selection, as was the case for the mutation R79E, the bombarded DFS160 synthetic ρ- was identified by the cross with the mit- COX2 tester strain NB97. If the Leu+ colonies had also received the mitochondrial plasmid, the presence of the plasmid within mitochondria could be confirmed by crossing over of the functional COX2 on the vector into the NB97 mitochondrial DNA, which contains functional COB DNA. The ρ- colony had to be localized on the original bombardment plate and verified by re-streaking and repeating the tester strain cross a minimum of three times. It was then crossed to the final recipient strain FG21 (see below), a derivative of W303-1B, and identified by its failure to grow on medium lacking arginine.
2.7 Cytoduction between recipient strain YTE31 and MR6, a derivative of W303-1B
Since most of the laboratory strains we use, including those containing the new center N mutations (1), are in the nuclear background of yeast strain W303-1B, we made a cytoduction as described elsewhere (2), mating the YTE31 strains carrying respiratory competent cytochrome b mutations with MR6 ρ0 (6), an arg8 derivative of W303-1B. The mating reaction was spread on YPDA plates such that a countable number of colonies were obtained. This master plate was replica plated on YPEG after two days to select for respiring colonies. Since strain YTE31 carries the kar1-1 mutation (15), which prevents fusion of the nuclei, the desired MR6 nuclear background could be selected for by growth on minimal medium supplied with the needed ingredients.
2.8 Creating recipient strains FG20 and FG21, derivatives of W303-1B that contain the YTE31 mitochondria
To additionally facilitate the process, we created Matα (FG20) and Mata, (FG21) recipient strains that have the MR6 nuclear background and the YTE31 mitochondria, with the cytochrome b gene replaced by ARG8m. The strain FG20 was obtained by mating the Matα MR6 ρ0 strain (6) with strain YTE31, and selection of Arg+ colonies with the nuclear background of MR6. The mating type of FG20 was switched with the HO plasmid to create strain FG21. We also created, by transformation with the HO plasmid, a MATa DFS160 strain. Thus, direct screening with the desired W303-1B nuclear background could be performed using DFS160 synthetic petites in either the a or α mating type. Strain YTMT2 is the COX2 tester strain for bombardment in DFS160 Mat a. The recipient strains are also necessary to obtain non-functional cytochrome b mutations in a W303-1B background.
2.9 Testing growth of the yeast strains on non-fermentable medium and measurement of ubiquinol-cytochrome c reductase activities
Growth of the cytochrome b mutated strains was tested in parallel on YPEG plates after they had been grown to stationary phase in liquid YPDA medium. Identical amounts of cells were spotted on plates in serial dilutions. Growth in liquid culture was measured in N3 medium, which is a buffered medium containing 2% glycerol and adenine, as described previously (1).
Membranes from the mutant yeast strains were prepared and ubiquinol-cytochrome c reductase activities measured as described previously (1). Cytochrome bc1 complex concentration was determined from the difference spectrum of the sodium dithionite-reduced minus ascorbate-reduced enzyme using an extinction coefficient of 25 mM-1 cm-1 at 563-578 nm (17) for cytochrome b. The assay buffer contained 50 mM potassium phosphate, pH 7.0, 250 mM sucrose, 0.2 mM EDTA, 1 mM NaN3, and 0.01% Tween-20. Measurements were performed with membranes diluted to a concentration of 5 nM cytochrome bc1 complex in assay buffer supplemented with 1 mM potassium cyanide and 30 πM cytochrome c. The reac- tion was started by adding decyl-ubiquinol to a final concentration of 50 πM. Reduction of cytochrome c was monitored at 550-539 nm with the Aminco DW2a™ spectrophotometer in the dual wavelength mode. An extinction coefficient of 21.5 mM-1 (18) was used to calculate cytochrome c reduction at 550-539 nm.
3. Results
3.1 Creation of a recipient strain for cytochrome b mutations where the cytochrome b gene is replaced by mitochondrially encoded ARG8
There is only inefficient, if any, mitochondrial transformation when exogenous DNA is delivered directly by bombardment into yeast cells containing ρ+ mitochondria (data not shown and Ref. 2). This problem is circumvented by bombarding a suitable strain lacking mtDNA (ρ0) with a plasmid containing the mutagenic DNA fragment. It is one of the extraordinary aspects of S. cerevisiae mitochondria that any plasmid can be replicated and stably propagated as synthetic ρ- molecules (19). When the synthetic ρ- mtDNA is brought into contact with ρ+ mtDNA by crossing, homologous recombination can occur at high frequency, leading to the introduction of the mutation of interest into a complete mitochondrial genome.
We used this procedure first to replace the cytochrome b gene with ARG8m. We used a pBluescript backbone and cloned an ARG8m PCR product into it that had 100 bps of cytochrome b 5’-UTR and 3’-UTR sequences flanking it. The resulting vector, pSCSI, is shown in Fig. 1A. This vector was introduced by biolistic transformation into the mitochondria of the ρ° strain DFS160 (2, 9). Since the efficiency of mitochondrial transformation is rather weak, co-transformation with a nuclear selection marker is performed, such as the LEU2 gene. Leu+ transformants originate from cells that have been hit and survived. A few of them (termed CAB50) contain in their mitochondria the plasmid pSCS1. These can confer, by crossing, arginine prototrophy to a suitable ρ+ strain, SAS1A (10), as illustrated in Fig. 1B. The mitochondria of the mated cells fuse, allowing ARG8m to replace the COB gene after homologous recombination. Some intermediate steps, not depicted in Fig. 1B, were necessary, since the mitochondrial DNA of SAS1A carries the COX1 gene with introns. For its correct splicing, introns of COB are necessary (11). Since the mutated COB genes we wanted to introduce were intron-less, the COX1 introns had to be removed beforehand from the Arg+ recombinants (CAB52-A) issued from the cross between CAB50 and SAS1A. To this end CAB52-A was crossed with a synthetic ρ- strain, XPM177-1, bearing an intron-less COX1 gene. CAB52-A recombinants lacking the COX1 introns were identified by virtue of their ability to recover respiratory competence in crosses with a cox1Δ∷ARG8m strain (XPM62a) containing an intron-less cytochrome b gene. One such cytoductant, where the cytochrome b gene was replaced by ARG8m and lacking the COX1 introns, was retained and named YTE31.
Fig. 1. Creating the recipient strain containing ARG8m instead of cytochrome b in its mitochondria.
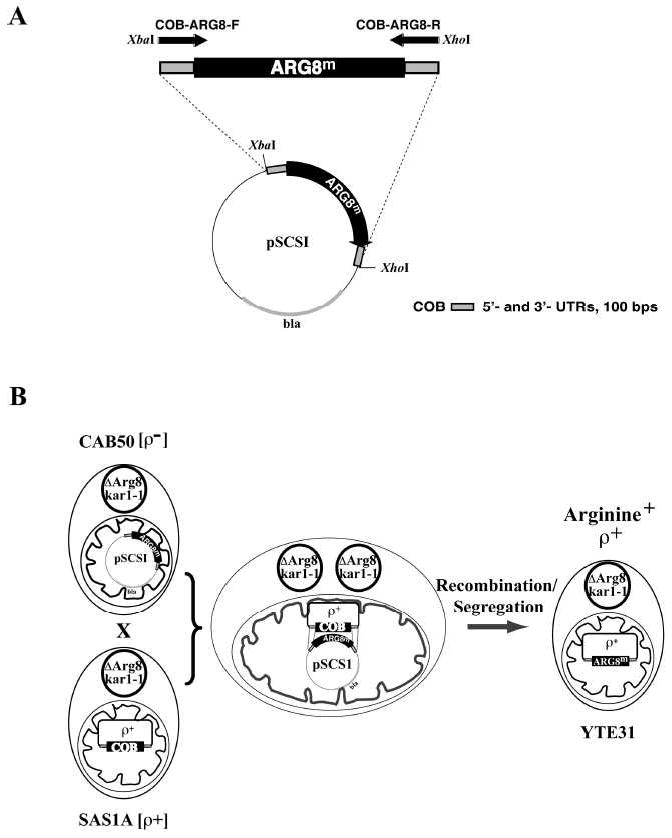
Panel A depicts the creation of a plasmid for biolistic transformation containing an ARG8m cassette flanked by cytochrome b 5’- and 3’- UTRs. The template for the ARG8 gene using mitochondrial codons was plasmid pDS24 (9). The primers COB-ARG8-F and COB-ARG8-R were used for the gene amplification, resulting in an ARG8m gene flanked by approximately 100 bps of COB 5’-UTR and 3’-UTR, respectively, encoded by the 3’- ends in both primers. Both primers also contained the restriction sites shown, via which the gene was cloned into the pBluescript vector KS to create the vector pSCSI. The plasmid also contains the β-lactamase gene (“bla“). Panel B depicts how ARG8m was introduced into the mitochondrial DNA of the recipient strain. The strain used for bombardment was DFS160, which is auxotrophic for leucine and arginine and does not contain mitochondrial DNA. The synthetic ρ- DFS160 containing the plasmid pSCSI was named CAB50. Replacement of cytochrome b by ARG8m can be confirmed by arginine prototrophy.
3.2 Creating template vector pMD2 that carries intronless cytochrome b and COX2
To facilitate the replacement of the ARG8m gene with the mutated COB gene, an intron-less version of cytochrome b was amplified by PCR, using strain CK520 as a template (12). The intron-less cytochrome b gene was cloned into a pBluescript vector via ApaI and HincII restriction sites. In order to be able to screen for cytochrome b mutations leading to respiratory deficiency (see below), the COX2 gene amplified from strain W303-1B was sequenced and inserted via PstI into the vector, named pMD1. Sequencing the intron-less cytochrome b gene revealed that it carried a well-characterized mutation at position 252, from glycine to aspartate (13), which had occurred when the intron-less mitochondrial genome was created (14). The mutation was removed using the site-directed mutagenesis kit and the primers listed in the Supplementary Primer Table, resulting in plasmid pMD2, shown in Fig. 2A.
Fig. 2. Introduction of cytochrome b mutations into recipient strain YTE31.
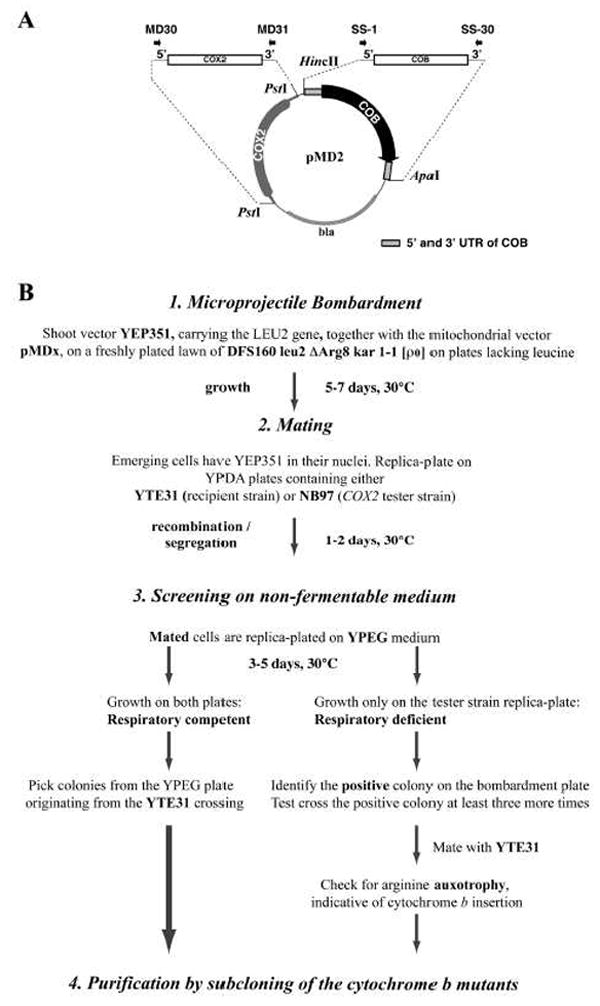
Panel A depicts the creation of a plasmid containing the cytochrome b gene (COB) cassette flanked by cytochrome b 5’- and 3’-UTRs and a functional COX2 gene, as a template for primer mutagenesis. The template for the COB gene was an intron-less version of the cytochrome b gene, which was amplified with primers which resulted in a COB gene flanked by approximately 340 bps of UTRs on each side, and HincII and ApaI sites for cloning into the Bluescript vector, named pCB6. The COX2 gene, which allows selection of cytochrome b mutants that are impaired or respiratory deficient, was amplified with primers that resulted in a PCR product containing the ORF of COX2 flanked by approximately 250 bps of the 5’- and 3’- UTRs. This was cloned via a PstI site into vector pCB6, resulting in vector pMD2. Panel B depicts a flowchart with the sequence of events needed to introduce plasmid pMDx, containing a cytochrome b mutation, into recipient strain YTE31.
3.3 Creation of strains with mutated cytochrome b genes
A flowchart of the procedure to introduce cytochrome b mutations in the mitochondrial genome is shown in Fig. 2B. The plasmid pMDx, carrying a cytochrome b mutation, COBm, was delivered into the mitochondria of the ρ0 strain DFS160 by bombardment. Pre-selection was achieved through the co-bombardment of plasmid YEP351 containing the LEU2 gene (Step 1 in Fig. 2b). When the surviving Leu+ colonies emerged after 5-7 days, they were replica-plated on YPDA medium containing a lawn of recipient strain YTE31 and the same medium containing a lawn of a mit- cox2 tester strain, NB97, respectively (Step 2 in Fig. 2B). After two days, the two mating plates were replica-plated on non-fermentable medium (Step 3 in Fig. 2b).
In case of a cytochrome b mutation that supports respiratory growth, respiring colonies were emerging on the two replica plates, at the same positions, as shown the Y16A mutation in Fig. 3A, upper panel. The respiring clones issued from the cross with YTE31 resulted from replacement of ARG8m with the mutated but still functional cytochrome b gene. Those issued from the cross with NB97 originated from a recombination between the COX2 gene of plasmid pMDx and the mutated cox2 locus of NB97, schematically depicted in Fig. 3B. The only thing that remained to be done was to pick a few respiring clones issued from the cross with YTE31 and to verify them by sequencing for the presence of the cytochrome b mutation. Without exception, all of the isolates proved to contain the mutated cytochrome b gene properly integrated into a complete mtDNA. It has to be noted that due to the presence of the nuclear kar1-1 mutation in both DFS160 and YTE31 most of the cells remain haploid after crossing with either the nucleus of DF160 or that of YTE31. In all analyzed cases, >80% of the isolates had the nucleus of YTE31. Thus only a very limited number of colonies (five) need to be analyzed to have the desired cytochrome b mutants in the YTE31 nuclear background.
Fig. 3. Screening for cytochrome b mutations.
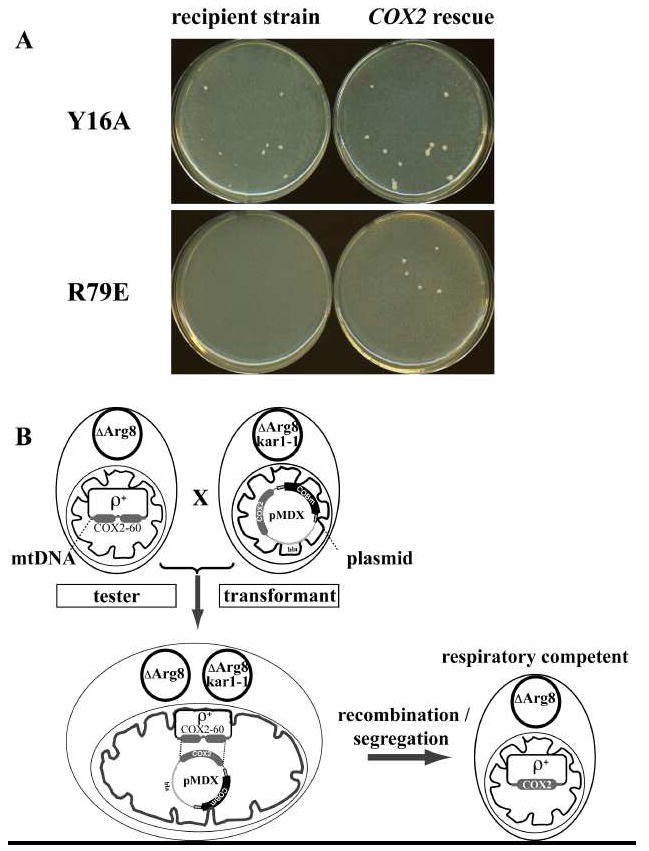
Panel A shows non-fermentable medium plates (YPEG) carrying the crosses of the synthetic ρ- with recipient strain YTE31 (left) or the COX2 rescue strain NB97 (right). The upper half shows the Y16A mutant strain, which is respiratory competent and grows in both crosses. The lower panel shows the R79E mutant strain, the growth of which is only possible with the rescue strain. Panel B shows schematically how the COX2 rescue works. After mating on fermentable medium, the cells are replica plated on non-fermentable medium. The tester strain, NB97, is ρ+ but contains a deletion in COX2 and thus is respiratory deficient unless corrected with a functional COX2 gene. Hence, the rescue is dependent on receiving the COX2 gene on the plasmid that was introduced by the biolistic transformation of the recipient strain and thus confirms the existence of the plasmid in that strain. One has to go back to the original plate to localize the corresponding colony, and perform this procedure at least three more times before finally mating the synthetic ρ- with YTE31.
When the cytochrome b mutation seriously impairs the bc1 complex, as was the case for the arginine 79 to glutamate exchange, shown in Fig. 3A, lower panel, the cross of the synthetic ρ-COBm with YTE31 did not produce clones with good respiratory growth. Only the cross with the mit- cox2 strain (NB97) produced such clones. In that case, some further work was needed to isolate the respiratory deficient cytochrome b mutant. First, the synthetic ρ- colonies responsible for the COX2 rescue, i.e. containing in their mitochondria the pMD-R79E plasmid, had to be carefully located on the original bombardment plate and purified by subcloning. The ρ- COBm was then crossed with the cytochrome b deletion strain YTE31. A few percent of the progeny from the crossing consisted of cells where the ARG8m gene had been replaced with the mutated cytochrome b gene. These cells could very easily be identified by their inability to grow in the absence of arginine and their ability to recover respiratory competence when crossed with a synthetic ρ- strain containing the wild-type cytochrome b gene.
3.4 Comparing growth of the strains with cytochrome b mutations using non-fermentable medium
We introduced three mutations at tyrosine 9, alanine, phenylalanine, and threonine, three mutations at tyrosine 16, alanine, phenylalanine, and iseuleucine, and two mutations at glycine 37, alanine and cysteine. We also introduced mutations at arginine 79 to glutamate, glutamine and lysine. For the study of the consequences of these cytochrome b mutations we decided to work in the nuclear background of the W303-1B strain. This has proven to be a reliable laboratory strain for the investigation of the S. cerevisiae bc1 complex (1, 21), especially since it is not prone to suppression of respiratory function when grown on glucose (21). Thus, the mitochondria of YTE31 containing the respective cytochrome b mutations were transferred by cytoduction into strain MR6/50, a ρ° derivative of W303-1B in which ARG8 is deleted (∃arg8). For further studies we have constructed the FG21 strain. This strain was obtained by cytoduction of the mtDNA of strain YTE31 into strain MR6/50. Thus, it will be possible with the FG21 strain to isolate directly the cytochrome b mutants in the ∃arg8 W303-1B nuclear background, avoiding the cytoduction step from YTE31.
The strains with cytochrome b mutations and the MR6 nucleus were tested in parallel for respiratory competence on non-fermentable medium. Fig. 4A shows the growth on non-fermentable medium, YPEG plates, at 30°C and 34°C, respectively. The cytochrome b mutations affecting center N, shown in the upper panel, Y9A, Y9F and Y9T, do not seem to be impaired when grown on YPEG plates compared to the wild-type strain. The same can be observed for the strains Y16F and Y16I. Slightly impaired in growth are the stains carrying the mutations Y16A, G37A and G37C, where the second dilution at 30°C appears to be somewhat slower growing. These differences are more pronounced at 34°C, where the Y16A mutant fails to grow. The reason we mutated tyrosine 9 and tyrosine 16 is that from looking at the crystal structure of the yeast cytochrome bc1 complex (Protein data bank code 1EZV, Ref. 22), we suspect that these two positions are involved in the dimer cooperativity. The change from a tyrosine to a phenylalanine in both cases was expected to be least detrimental, due to the retention of the aromatic ring. Replacing the tyrosine with the small amino acid alanine was expected to have the biggest impact. This could indeed be observed for the mutant strain Y16A. At 34°C, this mutant showed almost no growth on YPEG, although an alanine at this position is naturally occurring in bovine cytochrome b. The mutant strain Y16I was also slightly sensitive to higher temperatures, indicating that these mutations impose some instability due to structural changes.
Fig. 4. Testing the strains with cytochrome b mutations for their growth on non-fermentable medium.
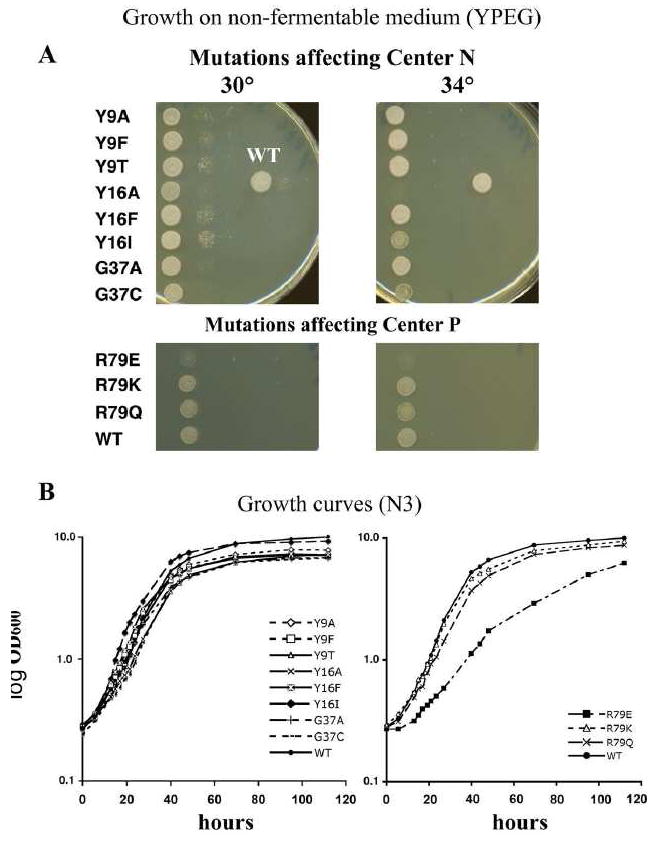
Shown in panel A are the strains with the cytochrome b mutations created in this study, grown on YPEG plates. The plates on the left depict the growth of serial dilutions of the strains at 30°C. The plates on the right are the same strains grown at 34°C. WT is the strain that was created by bombarding DFS160 with non-mutated, intron-less cytochrome b, followed by cytoduction and crossing as with the cytochrome b mutant strains. For these experiments, the cytochrome b mutations were in a MR6 nuclear background (see Material and Methods). Panel B shows the growth of the strains in the liquid non-fermentable medium N3. The left panel shows the center N mutants and the right panel shows the center P mutants. The growth curve for the wild-type strain is shown in both panels to permit comparison of the mutants.
Although the strains show growth differences at 34°C on non-fermentable medium on plates, there are only small differences in the growth rate and yields at 30° C on liquid medium. Since these growth curves were obtained with single cultures, they would need to be repeated with multiple cultures to determine whether these small apparent differences are significant.
Glycine at position 37 is located in helix A of the protein rather distant to center N, but mutations of glycine 37 often lead to center N inhibitor resistance. G37V has been described before as conferring resistance to antimycin (23, 24), and it was expected that a change to the similar amino acid, alanine, might also show some resistance. We therefore tested strain G37A and G37C on antimycin and ilicicolin H plates, respectively, and these mutations conferred resistance to both inhibitors (data not shown). This indicates that glycine 37 is one of the center N locations that, when mutated to a specific amino acid, can confer resistance to different center N inhibitors, which suggests a structural role of glycine 37. Resistance to more than one center N inhibitor was not observed in our previous study for the mutations G37D and G37S (1). The mutation of G37C leads to instability at 34°C, whereas the G37A mutation does not, which is probably due to the fact that cysteine contains a bulky sulfhydryl group, whereas alanine and valine have small, non-polar side chains. Further investigation of these mutants might give insight into the structure/function relationship and inhibitor resistance properties of cytochrome b.
The lower panel of Fig.4A shows the mutations we created at arginine 79. This arginine is highly conserved between cytochrome b’s of different species. It is, together with the heme bL propionate and several water molecules, involved in a hydrogen-bonding network that may receive protons from the cytochrome b residue glutamate 272 at the ubiquinol oxidase site (25). We mutated this arginine into glutamine, glutamate and lysine. The mutation R79E had to be obtained by applying the COX2 rescue method, since it shows very impaired growth on glycerol (Fig. 4A, lower panel). Although the R79E mutant grows on non-fermentable medium, the respiratory competence is too weak for the selection process on this medium, as was seen in Fig. 3A. This failure to grow on non-fermentable medium is even more pronounced at elevated temperatures, and the R79Q mutation was also impaired at the higher temperature (Fig. 4A). Only after prolonged incubation of the plate did the R79E mutant start growing on non-fermentable medium (data not shown)
Panel B of Fig. 4 shows the growth of the mutant strains in liquid non-fermentable medium (N3) with glycerol as carbon source at 30°C. The generation times are slightly different, and the growth yield also seems to differ a small amount between the wild-type strain (7.1 hours doubling time), the Y16I and Y9A (9.1 hours doubling time) mutants, and the rest of the center N mutation strains. Strains with the Y16A and G37C mutations have the longest generation times, 10.5 hours, whereas those with the Y16F and Y16I mutations are similar to the wild-type strain with 6.7 and 7 hours, respectively. Mutants Y9F, Y9T, and G37A have slightly higher doubling times with 7.6 hours, 8 hours, and 7.8 hours, respectively.
The growth in liquid N3 medium of the strains with mutations at center P is consistent with the behavior of these strains on plates. While the doubling time of the R79Q mutant (approximately 8.7 hours) seems only slightly longer than that of the wild-type yeast (7.1 hours) and the R79K mutant (7.9 hours), the growth of the R79E mutant is severely compromised with a doubling time of approximately 24 hours, and the yield does not reach wild-type levels even after 120 hours, although the mutant has not yet reached stationary phase at that time.
3.5 Effect of the cytochrome b mutations on the activity of the bc1 complex
Membranes were isolated from the mutant strains, and the ubiquinol-cytochrome c reductase activities of the bc1 complexes were measured. As shown in Table II, there are significant differences in the activities of the bc1 complexes in membranes from the various mutants. While the strains with the Y9F, Y9T, Y16F, and Y16I mutations exhibited activities comparable to that of the wild-type enzyme, the Y9A, Y16A, G37A and G37C mutations impaired the activity to various extents. The lowest activity of the center N mutants was observed with the G37C mutant, which showed only 41% activity compared to the wild-type strain. The lowest activity for the arginine 79 substitutions was observed for the glutamate mutant, consistent with its extremely low growth rate on non-fermentable medium, while the bc1 complex from the glutamine mutant reached activities of 67% of the wild-type, and the lysine mutation had activity only slightly less (88%) than that of the enzyme from the wild-type strain.
4. Discussion
We previously isolated several Qn site mutations of cytochrome b in S. cerevisiae by using the inhibitor ilicicolin H to select for inhibitor resistant yeast mutants (1). There have also been extensive screens for cytochrome b mutations decades ago, often using inhibitors (see Ref. 7 for a review). However, screens for mutations conferring inhibitor resistance are time-consuming and their outcome is limited to only a very small number of residues of the cytochrome b protein. In addition, many mutations of interest don’t have any connection with inhibitor resistance, and therefore can’t be obtained this way.
Several valuable S. cerevisiae strains carrying point mutations in cytochrome b have been created in recent years using biolistic bombardment (20, 26, 27), including mutation of the crucial glutamate 272 (27). In the previously used methods, the recipient strain contained either a wild-type cytochrome b gene, or a cytochrome b gene with a point mutation (mit-) impairing mitochondrial respiration (20, 27). The use of a wild-type recipient strain makes the process very laborious, especially when the mutations of interest have no or only moderate effects on the function of the bc1 complex, which is by far the most common situation as illustrated in the present study. That means that hundreds of isolates have to be characterized at the molecular level (e.g. by sequencing) to find the few issued from a recombination between the synthetic ρ- mtDNA bearing the cytochrome b mutation and the wild-type ρ+ mtDNA of the recipient strain.
With the method described here, which is based on the use of a cytochrome b deletion strain, all the respiring isolates, without exception, have the mutation of interest properly integrated into their mitochondrial genome. The former method, which uses a mit- cytochrome b mutant as a recipient strain (27), also proved to be helpful in the construction of respiratory competent cytochrome b mutants. Hence, both methods allow the detection of successful recombination events by restoration of respiratory competence in the recipient strain. However, several drawbacks limit the use of a mit- mutant as a recipient strain. First, mit- mutants can spontaneously revert due to either an intragenic or extragenic secondary mutation, whereas phenotypic suppression of the cytochrome b gene deletion strain is impossible. Given the poor efficiency of mitochondrial genetic transformation, this poses a considerable problem.
Furthermore, the tester mit- mutation needs to be relatively close to the site where an introduced cytochrome b mutation is supposed to be located, otherwise the restoration of respiratory competence can be the result of the correction of the mit- mutation without the integration of the mutation of interest (mutations within less than 100 base pairs of the mtDNA can be separated rather easily by recombination, see Ref. 24). Another drawback is the very small number of mit- exonic cytochrome b mutants available, and most of these mutations are clustered in very specific regions of cytochrome b.
In addition, our method allows the creation of any type of mutation in the cytochrome b gene, for example the deletion of a defined segment or the introduction of a combination of several mutations in different regions, something that can not be easily implemented with a mit- recipient strain, where most of the coding sequence of the cytochrome b gene is still present. Taken together, our method provides a simple phenotypic screening approach, revealing in all cases whether a given cytochrome b mutation results in a stringent respiratory deficiency or not. Only in the case of respiratory deficiency, a non-reverting strain lacking a segment of the COX2 gene is required. This strain indeed functions as a tester strain, since it is indicative of the presence of the plasmid in the mitochondria of the bombarded strain. It is not used as a recipient strain for the creation of cytochrome b mutations
To confirm the utility of this new method, we created a group of cytochrome b mutations that we expect might alter bc1 complex function. Tyrosine 9 and tyrosine 16 have been implicated in dimer cooperativity at center N (28). For tyrosine 9, three different point mutations were created, alanine, phenylalanine, and threonine, and likewise three mutations were created for tyrosine 16, alanine, phenylalanine, and isoleucine. No mutation at either of these locations has been reported before. We also introduced mutations at a highly conserved location in center P, arginine 79, which has been proposed to be involved in proton conduction at center P (25). Mutations at this site have also never been described before.
We also targeted mutations to glycine 37, since this residue is especially interesting in regard to inhibitor resistance at center N (1, 23). We replaced glycine 37 with alanine and cysteine (1, 23, 24). We found that both mutations confer resistance to ilicicolin and antimycin on non-fermentable plates (data not shown), and the cysteine mutation was previously shown to confer antimycin resistance (1). It was postulated that an alanine mutation at this position could lead to antimycin resistance, based on the experience with the glycine to valine exchange (23, 24). We introduced all of these mutations, as well as the wild-type sequence of intron-less cytochrome b, into the mitochondria of YTE31 (Fig. 1B and 2B). Since we wanted to have those mutations in a nuclear background resembling that of strain W303-1B, we did a cytoduction with its derivative, MR6 ρ0 (Table I and Ref. 6).
The particular positions in cytochrome b we had chosen to mutate as well as the amino acids we introduced were expected to possibly have detrimental effects on the bc1 complex (25, 28). While the R79E mutation at center P severely impaired bc1 activity and respiration, none of the center N cytochrome b mutations had an extremely detrimental effect on respiration as measured by growth on a non-fermentable substrate, although some mutations did cause noticeable changes in growth rates at 34° C and affect the activity of the bc1 complex (Fig. 4 and Table II). Fig. 4 B shows that there are small but noticeable differences in the growth yields of the various strains and a significant difference in doubling time for the R79E mutant. This does not come as a surprise for such a drastic mutation like arginine 79 to glutamine, but the reasons for differences in the yield of the center N mutations remain to be elucidated in future experiments. These growth comparisons would need to be reproduced with multiple cultures to determine whether these small apparent differences are significant.
Interestingly, pronounced differences can be observed at higher temperature between the mutants, even when they are at the same location like Y16A and Y16F. The Y16I mutation seems to impose some instability on the protein, since the corresponding strain has problems growing at higher temperatures (Fig. 4 A). Likewise, two of the current mutations, G37A and G37C, conferred ilicicolin H and antimycin resistance on plates (data not shown), whereas a previously obtained mutation at this position, G37D, showed very weak resistance, but was impaired for growth on non-fermentable medium (1). Furthermore, we were surprised that these mutations conferred resistance to more than one center N inhibitor, a fact that we had only observed before for the L198F mutation (1).
Our data illustrates that usually several mutations have to be created to find those worth characterizing further. There are multiple questions regarding cytochrome b function, such as the structural basis for regulatory interactions between monomers in the bc1 dimer, the role of highly conserved residues in proton conduction at center P, and the determinants of semiquinone stability at center N, that are likely to be resolved by creating additional mutations in this uniquely important subunit of the bc1 complex.
Supplementary Material
Acknowledgments
We thank Dr. X. Perez-Martinez for strains XPM177-1 and XPM62a, and Drs. T. Ellis and H.L. Fiumera for help with construction and verification of strain YTE31. We thank Dr. Brigitte Meunier for helpful advice concerning the primer mutagenesis reaction.
This work was supported by National Institutes of Health Research Grant GM 20379 to B.L.T. and GM 29362 to T.D.F. Jean-Paul di Rago was supported by The Conseil régional de la région Aquitaine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ding MG, di Rago J-P, Trumpower BL. Investigating the Qn site of the cytochrome bc1 complex in Saccharomyces cerevisiae with mutants resistant to ilicicolin H, a novel Qn site inhibitor. J Biol Chem. 2006;281:36036–36043. doi: 10.1074/jbc.M608026200. [DOI] [PubMed] [Google Scholar]
- 2.Bonnefoy N, Fox TD. Genetic transformation of Saccharomyces cerevisiae mitochondria. Methods in Enzymology. 2001;350(5):97–111. doi: 10.1016/s0076-6879(02)50958-7. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto K, Perlman PS, Butow RA. The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: Preferential transmission of mitochondrial DNA to the medial bud. J Cell Biol. 1998;142:613–623. doi: 10.1083/jcb.142.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dujon B, Slonimski PP, Weill L. Mitochondrial genetics. IX. A model for the recombination and segregation of mitochondrial genes in Saccharomyces cerevisiae. Genetics. 1974;78:415–437. doi: 10.1093/genetics/78.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dujon B. The Molecular Biology of the yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1981. p. 505. [Google Scholar]
- 6.Rak M, Tetaud E, Godard F, Sagot I, Salin B, Duvézin-Caubet S, Slonimski PP, Rytka J, di Rago J-P. Yeast cells lacking the mitochondrial gene encoding the ATP synthase subunit 6 exhibit a selective loss of complex IV and unusual mitochondrial morphology. J Biol Chem. 2007;282:10853–10864. doi: 10.1074/jbc.M608692200. [DOI] [PubMed] [Google Scholar]
- 7.Brasseur G, Saribas AS, Daldal F. A compilation of mutations located in the cytochrome b subunit of the bacterial and mitochondrial bc1 complex. Biochim Biophys Acta. 1996;1275:61–69. doi: 10.1016/0005-2728(96)00051-5. [DOI] [PubMed] [Google Scholar]
- 8.Wong L-JC. Pathogenic mitochondrial DNA mutations in protein-coding genes. Muscle Nerve. 2007;36:279–293. doi: 10.1002/mus.20807. [DOI] [PubMed] [Google Scholar]
- 9.Steele DF, Butler CA, Fox TD. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc Natl Acad Sci USA. 1996;93:5253–5257. doi: 10.1073/pnas.93.11.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiumera HL, Broadley SA, Fox TD. Translocation of mitochondrially synthesized Cox2 domains from the matrix to the intermembrane space. Mol Cell Biol. 2007;27:4664–4673. doi: 10.1128/MCB.01955-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labouesse M, Netter P, Schroeder R. Molecular basis of the ’box effect’, a maturase deficiency leading to the absence of splicing of two introns located in two split genes of yeast mitochondrial DNA. Eur J Biochem. 1984;144:85–93. doi: 10.1111/j.1432-1033.1984.tb08434.x. [DOI] [PubMed] [Google Scholar]
- 12.Labouesse M. The yeast mitochondrial leucyl-tRNA synthetase is a splicing factor for the excision of several group I introns. Mol Gen Genet. 1990;224:209–221. doi: 10.1007/BF00271554. [DOI] [PubMed] [Google Scholar]
- 13.Saint-Georges Y, Bonnefoy N, di Rago J-P, Chiron S, Dujardin G. A pathogenic cytochrome b mutation reveals new interactions between subunits of the mitochondrial bc1 complex. J Biol Chem. 2002;277:49397–49402. doi: 10.1074/jbc.M207219200. [DOI] [PubMed] [Google Scholar]
- 14.Séraphin B, Boulet A, Simon M, Faye G. Construction of a yeast strain devoid of mitochondrial introns and its use to screen nuclear genes involved in mitochondrial splicing. Proc Natl Acad Sci USA. 1987;84:6810–6814. doi: 10.1073/pnas.84.19.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conde J, Fink GR. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976;73:3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnefoy N, Bsat N, Fox TD. Mitochondrial translation of Saccharomyces cerevisiae COX2 mRNA is controlled by the nucleotide sequence specifying the pre-Cox2p leader peptide. Mol Cell Biol. 2001;21:2359–2372. doi: 10.1128/MCB.21.7.2359-2372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berden JA, Slater EC. The reaction of antimycin with a cytochrome b preparation active in reconstitution of the respiratory chain. Biochim Biophys Acta. 1970;216:237–249. doi: 10.1016/0005-2728(70)90215-x. [DOI] [PubMed] [Google Scholar]
- 18.Yu CA, Yu L, King TE. Preparation and Properties of Cardiac Cytochrome c1. J Biol Chem. 1972;247:1012–1019. [PubMed] [Google Scholar]
- 19.Fox TD, Sanford JC, McMullin TW. Plasmids can stably transform yeast mitochondria lack- ing endogenous mtDNA. Proc Natl Acad Sci USA. 1988;85:7288–7299. doi: 10.1073/pnas.85.19.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill P, Kessl J, Fisher N, Meshnick S, Trumpower BL, Meunier B. Recapitulation in Saccharomyces cerevisiae of cytochrome b mutations conferring resistance to atovaquone in Pneumocystis jiroveci. Antimicrob Agents Chemother. 2003;47:2725–2731. doi: 10.1128/AAC.47.9.2725-2731.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown TA, Trumpower BL. Strain-dependent variation in carbon source regulation of nucleus-encoded mitochondrial proteins of Saccharomyces cerevisiae. J Bacteriol. 1995;177:1380–1382. doi: 10.1128/jb.177.5.1380-1382.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunte C, Koepke J, Lange C, Roßmanith T, Michel H. Structure at 2.3 A resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure. 2000;8:669–684. doi: 10.1016/s0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 23.di Rago J-P, Colson A-M. Molecular basis for resistance to antimycin and diuron, Q-Cycle inhibitors acting at the Qi site in the mitochondrial ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. J Biol Chem. 1988;263:12564–12570. [PubMed] [Google Scholar]
- 24.di Rago J-P, Perea J, Colson A-M. Isolation and RNA sequence analysis of cytochrome b mutants resistant to funiculosin, a center i inhibitor of the mitochondrial ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. FEBS Lett. 1990;263:93–98. doi: 10.1016/0014-5793(90)80713-s. [DOI] [PubMed] [Google Scholar]
- 25.Palsdottir H, Lojero CG, Trumpower BL, Hunte C. Structure of the yeast cytochrome bc1 complex with a hydroxyquinone anion Qo site inhibitor bound. J Biol Chem. 2003;278:31303–31311. doi: 10.1074/jbc.M302195200. [DOI] [PubMed] [Google Scholar]
- 26.Kessl JJ, Ha KH, Merritt AK, Lange BB, Hill P, Meunier B, Meshnick SR, Trumpower BL. Cytochrome b mutations that modify the ubiquinol-binding pocket of the cytochrome bc1 complex and confer anti-malarial drug resistance in Saccharomyces cerevisiae. J Biol Chem. 2005;280:17142–17148. doi: 10.1074/jbc.M500388200. [DOI] [PubMed] [Google Scholar]
- 27.Wenz T, Hellwig P, MacMillan F, Meunier B, Hunte C. Probing the role of E272 in quinol oxidation of mitochondrial complex III. Biochemistry. 2006;45:9042–9052. doi: 10.1021/bi060280g. [DOI] [PubMed] [Google Scholar]
- 28.Covian R, Trumpower BL. Regulatory Interactions Between Ubiquinol Oxidation and Ubiquinone Reduction Sites in the Dimeric Cytochrome bc1 Complex. J Biol Chem. 2006;281:30925–30932. doi: 10.1074/jbc.M604694200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


