Abstract
Prolonged opioid exposure increases the expression of cholecystokinin (CCK) and its receptors in the central nervous system, where CCK may attenuate the antinociceptive effects of opioids. The complex interactions between opioid and CCK may play a role in the development of opioid tolerance. We designed and synthesized cyclic disulfide peptides and determined their agonist properties at opioid receptors and antagonist properties at CCK receptors. Compound 1 (Tyr-c[D-Cys-Gly-Trp-Cys]-Asp-Phe-NH2) showed potent binding and agonist activities at δ and µ opioid receptors while displaying some binding to CCK receptors. The NMR structure of the lead compound displayed similar conformational features of opioid and CCK ligands.
Keywords: Multivalent Ligands, Bifunctional Peptides, Overlapping Pharmacophores, G-Protein Coupled Receptors, Pain, Tolerance, NMR Conformation
Introduction
Neuropathic pain is an abnormal pain that can result from nerve injuries and current treatment with opioids are problematic due to the side-effects associated with doses necessary to achieve sufficient pain relief in patients [36, 51]. While mechanisms of neuropathic pain are still being elucidated, emerging studies indicate the involvement of neuroplasticity in which significant increased expression of neuromodulators and neurotransmitters in the central nervous system such as cholecystokinin (CCK) occurs [17, 46, 47, 49, 50]. CCK-8 is a linear octapeptide found in the periphery as a hormone and in the central nervous system (CNS) as a neurotransmitter where it is commonly associated with satiety and anxiety, respectively [34, 35]. CCK-8 has long been considered as an “anti-opioid” peptide because it diminishes opioid induced antinociception [14]. Additionally, CCK-8 has been shown to have direct pronociceptive actions. For example, sustained administration of morphine to animals results to up regulation of CCK and CCK receptors, which may lower the pain-relieving actions of the opiate by limiting the pronociceptive and anti-opiate actions of CCK [48].
We, therefore, hypothesize that effective treatment of chronic pain states such as neuropathic pain and improved antinociceptive actions would be associated with compounds which could act as agonists at opioid receptors while acting to counteract the up regulation/activity of endogenous CCK (i.e., CCK antagonist).
Previously, we reported the structure-relationships of linear peptides, with overlapping pharmacophores at the opioid and CCK receptor [1]. Several of these linear analogues demonstrated simultaneous potent agonist activity at opioid receptors and reduced agonist or antagonist activity at CCK receptors. To further examine the required bioactive conformation for binding at opioid and CCK receptors and improve the agonist properties at the opioid receptors and antagonist activity at CCK receptors, the lead linear CCK/opioid peptide analogues have been modified by cyclization. In addition to potentially improving the biological activity profiles of the peptides, cyclization induces a conformational constraint to the peptide that allows for the use of biophysical methods to define the three dimensional structural parameters (e.g. angles of psi and phi of the peptide backbone, chi angles of side chain groups, orientation and distances between the pharmacophore elements) that are necessary for the biological activities at the CCK and opioid receptors. This information can be useful in the de novo design of peptide mimics. Another advantage of cyclization is that it may allow the peptide to cross the blood brain barrier, as well as making the peptide stable to chemical and enzymatic degradation [16]. Herein we report the design and structure activity relationships of a series of cyclic analogues as an antagonist at CCK-1 and CCK-2 receptors and agonists at δ and µ opioid receptors, and compare our cyclic lead compound to opioid and CCK ligands.
2. Experimental
2.1 Materials
Rink Amide AM resin (200–400 mesh, 0.6–0.7 mmol/gram substitution) was purchased from Novabiochem (U.S.A). Nα-Fmoc protected amino acids including Nα-Fmoc-Cys(S-Trt)-OH and Nα-Fmoc-D-Cys(S-Trt)-OH were purchased from American Peptide Co., (Sunnyvale, CA); Nα-Fmoc-D-Trp(Ni-Boc)-OH was purchased from Novabiochem (U.S.A.); Nα-Fmoc-Pen(S-Trt)-OH and Nα-Fmoc-D-Pen(S-Trt)-OH were purchased from ChemImpex (Woodale, NJ); HBTU and HOBt were purchased from Peptide International (Louisville, KY). Potassium ferracyanide and thallium trifluoroacetate were purchased from Aldrich (St. Louis, MO). Peptide synthesis solvents were reagent grade, except for the HPLC grade CH3CN, were acquired from commercial sources and used without further purification unless otherwise noted. The purification of the crude peptides was achieved using a Hewlett-Packard 1100 series Liquid Chromatograph with a C18-bonded silica column semi-preparative column (Vydac 218TP1010, 300 Å, 1.0 ×25 cm, Separations Group, Hesperia, CA). The separations were monitored at 220 and 280 nm with a Hewlett-Packard 1100 series multiple variable wavelength UV detector and were integrated with a Hewlett-Packard 3396 series III integrator. Purity of the isolated peptides was assessed with analytical RP-HPLC in two different gradient systems as detected at 214, 230, 254, 280 nm. The structures of the pure peptides were confirmed by ESI-MS (Finnigan, Thermoelectron, LCQ classic) and high resolution fast atom bombardment FAB-MS (JEOL HX110 sector instrument) or MALDI-TOF, where were performed at the University of Arizona Mass Spectrometry and Protein Sequencing Facility. TLC was done on Analtech (Newark, NJ) silica gel F254 plates (250 µM layer thickness) using the following systems: (A) 1-butanol/water/acetic acid (4:1:1); (B) ethyl acetate/1-butanol/water/acetic acid (5:3:1:1); and (C) chloroform/methanol/acetic acid (7:1:2). The peptides were detected on the TLC plates using iodine vapor.
2.2 Design of cyclic peptides
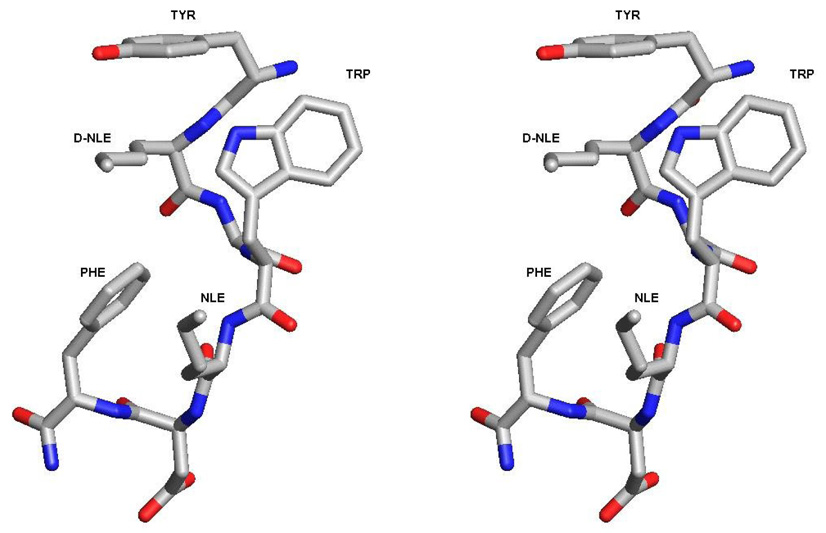
In our design of a globally constrained CCK/opioid peptide, we examined the structure-activity relationships of linear peptides at opioid and CCK receptors. One of our best compounds, Tyr1-D-Nle2-Gly3-Trp4-Nle5-Asp6-Phe7-NH2, (RSA504) had nanomolar binding at the δ and µ opioid receptors (Ki = 2.9 nM and 27.1 nM, respectively) and at the CCK-1 and CCK-2 receptors (Ki = 11.2 and 15.9 nM, respectively), as well as a balanced selectivity between CCK-1 and CCK-2 receptor types (nearly a 1:1 ratio) [1]. To determine whether the lead linear peptide was a reasonable choice for cyclization, we modeled its putative structure by conformational search using Monte Carlo. As seen in Figure 1, one of the lowest energy conformations for RSA504 showed that the side chain groups of D-Nle2 and Nle5 are oriented on the same face, and that the terminal alkyl groups are in close proximity to each other. Thus, positions 2 and 5 were determined to be appropriate sites of substitution for cyclization. Furthermore, these sites of cyclization are consistent with cyclic peptide ligands for opioid receptors, particularly to DPDPE in which positions 2 and 5 were substituted with D-Pen [30]. Also this design retains the free N-terminal Tyr1, which is important for agonist activity at opioid receptors [8, 18, 28]. From the perspective of the CCK receptors, the Phe8 C-terminal end was not used for cyclization since Phe8 is important for the recognition of peptide ligands at CCK receptors, much like other reported cyclic analogues of CCK-8 [9, 10]. Cyclization was implemented on the leading linear peptide by substituting position 2 and position 5 with D-Cys and Cys, respectively, as well as the more topographically constrained β,β dimethyl analogues of D-Pen and Pen. The free sulfhydryl groups of the linear peptides were oxidized to make a side chain to side chain cyclic disulfide bond. The cyclic peptides synthesized and examined for bioactivity are listed in Table 1.
Figure 1.
Stereoview of the lowest energy conformation of RSA504 (Tyr-D-Nle-Gly-Trp-Nle-Asp-Phe-NH2) based on a Monte Carlo conformational search. D-Nle and Nle are oriented on the same side and the terminal alkyl groups are in close proximity to each other. These sites were used for substituting residues appropriate for cyclization.
Table 1.
Structure of Peptides Discussed in This Study. Compounds 1 – 6 were Designed and Synthesized
| Compound | Sequence |
|---|---|
| CCK-8 | Asp26-Tyr(SO3H)27-Met28-Gly29-Trp30-Met31-Asp32-Phe33-NH2 |
| [Met5]-enkephalin | Tyr1-Gly2-Gly3-Phe4-Met5-OH |
| DPDPE | Tyr-c[D-Pen-Gly-Phe-Pen]-OH |
| RSA504 | Tyr1-D-Nle2-Gly3-Trp4-NMeNle5-Asp6-Phe7-NH2 |
| 1 | Tyr-c[D-Cys-Gly-Trp-Cys]-Asp-Phe-NH2 |
| 2 | Tyr-c[D-Cys-Gly-Trp-D-Cys]-Asp-Phe-NH2 |
| 3 | Tyr-c[D-Pen-Gly-Trp-Cys]-Asp-Phe-NH2 |
| 4 | Tyr-c[D-Cys-Gly-Trp-Pen]-Asp-Phe-NH2 |
| 5 | Tyr-c[D-Cys-Gly-D-Trp-Cys]-Asp-Phe-NH2 |
| 6 | Tyr-c[D-Cys-Gly-D-Trp-Pen]-Asp-Phe-NH2 |
2.3 Peptide Synthesis
Standard tert-butyl side chain protected Fmoc-amino acids were used [1] and the disulfide cyclization was achieved by potassium ferricyanide oxidation [27] or thallium trifluoroacetate assisted “on-resin” oxidation [2]. Briefly, the Rink amide resin was swelled and the initial Fmoc-deprotected with 25% (v/v) piperidine in DMF solution. Most coupling reactions were achieved using a three-fold excess (relative to resin substitution) of Fmoc-amino acid, HBTU, and HOBt in the presence of six-fold excess DIEA in DMF. Because Cys and Pen residues prone to racemization, the coupling of Cys or Pen residue was done with Fmoc-amino acid (3 eq.), HBTU (3 eq.), and HOBt (3 eq.) in a solution of DMF/DCM (1:1) with no preactivation, and the base used was 2,4,6-trimethylpyridine (TMP, 6 eq collidine) [4]. The cleavage from the resin was achieved in the 95% TFA in the presence of thiol and silane based scavengers for 1.5 hours. The peptide was precipitated in cold diethyl ether and was isolated by centrifugation.
For the disulfide cyclization of peptides 1, 2, 4, 5, and 6, the crude peptide was dissolved. The dissolved peptide was slowly added to the 0.01 M aqueous solution of K3Fe(CN)6 with an aid of a syringe pump set to dispense at a rate of 3 mL/ hour. The pH of the solution was monitored and adjusted to pH~8.5 by adding concentrated ammonium hydroxide (NH4OH). After the oxidation was completed, as monitored by HPLC, the pH was adjusted to about 5.0 with acetic acid. An ion exchange resin (IRA-68 Amberlite resin, Cl-form) was added to remove excess K3Fe(CN)6 and other iron salts. After one hour, the ion exchange was filtered through a course frit funnel. The volume was reduced by rotary evaporation and lyophilization. The crude peptides were dissolved with minimal acetonitrile and DMSO followed by a slow addition of water before loading in HPLC.
For peptide 3, Fmoc-D-Pen(Acm)-OH, cyclization was done “on-resin” to keep the Tyr and Trp fully proctected during the orthogonal deprotection of the Acm group. Linear peptide was constructed by standard coupling of Fmoc/tBu protected amino acids. Fmoc D-Pen and Fmoc-Cys were side chain protected with S-Acm. The final N-terminal Fmoc group was not deprotected. The resin was swelled with cold solution of DMF and anisole (19:1, v/v) to which thallium trifluoroacetate was added (4 eq., 0.315 grams). After a 15 hour reaction, the resins were washed and the N-terminal Fmoc group was deprotected. After cleavage from the resin and deprotection of the side chain groups, the peptide was precipitated and dried. The crude peptides were dissolved with minimal acetonitrile and DMSO followed by a slow addition of water before loading in HPLC. Fmoc-D-Pen(Trt)-OH may be used alternatively using solution phase oxidation.
2.4 Bioassays
The binding affinities of the CCK/opioid analogues were determined by competitive binding against radiolabeled DPDPE (δ opioid receptors) or DAMGO (µ opioid receptors) in membranes from transfected cells that stably expressed the hDOR or the rMOR, respectively, and against radiolabeled sulfated CCK-8 at the human CCK-1 or the human CCK-2 receptors in transfected HEK293 cells as previously reported [1]. The functional biological activities of the cyclic derivatives were assessed using GTP-γ-S binding assay and PI assay for opioid receptors and CCK receptors [1], respectively (Table 2 and Table 3). Opioid agonist activity at δ and µ receptors were also determined in vitro using electrically stimulated MVD and GPI respectively. CCK antagonist activity was determined in vitro using unstimulated GPI/LLMP against sulfated CCK-8 (Table 4) and binding affinities and PI assay for the compounds at hCCK-and hCCK2 receptors were determined (Table 5).
Table 2.
Binding Affinities and Functional Activities of the CCK/Opioid Peptides at the δ and µ Opioid Receptors
| Binding Affinity | [35S]GTP-γ-S binding | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peptidea | hDORb, [3H]DPDPEc | rMORb, [3H]DAMGOd | hDORb | rMORb | ||||||
| Log IC50e | Ki/nMf | Log IC50e | Ki/nMf | Log EC50g | EC50/nM | ECmaxh | Log EC50g | EC50/nM | Emaxh | |
| 1 | 0.2 | 2.2 | 780 | 190 | 42 | 69 | ||||
| 2 | 3.8 | 9.2 | 200 | 110 | 25 | 110 | ||||
| 3 | 0.3 | 1300 | 14 | 150 | 110 | 42 | ||||
| 4 | 2.6 | 1.4 | 1.8 | 44 | 1.7 | 45 | ||||
| 5 | 57 | 43. | n/d | n/d | 42 | 69 | ||||
| 6 | 510 | 1500 | 2000 | 43 | 5400 | 58 | ||||
| RSA504 | 23. | 27 | 5.5 | 81 | 0.47 | 110 | ||||
| DPDPE | n.d. | - | n.d. | - | −8.8±0.25 | 1.6 | 69±4.2 | - | - | |
| DAMGO CCK-8 | n.d. | - | n.d. | - | n.d. | - | - | −7.44±0.19 | 37 | 150±9.4 |
Refer to Table 1 for the structure of the peptides.
Carried out using membrane preparations from transfected HN9.10 cells that constitutively expressed the respective opioid receptor type.
Kd = 0.50 ± 0.1 nM.
Kd = 0.85 ± 0.2 nM.
Logarithmic values determined from the non-linear regression analysis of data collected from at least 2 independent experiments.
Caculated using the Cheng and Prusoff equation to correct for the concentration of the radioligand used in the assay.
Logarithmic values determined from the non-linear regression analysis of data collected from at least 2 independent experiments. The EC50 value is converted from the respective log EC50.
[35S]GTP-γ-S bound / basal [35S]GTP-γ-S bound] X 100 ± standard error. n.c. incomplete competition of the radioligand at the highest test concentration of the peptide. An IC50 value cannot be determined. n.s. non-saturable dose-response curve. In these cases,stimulation of [35S]GTP-γ-S binding was observed only at the highest test dose(s) of the peptide (1 ×10−5 and 1 × 10−4 M). An EC50 or Emax value cannot be determined. n.d. not determined.
Table 3.
In-Vitro Functional Activity of CCK/Opioid Peptides at the MVD and GPI
| opioid, IC50/nMa | ||
|---|---|---|
| No. | ||
| MVD (δ) | GPI (µ) | |
| 1 | 0.45±0.06 | 63±5.5 |
| 2 | 120±7.5 | 280±9.1 |
| 3 | 9.50±1.8 | 150±24 |
| 4 | 15±2.3 | 2900±81 |
| 5 | 2400±500 | 18.6% at 1µM |
| 6 | 27% at 1 µM | 5% at 1 µM |
| RSA504 | 23±9.7 | 210±52 |
| SNF9007 | 29±10 | 220±81 |
| Biphalin | 2.7±1.5 | 8.8±0.3 |
Concentration at 50% inhibition of muscle concentration at electrically stimulated isolated tissue. n.d.: not determined.
Table 4.
Functional Activity of CCK/Opioid Peptides at the MVD and GPI
| Opioid Agonist,IC50 (nM) | CCK activity at GPIa | ||||
|---|---|---|---|---|---|
| No. | Peptide | MVD(δ) | GPI(µ) | CCK-AGONIST(A50, nM) | CCK-ANTAGONIST (Ke,nM), in absenceof naloxone |
| Asp-Tyr-D-Phe-Gly-Trp-MeNle-Asp-Phe-NH2 | 58 | 180 | IA | 31 | |
| Tyr-D-Nle-Gly-Trp-Nle-Asp-Phe-NH2 | 23±9.7 | 210±52 | IA | 190±80 | |
| 1 | Tyr-c[D-Cys-Gly-Trp-Cys]-Asp-Phe-NH2 | 0.45±0.06 | 63±5.5 | IA | 7.6±2.3 |
| 2 | Tyr-c[D-Cys-Gly-Trp-Cys]-Asp-Phe-NH2 | 120±7.5 | 280±9.1 | IA | 23±9.1 |
| 3 | Tyr-c[D-Pen-Gly-Trp-Cys]-Asp-Phe-NH2 | 9.5±1.78 | 150±24.4 | IA | 19±6.7 |
| 4 | Tyr-c[D-Cys-Gly-Trp-Pen]-Asp-Phe-NH2 | 15±2.3 | 2900±80.7 | IA | 24±7.8 |
| 5 | Tyr-c[D-Cys-Gly-D-Trp-Cys]-Asp-Phe-NH2 | 2400±493 | 18.6% at 1uM | IA | 150 ±58 |
| 6 | Tyr-c[D-Cys-Gly-D-Trp-Pen]-Asp-Phe-NH2 | 27% at 1µM | 5% at 1uM | IA | IA |
IA, inactive with 0% response at 1 µM.
In the presence of naloxone, the compounds were inactive as antagonists.
Table 5.
Binding Affinities and Functional Activities of the CCK/Opioid Peptides at the CCK-1 and CCK-2 Receptors
| Binding Affinity, [125I]CCK-8(SO3)b | PI assay | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peptidea | hCCK-1c | hCCK-2c | hCCK-1d | hCCK-2d | ||||||
| LogIC50e | Ki/nMf | LogIC50e | Ki/nMf | LogEC50g | EC50/nM | Emaxh | LogEC50g | EC50/nM | Emaxh | |
| 1 | 32,000 | nc | nr | -- | nr | -- | ||||
| 2 | 2,500 | 4,900 | nr | -- | nr | -- | ||||
| 3 | >10,000 | >10,000 | nr | -- | nr | -- | ||||
| 4 | nc | nc | nr | -- | 5500 | -- | ||||
| 5 | 2,300 | >10,00 | ns | -- | 16,000 | 3.8 | ||||
| 6 | nc | nc | nr | -- | nr | -- | ||||
| RSA504 | 11.2 | 16 | 2540 | 15 | 1930 | 10.1 | ||||
| CK8 (SO3) | n.d. | - | n.d. | - | −7.55±0.15 | 28 | 59±3.0 | −7.57±0.24 | 27 | 16±1. |
Refer to Table 1 for the structure of the peptides.
Kd = 1.9 ± 0.2 nM for hCCK-1; Kd = 1.3 ± 0.4 nM for hCCK-2.
Carried out using whole cell lysate preparations from transfected HEK 293 cells that constitutively expressed the respective receptor type.
Carried out on hCCK-1 or hCCK-2 expressing cells cultured in 24 well titer plates.
Logarithmic values determined from the non-linear regression analysis of data collected from at least 2 independent experiments.
Anti-logarthmic value of the respective IC50.
Logarithmic values determined from the non-linear regression analysis of data collected from at least 2 independent experiments. The EC50 value is converted from the respective log EC50.
Saturable, maximum level of [3H]inositol phosphates produced in the cells upon incubation with the peptide, expressed as a ratio ± standard error of the amount of [3H]inositol phosphates produced in the presence of peptide over the basal level of [3H]inositol phosphates in untreated cells. n.c. incomplete competition of the radioligand at the highest test concentration of the peptide. An IC50 value cannot be determined. n.s. non-saturable dose-response curve. In these cases, stimulation of [3H]phosphotidylinositol hydrolysis was observed only at the highest test dose(s) of the peptide (1 ×10−5 and 1 ×10−4 M). An EC50 or Emax value cannot be determined. n.r. no stimulatory (or inhibitory) response at the highest test concentration of the peptide. n.d. not determined.
2.5 NMR studies
Peptide solution was prepared at a concentration of ca. 9.6 mM of the peptide (Tyr-c[D-Cys-Gly-Trp-Cys]-Asp-Phe-NH2) in DMSO-d6(Cambridge Isotopes). All NMR spectra were recorded on a Bruker Avance 600 MHz spectrometer. Proton spectra were obtained at 290, 293, and 298K. TOCSY[6] and NOESY [23] spectra were recorded in phase sensitive mode at 290K with mixing times of 62 ms and 150ms, respectively. The TOCSY experiment was performed with 4096 data points in F2 and 512 data points in F1, and 8–16 scans were collected at each increment. The NOESY experiment was performed with 1048 data points in F2 and 512 data points in F1, and 16–64 scans were collected at each increment. 1H-NMR chemical shifts were reported relative to internal DMSO peak at 2.49 ppm. The qualitative and quantitative analyses of TOCSY and NOESY spectra were obtained using the program package FELIX2000. The NOE-based distance restraints were obtained from the NOESY spectra collected with a mixing time of 150 ms. The NOE cross-peaks were integrated with the FELIX2000 program and they were converted into upper distance bounds. Restrained molecular dynamics was used to refine the structures initially obtained with the hybridized distance geometry and simulated annealing protocol in DGII program within the Insight II (Accelrys Inc., San Diego) software package on a Silicon Graphics Octane computer. One hundred structures were generated and were refined using simulated annealing AMBER force field in the Discover module of Insight II. The iterative calculations were performed until distance violations did not exceed 0.16 Å. CCK-8 structure at CCK-A receptor was obtained from the Protein Databank with code 1D6G [40].
3. Results
3.1 Structure-function activity relationships at opioid and CCK receptors
When compared to the linear analogues most of the cyclic disulfide analogues had significantly increased binding affinities at both δ and µ opioid receptors in the low nanomolar range (Table 2). The cyclic [D-Cys2, Cys5] analogue 1 had significantly increased affinity to impressive nanomolar range for δ (Ki = 0.2 nM) and µ (Ki = 2.2 nM) opioid receptors with a nearly a ten fold selectivity for the δ opioid receptor. However, when a D-Cys was substituted at position 5 to produce the cyclic [D-Cys2, D -Cys5] analogue 2, the binding affinity slightly decreased compared to 1, though it still had high affinity, at δ (Ki = 3.8 nM) and µ (Ki = 9.2 nM) opioid receptors. To introduce more constraint to the cyclic disulfide bridge, D-Pen or Pen were substituted at positions 2 or 5 to produce the cyclic analogue 3 and the cyclic [D-Cys2, Pen5] analogue 4, respectively. Substitution of D-Pen2 in the N-terminal end gave 3 which had a binding affinity that was very high at the hDOR δ (Ki = 0.3 nM), while the binding affinity was quite poor at the rMOR (Ki = 1300 nM). Surprisingly, when the bulkier Pen residue was on the C-terminal end in 4, the binding affinity was very potent both at δ (Ki = 2.6 nM) and µ (Ki = 1.4 nM) opioid receptors, with a slight preference for the µ receptor. In an attempt to introduce antagonist property at CCK receptors, the D-Trp was substituted at position 4 producing analogues c[D-Cys2, D-Trp4, Cys5] 5 and c[D-Cys2, D-Trp4, Pen5] 6. However, 5 and 6 resulted in a significant loss in binding affinities at both δ and µ opioid receptors when compared to the other cyclic disulfide analogues.
In the GTP-γ-S assays (Table 2), 1 and 2 had moderate activities at the hDOR (EC50 =780 and 200 nM, respectively, and good activities at the rMOR (EC50 = 42 and 25 nM, respectively). When Pen residues were substituted in 3 and 4, the activities at the hDOR increased (EC50 = 14.4 nM and 1.8 nM). At the rMOR, the activity of 3 moderately decreased (EC50=111 nM) while 4 increased (EC50 = 1.7). When D-Trp was substituted in 6, the activities at the hDOR and rMOR decreased to the micromolar range (EC50 = 2000 and 5400 nM, respectively).
In the in vitro assays (Table 3), the cyclic [D-Cys2,Cys5] 1 showed excellent agonist activity in the MVD (IC50 = 0.45 nM) while maintaining good agonist activity in the GPI (IC50 = 63 nM). When both positions 2 and 5 were substituted with D-Cys resulting in the cyclic analogue 2, the agonist activities significantly decreased in the MVD (IC50 = 120 nM) and GPI (IC50 = 280 nM) when compared to 1. When D-Pen or Pen residues were substituted at positions 2 or 5 to produce analogues 3 and 4, respectively, the bioactivity of both 3 and 4 were good in the MVD (IC50 = 9.5 and 15 nM, respectively). The agonist properties of 3 and 4, however, were less potent in the GPI (IC50 = 150 and 2900 nM, respectively). When D-Trp was substituted at position 4 in cyclic analogues 5 and 6, there was a significant loss in bioactivities in the MVD and GPI to the high micromolar range and some with a partial response at 1 µM.
In the binding affinity and functional assays at the cloned CCK receptors (Table 5), the cyclic disulfide analogues 1 – 6 showed a significant loss in binding affinities when compared to the linear compounds (Table 5). These cyclic disulfide analogues showed very poor CCK agonist and antagonist properties against sulfated CCK-8 in the unstimulated GPI/LMMP (Table 4) assays.
4. Discussion
4.1 CCK/opioid cyclic analogues 1–4 are potent opioid ligands
Analogues 1 – 6 cyclic compounds are conformationally restricted by virtue of their 14-membered [i, i+3] disulfide containing rings. The Pen-containing analogues are further constrained by the β,β – dimethyl substituents. As mentioned in the results section, the cyclic disulfide analogues 1 – 6 produced potent agonist properties in δ and µ opioid receptors both in GTP-γ-S binding assays and in the MVD and GPI functional assays, and were potent antagonist CCK activity in the MVD and GPI in vitro tissue assays. Since the design of these disulfide analogues were based on very potent δ opioid ligand DPDPE [29] the potent activities at the MVD was expected. Furthermore, CCK/opioid analogues 1 – 4 have similar bioactivities and selectivity at the MVD and GPI, when compared to the corresponding cyclic disulfide enkephalin or enkephalinamide analogues [29, 30, 42].
Furthermore and surprisingly, these novel cyclic disulfide CCK/opioid analogues retained potent bioactivities in the MVD and GPI even when position 4 is a Trp, which is a bulkier aromatic residue than Phe. In fact, analogue 1 is six time more potent than DPDPE. This increased potency at the MVD is consistent with the observations when bulkier analogues, such as Nal [26] and para-substituted Phe [7, 19] are substituted in position 4 of enkephalin analogues.
Moreover, these cyclic CCK/opioid analogues have a unique “address” sequence at the C-terminal end of the peptide for the opioid receptors [44]. Thus, these CCK/opioid cyclic disulfide analogues can be compared to a series of highly δ receptor selective ligands in which a Phe6 has been added to c[D-Pen2, L-Cys5]-enkephalin [5]. When compared, the charged side chain residue of the -Asp6-Phe7-NH2 does not seem to significantly affect potency at the µ opioid receptors at the GPI, but losses some potencies at the δ opioid receptors at the MVD.
D-Trp was introduced again in the cyclic disulfide as an approach to make the CCK activity to that of a pure antagonist. While substitution of D-Trp at position 4 was an effective strategy in introducing antagonist activity in with the linear series, it was not an effective approach with the cyclic disulfide analogues. Substitution of D-Trp4 resulted to 5 and 6 which had a dramatic decrease in the agonist bioactivity at the δ and µ opioid receptors. This drop in potency is consistent with the poor potency of (2R, 3S) and (2R, 3S)-βMePhe analogues of DPDPE in which the potency dropped significantly in the MVD and GPI [7]. This loss in potency was somewhat expected, since the substitution of D-Trp only induced the antagonist property of CCK receptors when position 5 is NMeNle but not when position 5 is Nle. In these cyclic disulfide analogues, position 5 is not N-methylated (i.e. position 5 is not NMeCys or NMePen). In the case of analogues 5 and 6, the local constraint and conformations of positions 4 and 5 may have a greater role in the bioactive conformation than the global constraint introduced by the side chain to side chain disulfide [i,i+3] cyclization.
4.2 Compound 1 has unique conformational features as an opioid ligand
The NMR studies of 1 were done in DMSO-d6, primarily because 1 is not very soluble in aqueous solvents. DMSO is a widely used solvent in NMR studies of peptides, including enkephalins and CCK, because its biophysical properties are comparable to extracellular aqueous solutions. In addition, a comparative analysis of NMR studies of CCK in DMSO and aqueous solvents (and other mixtures of solvents) showed that different solvents do not significantly modify the conformation of CCK [3] and similar findings were made for DPDPE [11, 32].
The 1H chemical shift assignments for compound 1 (RSA101c) were made utilizing 2D NMR experiments and the values are summarized in Table 6. The intraresidue geminal (2J) and vicinal (3J) are provided in the Supporting Information. Compound 1 is a cyclized peptide resulting to limited conformational freedom for its chemical moieties. Analysis of the nonequivalent Gly may provide information regarding the surrounding environment of these protons. The two nonequivalent Gly Hs of compound 1 have chemical shifts of 4.31 and 3.24 ppm, a difference of 1.07 ppm. Previous studies of DPDPE had reported a chemical shift difference between the two nonequivalent Gly Hαs of DPDPE, in H2O/D2O, to be 0.80 ppm [21] and, in DMSO, 1.19 ppm [32], while other analogues of DPDPE had 0.45 – 0.84 ppm differences [20, 31]. As for the four stereoisomers of TMT-DPDPE in DMSO, the chemical shift difference between the two nonequivalent Gly Hαs ranged from 1.05 – 1.18 ppm [41]. For DPDPE, it was reasoned that the bulky disulfide bond and bulky geminal dimethyl groups of the Pen residues tended to limit backbone flexibility, forcing the carboxyl groups away from the 14-membered ring. As a consequence of this, the Gly Hα was fixed in the deshielding zone of the carbonyl groups, leading to a strong non-equivalence. In the case of compound 1 (RSA101c) since the difference in chemical shift is about ca. 0.2 ppm greater than DPDPE, other factors may have contributed. It can be suggested that the aromatic side chain of Trp4, or even Phe7, may be oriented in such a way that that the ring current effect is deshielding one of the Gly Hα, causing a larger nonequivalence in chemical shift [11, 21, 31].
Table 6.
1H Chemical Shifts for Compound 1 (T = 298 K, DMSO-d6)
| Chemical shifts (ppm) | ||||||
|---|---|---|---|---|---|---|
| NH | Hα/Hα′ | Hβ | Hβ′ | H(Ar) | ||
| Tyr1 | 4.21 | 2.86 | 2.79 | 7.00, 6.67 | ||
| D-Cys2 | 8.62 | 4.78 | 3.04 | 2.73 | -- | |
| Gly3 | 8.75 | 4.31/3.24 | -- | -- | -- | |
| Trp4 | 8.78 | 4.48 | 3.15 | 2.95 | 7.12 ,7.30 ,7.04, 6.9, 7.53, 9.37 | |
| Cys5 | 7.51 | 4.47 | 2.94 | 2.89 | -- | |
| Asp6 | 8.42 | 4.48 | 2.69 | 2.42 | -- | |
| Phe7 | 8.02 | 4.31 | 3.03 | 2.81 | 7.30, 7.26, 7.19 | |
Temperature coefficients of the NH chemical shifts may provide insight to possible conformations stabilized by hydrogen bonds. Large values of this parameter (≥6 ppb/K) are generally indicative of exposure and proton exchange with the solvent, while small values (≤3 ppb/K) suggest inaccessibility to the solvent or participation in an intramolecular hydrogen bond. The Δδ/ΔT (-ppb/K) values for 1, as well as comparative data for DPDPE and non-sulfated Tyr CCK-8, are summarized in Table 7. The large temperature dependence for Gly3, Trp4 and Phe7 indicates that the amide protons in these peptides are exposed to solvent. These amide protons may not have a role in stabilizing a turn. The high temperature coefficient for Gly3 is surprisingly higher than the corresponding values for DPDPE and non-sulfated CCK-8. The temperature coefficient for D-Cys2 was moderate at 2.93 ppb/K. These values are within the range of DPDPE (2.6 to 5 ppb/K) [21]. For Cys5, the amide proton had a very small temperature coefficient (ca. 0 ppb/K). This may suggest that this amide proton had an intramolecular hydrogen bond, as seen typically in cyclic peptides. Also, this suggests that the Cys5 amide proton might be buried deep in the hydrophobic pocket of the cyclic peptide. This temperature coefficient is similar to the amide proton of D-Pen5 in DPDPE (0.9 ppb/K). The Asp6 amide proton with a temperature coefficient of 4.5 ppb/K might be involved in stabilizing the turn by hydrogen bonding. As mentioned before, 1 and DPDPE have similarly small coefficients for Cys5 and D-Pen5, respectively [15, 32]. On the contrary, because CCK-8 is a linear peptide[15], as expected, it had large temperature coefficients compared to 1. Similar observations and comparisons can be made with [β–MePhe4]DPDPE [33] and [βMePhe2,Nle5]SNF-9007 (CCK-8 analogue) [33].
Table 7.
Temperature Coefficients of NH protons for Compound 1in DSMO-d6.
| Δδ/ΔT (−pb/K) | |||
|---|---|---|---|
| Residues | |||
| Compound 1 | DPDPE | NS-CCK-8 | |
| Asp0 | -- | -- | -- |
| Tyr1 | -- | -- | 7.6 |
| D-Cys2/D-Pen/β-MePhe | 2.9 | 3.2 | 4.8 |
| Gly3 | 7.5 | 2.6 | 2.0 |
| Trp4/β-MePhe /Trp | 5.6 | 5.1 | 7.6 |
| Cys5/D-Pen/Nle | 0.0 | −0.3 | 8.8 |
| Asp6 | 4.5 | -- | 4.0 |
| Phe7 | 7.1 | -- | 4.8 |
DPDPE: Tyr-c[D-Pen-Gly-Phe-D-Pen]-OH,21
NS-CCK-8: Asp-Tyr-Met-Gly-Trp-Met-Asp-Phe-NH215
The coupling constants 3JHαNH and 3JHαβ are summarized in Table S2. These coupling constants are related to the backbone conformation and the mean orientation of the side chain groups. The population percentages of the three staggered rotamers around the Cα−Cβ bond were calculated with the Pachler parameters [37, 38]. For aromatic side chain groups (Tyr, Trp, Phe) different parameters were used [12]. The calculated rotamer populations for gauche (−) and trans were assigned based on the stereospecific assignment of the Hβs, using 2D-NMR experiments [25]. The calculated values are summarized in Table 8.
Table 8.
Calculated Side Chain Rotamer Populations (%) about the Cα−Cβ bond (χ1) in Compound 1
| Rotamer populations (%) | |||
|---|---|---|---|
| gauche (−) | trans | gauche (+) | |
| Tyr (1) | 35* | 34* | 31 |
| D-Cys (2) | 15 | 85 | 0.00 |
| Trp (4) | 60 | 15 | 25 |
| Cys (5) | 84 | 9.0 | 7 |
| Asp (6) | 32 | 46 | 22 |
| Phe (7) | 57 | 10 | 33 |
Rotamer populations were calculated from the measured homonuclear coupling constants using the Pachler equations. Pg(−) = (JHαHβ(Pro-R) – scJHαHβ)/(apJHαHβ - scJHαHβ; Pt = (JHαHβ(Pro-S) – scJHαHβ)/(apJHαHβ-scJHαHβ; Pg(+) = 1 – Pg(−) – Pt. For the aliphatic residues, the values of scJHαHβ=2.6 Hz and apJHαHβ = 13.6 Hz were used. For the aromatic residues, the values of scJHαHβ=3.55 Hz and apJHαHβ= 13.9 Hz were used. Gauche(−) and trans conformers were assigned by 2D-NMR methods as described in Kövér et. al. [23][24].
Cannot distinguish between gauche (−) and trans rotamers because the β,β’ could not be stereochemically assigned.
Tyr1 and Trp4 of 1 had significant gauche (+) conformations at 30 and 25%, respectively. These values are consistent with the gauche (+) conformations of Tyr2 and Trp4 residues of the [β–MePhe2]CCK-8 analogue [24]. For the (2R,3R)-[β–MePhe2,Nle5]SNF-9007, the gauche (+) populations were 28 and 22% for Tyr and Trp, respectively. For (2R,3S)-[β–MePhe2,Nle5]SNF-9007, the gauche (+) populations were 18% and 27%. For a more meaningful comparison to reported rotamer populations of the aromatic residues, the rotamer populations for 1 were calculated using only the Pachler parameters, which treats all residues the same (i.e. there are no correction values for aromatic residues. These values and the literature values for analogous peptides are summarized in Table 9.
Table 9.
Calculated Rotamer Populations for Tyr and Trp of Analogous Peptides in DMSO-d6.
| Rotamer | Compound 1a | DPDPEb | DPDPEc | CCK-7(ns)d | |
|---|---|---|---|---|---|
| Gauche (−)e | 42 | 39 | 27.0(73.0) | 25 | |
| Tyr | Transe | 41 | 60 | 73.0(27.0) | 58 |
| Gauche (+) | 17 | 3 | 0 | 17 | |
| Gauche (−)e | 65 | 69 | 73.0(27.0) | 20 | |
| Trp/Phe | Transe | 23 | 17 | 27.0(73.0) | 63 |
| Gauche (+) | 11 | 14 | 0 | 17 | |
The 17% gauche (+) conformation for Tyr1 of 1 is important because it is in great contrast to the gauche (+) conformations of the corresponding Tyr in DPDPE. In DPDPE, Tyr and Phe had little or no contribution from the gauche (+) conformation [21]. In another DPDPE study, Tyr1 had 1% gauche (+) conformation [32]. In c[D-Cys2, Cys5]-enkephalinamide, Tyr1 had 4% gauche (+) conformation [31]. The up to 5-fold difference in the gauche (+) population is unexpected since DPDPE and compound 1 have similar structures; both are 14-membered cyclic disulfides with aromatic residues at positions 1 and 4. Furthermore, a comparison against the potent (2S,3S)-isomer of [β-Me-p-NO2Phe4]DPDPE with 10% gauche (+) population for Tyr, also can be made [22, 45]. In this case, the increase in the gauche (+) may be a result of a substitution of a constrained aromatic residue at position 4. Similarly, the increased stability of the gauche (+) conformation of Tyr in 1 may be attributed to the bulkier aromatic Trp and/or the C-terminal Asp6-Phe7-NH2 residues. This newly observed access to the gauche (+) conformation of the Tyr at position 1 of 1 offers new insight regarding receptor recognition and the unique biological activities at the opioid receptors and CCK receptors.
4.3 Conformation of lead cyclic peptide
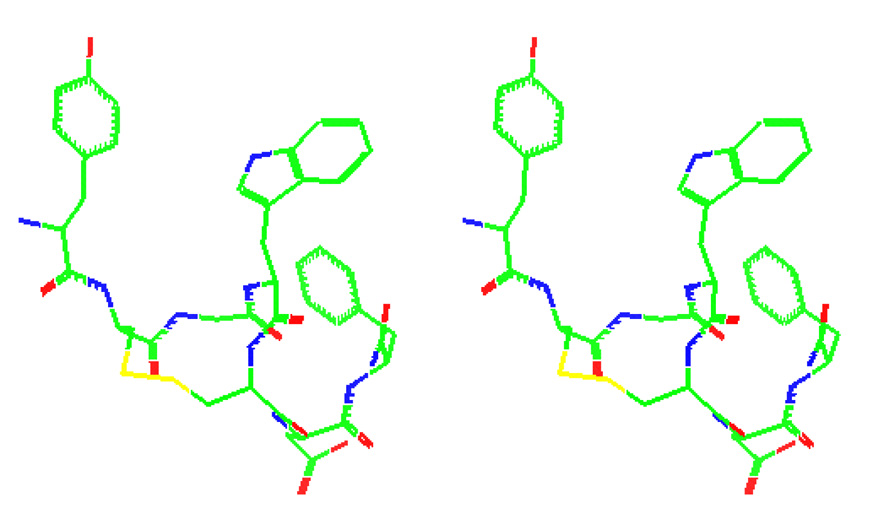
The structure of compound 1 was calculated using hybridized distance geometry and simulated annealing protocol. From the 2D-NMR experiments, 62 unique distance restraints, 2 dihedral angle restraints, and four stereospecifically assigned β-CH2 groups were determined and included throughout the structure refinement process. The top 20 structures with the lowest restraint energy were selected to represent the solution structures of compound 1. In all structures, the disulfide bond was right-handed. The lowest energy structure of compound 1 after MD simulation is depicted in Figure 2. The peptide backbone forms two consecutive turns making a helical-like structure. The same turn patterns were observed in previous NMR studies with CCK-7. In those reports, the primary sequence of CCK-8 was sufficient to form β- and γ-turns around the Gly-Trp-Met-Asp and Met-Asp-Phe-NH2 sequences. Furthermore, the backbone is folded such that the Tyr1 and Trp4 aromatic rings were closely oriented, which was supported by the observed NOEs between protons in these 2 aromatic rings. The distance between the centroids of Tyr and Trp was measured to be 7.4 Å. The close proximity of the aromatic rings of Tyr and Trp was also observed in NMR studies of CCK-8 [3]. In that study, Tyr and Trp were reported to be perpendicular to each other, in an edge-to-face orientation which is caused by the electrostatic attraction between the positively charged protons of Tyr and the negatively charged carbon atoms on Trp. Interestingly, this observation of close proximity of the aromatic residues at position 1 and 4 is consistent with the observed interaction of hydrophobic side chain groups of Tyr1 and Phe4 in DPDPE [21]. However, fluorescence studies of CCK-8, CCK-7 (ns), and CCK had suggested that Tyr and Trp are far apart (R = 15 Å) [15, 43]. The global constraint introduced to 1 by disulfide bridge may have prevented a dynamic interaction between Tyr and Trp, which may have contributed to the detrimental binding to CCK receptors.
Figure 2.
The stereoview of the lowest energy NMR structure of compound 1.
4.4 Compound 1 has common structural features to CCK-8
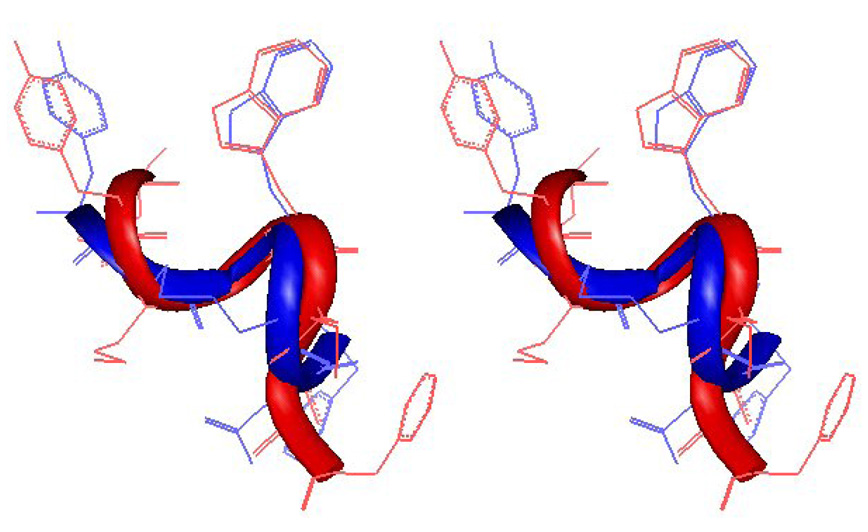
The NMR structure of the extensive NOE-restrained molecular dynamics simulation of compound 1 was compared to the reported structure of CCK-8 at CCK-A receptor. The backbone of lowest energy structure of compound 1 turn residues (D-Cys2-Gly3-Trp4-Cys5) can be superimposed reasonably well (rmsd 1.13 Å, see Figure 3) onto that of the corresponding residues (Met3-Gly4-Trp5-Met6) of CCK-8 bound with the third extracellular loop of the human CCK-2 receptor [39]. Coincidentally and interestingly, the spatial orientations of the Trp side chain groups of both peptides were very similar to each other. Figure 3 supports our hypothesis that, at least in part of the residues 1–4, opioid and CCK ligands have overlapping pharmacophores. However, despite the similarities of the residues in the cyclic peptide, the exocyclic residues Asp6 and Phe7 of compound 1 do not overlap well with backbone and side chain groups of the corresponding residues in CCK-8. The turns in these residues are obviously different, orienting the side chain groups differently. This difference in the C-terminal structure of compound 1 may account for the lack of potent binding affinities and activities at the CCK receptors.
Figure 3.
Superimposed structures of compound 1 (blue) and CCK-8 (red) [40]. The two structures have similar turns along the backbone between residues 1 – 4 and similar orientations of Tyr and Trp residues. The C-terminal Asp-Phe residues have different orientations.
5. Conclusions
Conformationally constrained cyclic disulfide analogues of the CCK/opioid peptides were designed and synthesized in our attempt to make a compound that simultaneously interacts with opioid receptors as agonists and CCK receptors as antagonists. While the cyclic analogues maintained strong interactions with δ and µ opioid receptors, these constrained peptides showed decreased interaction with CCK-1 and CCK-2 receptors and no CCK antagonist properties. Among the cyclic analogues, compound 1 displayed the highest potency at opioid receptors in the MVD and GPI tissue assays, with very good selectivity for the δ opioid receptor. The NMR structure of 1 was determined and it showed similar structural features to both opioid and CCK receptor ligands at the N-terminal side. However, disulfide cyclization may have introduced a tight, unfavorable constrain at the C-terminal side that have resulted to poor interaction with CCK receptors. Cyclization with a cyclic lactam may be more favorable for CCK receptor interaction because the ring size is bigger and more flexible.
Supplementary Material
One table with analytical data of the peptide ligands; one table with the J coupling constants; one table with the chemical shift difference between the two Hα protons of Gly; One figure showing the centroid distance between Tyr1 and Trp4 in the NMR structure of compound 1.
Acknowledgments
This research was supported in part by grants from the USPHS, National Institute of Drug Abuse, DA12394 and DA06284, and from University of Arizona NMR grant (NSF 9729350). K.E.K. was supported by a grant from National Science Foundation and the Hungarian Scientific Research Fund, OTKA NK-68578. The opinions expressed are those of the authors and not necessarily those of NIDA. Authors are thankful to Ms. Margie Colie for her assistance with the manuscript.
A List of Abbreviations
- BuOH
butanol
- Bzl
benzyl
- CCK
cholecystokinin
- CHO
Chinese hamster ovary
- DIEA
N,N-diispropylethylamine
- DMF
N,N-dimethylformamide
- DMSO
dimethylsulfoxide
- hDOR
human δ opioid receptor
- DPDPE
c[D-Pen2, D-Pen5]-enkephalin
- DAMGO
[D-Ala2, NMePhe4, Gly5-ol]-enkephalin
- GPCR
G-protein coupled receptor
- GPI
guinea pig isolated ileum
- HEK
human embryonic kidney
- HOBt
1-hydroxybenzotriazole
- LMMP
longitudinal muscle with myenteric plexus
- rMOR
rat µ opioid receptor
- MVD
mouse vas deferens
- NMM
N-methylmorpholine
- Pen
penicillamine
- PI
phosphoinositol
- SAR
structure-activity relationships
- TFA
trifluoroacetic acid
- TIS
triispropylsilane
- TMP
trimethylpyridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agnes RS, Lee YS, Davis P, Ma S-W, Badghisi H, Porreca F, Lai J, Hruby VJ. Structure-activity relationships of bifunctional peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. J Med Chem. 2006;49:2868–2875. doi: 10.1021/jm050921q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albericio F, Hammer RP, Garcia-Echeverria C, Molins MA, Chang JL, Munson MC, Pons M, Giralt E, Barany G. Cyclization of disulfide-containing peptides in solid-phase synthesis. Int J Pept Protein Res. 1991;37:402–413. doi: 10.1111/j.1399-3011.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 3.Albrizio S, Carotenuto A, Fattorusso C, Moroder L, Picone D, Temussi PA, D'Ursi A. Environmental mimic of receptor interaction: conformational analysis of CCK-15 in solution. J Med Chem. 2002;45:762–769. doi: 10.1021/jm0109457. [DOI] [PubMed] [Google Scholar]
- 4.Angell YM, Alsina J, Albericio F, Barany G. Practical protocols for stepwise solid-phase synthesis of cysteine-containing peptides. J Peptide Res. 2002;60:292–299. doi: 10.1034/j.1399-3011.2002.02838.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartosz-Bechowski H, Davis P, Slaninova J, Malatynska E, Stropova D, Porreca F, Yamamura HI, Hruby VJ. Cyclic enkephalin analogs that are hybrids of DPDPE-related peptides and metenkephalin-Arg-Gly-Leu: prohormone analogs that retain good potency and selectivity for delta opioid receptors. J Pept Res. 1999;53:329–336. doi: 10.1034/j.1399-3011.1999.00033.x. [DOI] [PubMed] [Google Scholar]
- 6.Bax A, Davis DG. MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J Magn Reson. 1985;65:355–360. [Google Scholar]
- 7.Boteju LW, Nikiforovich GV, Haskell-Luevano C, Fang SN, Zalewska T, Stropova D, Yamamura HI, Hruby VJ. The use of topographical constraints in receptor mapping: investigation of the topographical requirements of the tryptophan 30 residue for receptor binding of Asp-Tyr-D-Phe-Gly-Trp-(N-Me)Nle-Asp-Phe-NH2 (SNF 9007), a cholecystokinin (26–33) analogue that binds to both CCK-B and delta-opioid receptors. J Med Chem. 1996;39:4120–4124. doi: 10.1021/jm960078j. [DOI] [PubMed] [Google Scholar]
- 8.Buscher HH, Hill RC, Romer D, Cardinaux F, Closse A, Hauser D, Pless J. Evidence for analgesic activity of enkephalin in the mouse. Nature. 1976;261:423–425. doi: 10.1038/261423a0. [DOI] [PubMed] [Google Scholar]
- 9.Charpentier B, Dor A, Roy P, England P, Pham H, Durieux C, Roques BP. Synthesis and binding affinities of cyclic and related linear analogs of CCK8 selective for central receptors. J Med Chem. 1989;32:1184–1190. doi: 10.1021/jm00126a007. [DOI] [PubMed] [Google Scholar]
- 10.Charpentier B, Pelaprat D, Durieux C, Dor A, Reibaud M, Blanchard JC, Roques BP. Cyclic cholecystokinin analogs with high selectivity for central receptors. Proc Natl Acad Sci U S A. 1988;85:1968–1972. doi: 10.1073/pnas.85.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins N, Flippen-Anderson JL, Hasseth RC, Deschamps JR, George C, Kover KE, Hruby VJ. Conformational determinants of agonist versus antagonists properties of [D-Pen2, D-Pen5]enkephalin (DPDPE) analogs at opioid receptors. Comparison of X-ray crystallographic structure, solution 1H NMR data, and molecular dynamic simulations of [L-Ala3]DPDPE and [D-Ala3]DPDPE. J Am Chem Soc. 1996;118:2143–2152. [Google Scholar]
- 12.Cung MT, Marraud M. Conformational dependence of the vicinal proton coupling constant for the Ca−Cb bond in peptides. Biopolymers. 1982;21:953–967. [Google Scholar]
- 13.De Leeuw FA, Altona C. Component vicinal coupling constants for calculating side-chain conformations in amino acids. Int J Pept Protein Res. 1982;20:120–125. doi: 10.1111/j.1399-3011.1982.tb02663.x. [DOI] [PubMed] [Google Scholar]
- 14.Faris PL, Komisaruk BR, Watkins LR, Mayer DJ. Evidence for the neuropeptide cholecystokinin as an antagonist of opiate analgesia. Science. 1983;219:310–312. doi: 10.1126/science.6294831. [DOI] [PubMed] [Google Scholar]
- 15.Fournie-Zaluski MC, Belleney J, Lux B, Durieux C, Gerard D, Gacel G, Maigret B, Roques BP. Conformational analysis of cholecystokinin CCK(26–33) and related fragments by 1H NMR spectroscopy, fluorescence-transfer measurements, and calculations. Biochemistry. 1986;25:3778–3787. doi: 10.1021/bi00361a008. [DOI] [PubMed] [Google Scholar]
- 16.Greene DL, Hau VS, Abbruscato TJ, Bartosz H, Misicka A, Lipkowski AW, Hom S, Gillespie TJ, Hruby VJ, Davis TP. Enkephalin analog prodrugs: assessment of in vitro conversion, enzyme cleavage characterization and blood-brain barrier permeability. J Pharmacol Exp Therap. 1996;277:1366–1375. [PubMed] [Google Scholar]
- 17.Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–1989. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- 18.Hruby VJ, Agnes RS. Conformation-activity relationships of opioid peptides with selective activities at opioid receptors. Biopolymers. 1999;51:391–410. doi: 10.1002/(SICI)1097-0282(1999)51:6<391::AID-BIP3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Hruby VJ, Bartosz-Bechowski H, Davis P, Slaninova J, Zalewska T, Stropova D, Porreca F, Yamamura HI. Cyclic enkephalin analogues with exceptional potency and selectivity for delta-opioid receptors. J Med Chem. 1997;40:3957–3962. doi: 10.1021/jm9704762. [DOI] [PubMed] [Google Scholar]
- 20.Hruby VJ, Fang S, Toth G, Jiao D, Matsunaga TO, Collins N, Knapp R, Yamamura HI. Highly potent and selective cholecystokinin analogs for the CCK-B receptor; Pept 1990, Proc Eur Pept Symp; 1991. pp. 707–709. [Google Scholar]
- 21.Hruby VJ, Kao L-F, Montgomery Pettitt B, Karplus M. The conformational properties of the delta opioid peptide cyclic [D-Pen2,D-Pen5]enkephalin in aqueous solution determined by NMR and energy minimization calculations. J Am Chem Soc. 1988:3351–3359. [Google Scholar]
- 22.Hruby VJ, Toth G, Gehrig CA, Kao LF, Knapp R, Lui GK, Yamamura HI, Kramer TH, Davis P, Burks TF. Topographically designed analogs of cyclic-[D-Pen2,D-Pen5] enkephalin. J Med Chem. 1991;34:1823–1830. doi: 10.1021/jm00110a010. [DOI] [PubMed] [Google Scholar]
- 23.Jeener J, Meyer BH, Bachman P. Ernst RR. Investigation of exchange processes by two-dimensional NMR spectroscopy. J Chem Phys. 1979;71:4546–4553. [Google Scholar]
- 24.Kövér KE, Jiao D, Fang S, Hruby VJ. Conformational properties of the unnatural amino acid beta-methylphenylalanine in a linear octapeptide system; correlations of 13C-NMR chemical shifts with the side-chain stereochemistry of these amino acid residues. J Org Chem. 1994;59:991–998. [Google Scholar]
- 25.Kövér KE, Jiao D, Fang S, Hruby VJ. Conformational analysis of four beta-methlphenylalanine stereoisomers in a bioactive peptide by z-filtered relay NMR spectroscopy. Mag Reson Chem. 1993;31:1072–1076. [Google Scholar]
- 26.Mierke DF, Said-Nejad OE, Schiller PW, Goodman M. Enkephalin analogs containing beta-naphthylalanine at the fourth position. Biopolymers. 1990;29:179–196. doi: 10.1002/bip.360290123. [DOI] [PubMed] [Google Scholar]
- 27.Misicka A, Hruby VJ. Optimization of disulfide bond formation. Polish J of Chem. 1994;68:893–899. [Google Scholar]
- 28.Morgan BA, Smith CF, Waterfield AA, Hughes J, Kosterlitz HW. Structure-activity relationships of methionine-enkephalin. J Pharm Pharmacol. 1976;28:660–661. doi: 10.1111/j.2042-7158.1976.tb02827.x. [DOI] [PubMed] [Google Scholar]
- 29.Mosberg HI, Hurst R, Hruby VJ, Galligan JJ, Burks TF, Gee K, Yamamura HI. Conformationally constrained cyclic enkephalin analogs with pronounced delta opioid receptor agonist selectivity. Life Sci. 1983;32:2565–2569. doi: 10.1016/0024-3205(83)90239-4. [DOI] [PubMed] [Google Scholar]
- 30.Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF. Bispenicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosberg HI, Schiller PW. 1H N.M.R. investigation of conformational features of cyclic enkephalinamide analogs. Int J Pept Protein Res. 1984;23:462–466. doi: 10.1111/j.1399-3011.1984.tb02746.x. [DOI] [PubMed] [Google Scholar]
- 32.Mosberg HI, Sobczyk-Kojiro K, Subramanian P, Crippen GM, Ramalingam K, Woodard RW. Combined use of stereospecific deuteration, NMR, distance geometry, and energy minimization for the conformational analysis of the highly delta-opioid receptor selective peptide [D-Pen2,D-Pen5]enkephalin. J Am Chem Soc. 1990;112:822–829. [Google Scholar]
- 33.Nikiforovich GV, Hruby VJ. Models for the A-and B-receptor-bound conformations of CCK-8. Biochem Biophys Res Commun. 1993;194:9–16. doi: 10.1006/bbrc.1993.1777. [DOI] [PubMed] [Google Scholar]
- 34.Noble F, Roques BP. CCK-B receptor: chemistry, molecular biology, biochemistry and pharmacology. Prog Neurobiol. 1999;58:349–379. doi: 10.1016/s0301-0082(98)00090-2. [DOI] [PubMed] [Google Scholar]
- 35.Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, Roques BP. International Union of Pharmacology. XXI. structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev. 1999;51:745–781. [PubMed] [Google Scholar]
- 36.Ossipov MH, Porreca F. Challenges in the development of novel treatment strategies for neuropathic pain. NeuroRx. 2005;2:650–661. doi: 10.1602/neurorx.2.4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pachler KGR. Nuclear magnetic resonance (N.M.R.) study of some a-amino acids. I. Coupling constants in alkaline and acidic medium. Spectrochim Acta. 1963;19:2085–2092. [Google Scholar]
- 38.Pachler KGR. Nuclear magnetic resonance study of some a-amino acids. II. Rotational isomerism. Spectrochim Acts. 1964;20:581–587. [Google Scholar]
- 39.Pellegrini M, Bisello A, Rosenblatt M, Chorev M, Mierke DF. Conformational studies of RS-66271, an analog of parathyroid hormone-related protein with pronounced bone anabolic activity. J Med Chem. 1997;40:3025–3031. doi: 10.1021/jm970181o. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini M, Mierke DF. Molecular complex of cholecystokinin-8 and N-terminus of the cholecystokinin A receptor by NMR spectroscopy. Biochemistry. 1999;38:14775–14783. doi: 10.1021/bi991272l. [DOI] [PubMed] [Google Scholar]
- 41.Qian X, Shenderovich MD, Kövér KE, Davis P, Horváth R, Zalewska T, Yamamura HI, Porreca F, Hruby VJ. Probing the stereochemical requirements for receptor recognition of opioid agonists through topographic modifications in position 1. J Am Chem Soc. 1996;118:7280–7289. [Google Scholar]
- 42.Schiller PW, Eggimann B, DiMaio J, Lemieux C, Nguyen TMD. Cyclic enkephalin analogs containing a cystine bridge. Biochem Biophys Res Commun. 1981;101:337–343. doi: 10.1016/0006-291x(81)91265-1. [DOI] [PubMed] [Google Scholar]
- 43.Schiller PW, Natarajan S, Bodanszky M. Determination of the intramolecular tyrosine-tryptophan distance in a 7-peptide related to the C-terminal sequence of cholecystokinin. Int J Pept Protein Res. 1978;12:139–142. doi: 10.1111/j.1399-3011.1978.tb02877.x. [DOI] [PubMed] [Google Scholar]
- 44.Schwyzer R. Molecular mechanism of opioid receptor selection. Biochemistry. 1986;25:6335–6342. doi: 10.1021/bi00368a075. [DOI] [PubMed] [Google Scholar]
- 45.Shenderovich MD, Kover KE, Nikiforovich GV, Jiao D, Hruby VJ. Conformational analysis of beta-methyl-para-nitrophenylalanine stereoisomers of cyclo[D-Pen2, D-Pen5] enkephalin by NMR spectroscopy and conformational energy calculations. Biopolymers. 1996;38:141–156. doi: 10.1002/(sici)1097-0282(199602)38:2<141::aid-bip2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 46.Stanfa L, Dickenson A, Xu XJ, Wiesenfeld-Hallin Z. Cholecystokinin and morphine analgesia: variations on a theme. Trends in Pharmacol Sci. 1994;15:65–66. doi: 10.1016/0165-6147(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 47.Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 48.Watkins LR, Kinscheck IB, Kaufman EF, Miller J, Frenk H, Mayer DJ. Cholecystokinin antagonists selectively potentiate analgesia induced by endogenous opiates. Brain Res. 1985;327:181–190. doi: 10.1016/0006-8993(85)91512-4. [DOI] [PubMed] [Google Scholar]
- 49.Wiesenfeld-Hallin Z, de Arauja-Lucas G, Alster P, Xu XJ, Hokfelt T. Cholecystokinin/opioid interactions. Brain Res. 1999;848:78–89. doi: 10.1016/s0006-8993(99)01978-2. [DOI] [PubMed] [Google Scholar]
- 50.Wiesenfeld-Hallin Z, Xu XJ. The role of cholecystokinin in nociception, neuropathic pain and opiate tolerance. Regulatory Peptides. 1996;65:23–28. doi: 10.1016/0167-0115(96)00068-7. [DOI] [PubMed] [Google Scholar]
- 51.Wiesenfeld-Hallin Z, Xu XJ, Hokfelt T. The role of spinal cholecystokinin in chronic pain states. Pharmacol Toxicol. 2002;91:398–403. doi: 10.1034/j.1600-0773.2002.910619.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
One table with analytical data of the peptide ligands; one table with the J coupling constants; one table with the chemical shift difference between the two Hα protons of Gly; One figure showing the centroid distance between Tyr1 and Trp4 in the NMR structure of compound 1.