Introduction
Anorexia nervosa (AN) and bulimia nervosa (BN) are related disorders of unknown etiology that most commonly begin during adolescence in women (DSM-IV; Table 1[MSOffice1]). They are frequently chronic and often disabling conditions that are characterized by aberrant patterns of feeding behavior and weight regulation, and deviant attitudes and perceptions toward body weight and shape. In AN, an inexplicable fear of weight gain and unrelenting obsession with fatness, even in the face of increasing cachexia, accounts for a protracted course, extreme medical and psychological morbidity, and standardized mortality rates exceeding those of all other psychiatric disorders. BN usually emerges after a period of food restriction, which may or may not have been associated with weight loss. Binge eating is followed by either self-induced vomiting, or by some other means of compensation for the excess of food ingested. Although abnormally low body weight is an exclusion for the diagnosis of BN, some 25% to 30% of bulimics have a prior history of AN.
Table 1.
| DSM IV, Diagnostic Criteria for Anorexia Nervosa | |
|---|---|
| A | Refusal to maintain body weight at or above a minimally normal weight for age and height (e.g., weight loss leading to maintenance of body weight less than 85% of that expected; or failure to make expected weight gain during period of growth, leading to body weight less than 85% of that expected.) |
| B | Intense fear of gaining weight or becoming fat, even though underweight. |
| C | Disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or denial of the seriousness of the current low body weight. |
| D | In postmenarcheal females, amenorrhea, i.e., the absence of at least three consecutive menstrual cycles. (A woman is considered to have amenorrhea if her periods occur only following hormone, e.g., estrogen, administration.) |
Specify Type:
|
|
| DSM IV, Diagnostic Criteria for Bulimia Nervosa | |
| A | Recurrent episodes of binge eating. An episode of binge eating is characterized by both of the following |
|
|
| B | Recurrent inappropriate compensatory behavior in order to prevent weight gain, such as self-induced vomiting; misuse of laxatives, diuretics, enemas, or other medications; fasting, or excessive exercise |
| C | The binge eating and inappropriate compensatory behaviors both occur, on average, at least twice a week for 3 months |
| D | Self-evaluation is unduly influenced by body shape and weight |
| E | The disturbance does not occur exclusively during episodes of anorexia nervosa |
Specify Type:
|
|
Because AN and BN present most often during adolescence in women, they are often theorized to be caused by cultural pressures for thinness (1) since dieting and the pursuit of thinness are common in industrialized countries. Still, AN and BN affect only an estimated 0.3% to 0.7% and 1.5% to 2.5%, respectively, of females in the general population (2). This disparity between the high prevalence of pressures for thinness and the low prevalence of eating disorders (EDs), combined with clear evidence of AN occurring at least several centuries ago (3), the stereotypic presentation, substantial heritability, and developmentally specific age-of-onset distribution, underscores the possibility of contributing biological vulnerabilities.
Considering that transitions between syndromes occur in many, it has been argued that AN and BN share at least some risk and liability factors (4, 5). In fact, AN and BN are cross transmitted in families. (6, 7) Moreover there is an increased prevalence of AN and BN as well as subthreshold forms of ED in relatives, consistent with the possibility of a continuum of transmitted liability in at risk families manifesting a broad spectrum of eating disorder phenotypes (7). Twin studies can differentiate genetic from environmental effects by comparing concordance for a trait, or disorder, between identical (monozygotic; MZ) and fraternal (dizygotic; DZ) twins. Twin studies of AN and BN suggest there is approximately a 50 to 80% genetic contribution to liability (4, 8-11) accounted for by additive genetic factors. These heritability estimates are similar to those found in schizophrenia and bipolar disorder, suggesting that AN and BN may be as genetically-influenced as disorders traditionally viewed as biological in nature.
Clinical Symptoms and Puzzling behaviors
The DSM-IV diagnostic criteria for AN and BN focus on eating behavior and body image distortions. Because of their unusual and prominent nature, these symptoms tend to capture much attention. The pathogenesis of the disturbed eating behaviors is poorly understood (5, 12). Individuals with AN rarely have complete suppression of appetite, but rather exhibit an ego-syntonic resistance to feeding drives while simultaneously being preoccupied with food and eating rituals to the point of obsession. Individuals with AN severely restrict food intake, particularly fats and carbohydrates, but rarely stop eating completely; rather they restrict their caloric intake to a few hundred calories a day. They tend to be vegetarians, have monotonous choices in food intake, select unusual combinations of foods and flavors, and have ritualized eating behaviors. Similarly, BN is not associated with a primary, pathological increase in appetite; rather, like individuals with AN, individuals with BN have a seemingly relentless drive to restrain their food intake, an extreme fear of weight gain, and often have a distorted view of their actual body shape. Loss of control with overeating in individuals with BN usually occurs intermittently and typically only some time after the onset of dieting behavior. Restrained eating behavior and dysfunctional cognition relating weight and shape to self-concept are shared by all types of patients with EDs.
AN and BN individuals commonly have clusters of other puzzling symptoms. Excessive exercise and motor restlessness are common in AN (13). While not well studied, excessive exercise is thought to be associated particularly with the purging subtype of AN, as well as with a constellation of anxious/obsessional temperament. Individuals with AN often have resistance to treatment (14). In part this is due to the ego syntonic nature of the disorder, which is demonstrated by the patient’s denial of being underweight and refusal to accept the seriousness of the medical consequences of the disorder. Consequently, few control trials of any therapy have been performed, in part, because it has been difficult to enlist cooperation of individuals with AN, and in part because psychological and pharmacological strategies that have been successful in other disorders appear to be less effective in this illness.
Mood and impulse control
Individuals with AN and BN have elevated rates of lifetime diagnoses of anxiety and depressive disorders, and obsessive-compulsive disorder (6, 15-17). In addition, individuals with AN and BN are both consistently characterized by perfectionism, obsessive-compulsiveness, neuroticism, negative emotionality, harm avoidance, low self-directedness, low cooperativeness, and traits associated with avoidant personality disorder (PD). Consistent differences that emerge between ED groups are high constraint and persistence, low novelty seeking, constriction of affect and emotional expressiveness, ahendonia and asceticism, and reduced social spontaneity in restrictor-type AN. Individuals with BN are more likely to have high impulsivity, sensation seeking, novelty seeking, and traits associated with borderline PD in BN, and substance abuse (18).
In summary, individuals with restricting type AN are more likely to have restricted eating, constricted affect and emotional mood expression, and impulse over control, as well as personality traits of marked rigidity, conformity, and reduced social spontaneity. Individuals with BN may show similar traits, but in addition, may exhibit histories of episodic overeating, extremes of intense affect, and impulse dysregulation. Thus several domains (eating, affect, impulse control) are involved in systematic ways, specifically, over control in AN, and switches between over control and under control in BN, which raises the question of whether there is a disturbance of modulation of multiple systems.
Neurocognition
Individuals with AN have an obsessive, perseverative, and rigid personality style and have difficulty shifting sets. While those with AN do well on goal directed behavior, they have difficulties incorporating feedback and modifying their behavior. For example, they often feel that they should be able to do things perfectly without making mistakes, and they have little appreciation for the fact that mistakes are a normal learning experience. Moreover, they often fail to accurately recognize and incorporate affective and social stimuli in the environment, as confirmed by laboratory tests (19, 20). Those ill with and REC from (REC) AN tend (21) to have delayed setshifting, which allows for the adaptation of behavior in line with changing demands of the environment. Furthermore, individuals with AN have enhanced ability to pay attention to detail or use a logical/analytic approach but exhibit worse performance with global strategies (19, 22).
State and Trait
It has long been debated whether symptoms in individuals with AN and BN are cause or consequence of malnutrition. Confounding this understanding is the issue that most studies of symptoms have been done when individuals are ill with an ED. Recent studies have shown that the majority of people with AN and BN exhibit childhood perfectionism, obsessive-compulsive personality patterns, and anxiety that predate the onset of AN and BN (16, 23, 24). Moreover, studies done on 3 continents (Table 2) have shown that in AN and BN individuals with a lifetime history of an anxiety disorder diagnosis, the anxiety disorder most often began in childhood before the onset of the ED (25-28). The most common (15) premorbid childhood disorders were OCD and social phobia. In summary, such symptoms may be susceptibility factors that make people vulnerable to developing an ED. Malnutrition tends to exaggerate premorbid behavioral traits,(29) , not cause them.
Table 2.
Lifetime and Premorbid Rates of Anxiety Disorders (AD)
It is important to note that such temperament and personality traits persist after recovery from an ED. While a definition of recovery has not been formalized, investigators tend to include people after they were at a stable and healthy body weight for months or years, were not malnourished, and had not engaged in pathological eating behavior during that period of recovery. Some investigators include a criterion of normal menstrual cycles and a minimal duration of recovery, such as one year. Investigators (30-33) have found that women who were long-term REC from AN and BN have a persistence of anxiety, perfectionism, and obsessional behaviors (particularly symmetry, exactness, and order).
Gender, age, and puberty
AN and BN most commonly develop during adolescence or young adulthood (34) in proximity to puberty. Adolescence (35) is a time of profound biological, psychological and sociocultural change, and it demands a considerable degree of flexibility to successfully manage the transition into adulthood. Psychologically, change may challenge the rigidity of those at risk for AN and BN, and thus open a window of vulnerability (35). Importantly, biological changes may significantly enhance the risk of onset of an ED, particularly in women. This latter possibility is supported by twin studies which found essentially no genetic influence on overall levels of ED symptoms in 11 year-old twins, but significant genetic effects (>50%) in 17 year old twins (36). These findings collectively imply that puberty may play a role in the genetic diathesis for ED symptoms. The changes associated with adolescence differ in males and females and may therefore contribute to the sexual dimorphism of AN. Menarche is associated (35) with a rapid change in body composition and neuropeptides modulating metabolism. Little is known about whether the rise in estrogen levels associated with puberty in females is contributory. Estrogens modulates serotonergic function (37) as well as stress-related neuropeptides such as cortisol releasing hormone (CRH) (38) via a variety of mechanisms. Moreover, a major phase of synaptogenesis, pruning and myelination of predominantly frontal and limbic areas occurs around the time of puberty and adolescence and is thought to have a functional role in the integration of emotional processing with cognition (39).
Neurobiology
There is growing acknowledgement that neurobiological vulnerabilities make a substantial contribution to the pathogenesis of AN and BN(3). But we have little understanding of how such vulnerabilities result in disturbances of brain pathways or what systems are primarily involved. For example, are there disturbances of pathways related to the modulation of feeding behaviors, or mood, or temperament, or obsessionality, or impulse control? Are there primary disturbances of pathways that may modulate some factors related to body proprioception, and thus result in body image distortions? In the past, answering these questions has been thwarted by the inaccessibility of the brain. Technology capable of characterizing the complexity of brain circuits in humans, such as imaging or genetics, have only recently become available. Still, past studies have been useful in terms of grossly identifying systems that may be involved in ED. Because these technologies tend to only be able to characterize one molecule at a time, they cannot answer questions about complex interactions and function.
Neuropeptides
Central nervous system (CNS) neuropeptide dysregulation could contribute to abnormal function of gonadal hormones, cortisol, thyroid hormones and growth hormone in ED (40, 41). Moreover, mechanisms for controlling food intake involve a complicated interplay (42) between peripheral systems (including gustatory stimulation, gastrointestinal peptide secretion, and vagal afferent nerve responses) and CNS neuropeptides and/or monoamines. Studies in animals show that neuropeptides, such as CRH, leptin, the endogenous opioids (such as beta-endorphin), and neuropeptide-Y (NPY) modulate feeding behaviors and energy metabolism (43, 44). One of the few strategies capable of assessment of neuropeptides in vivo in humans is to measure their concentrations in cerebrospinal fluid (CSF). In fact, when malnourished and underweight, AN individuals have altered concentrations of CRH, NPY, beta-endorphin, and leptin (Figure 1). However, these disturbances tend to normalize after recovery (45). This observation can be interpreted to suggest that such disturbances are consequences rather than causes of malnutrition, weight loss and/or altered meal patterns. It should be noted that many systems known to modulate feeding and related functions remain to be explored in ED. For example, genotype studies raise the possibility that altered melanocortin (46) or cannabinoid (47) systems could play a role in ED.
Figure 1.
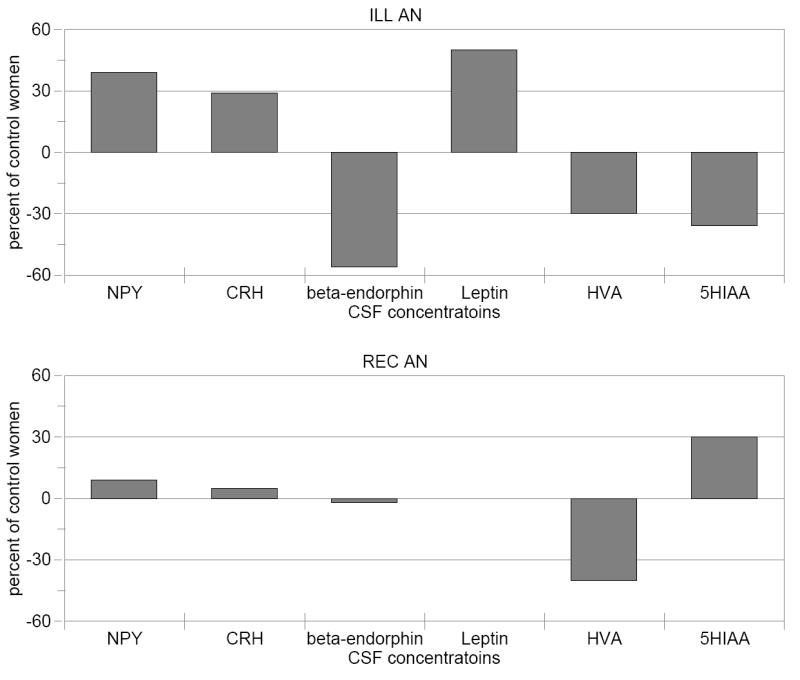
Comparison of CSF concentrations of neuropeptide and monoamine metabolites in individuals who were ill (underweight) and long-term recovered from AN. CSF values in AN are compared to healthy control women, where control mean values are set to 0.
Serotonin
Much technology has focused on characterizing monoamine function. In part, this is because many of the medications used to treat psychiatric disorders act on these systems. The monoamine systems, serotonin (5-HT), dopamine (DA), and norepinephrine (NE), are complex pathways with multiple receptors, transporters, enzymes, and intracellular cascades, etc. Consequently, our understanding of the pathophysiology of these systems in psychiatric disorders is limited. The cell bodies of monoamine neurons are located in the brainstem and project to cortical and striatal limbic regions (48). Each has multiple receptors, grouped on the basis of shared genetic sequences and second messengers.
Theoretically, 5-HT disturbances could contribute to appetite dysregulation (49, 50), anxious and obsessional behaviors and extremes of impulse control (51-55). Considerable evidence suggest that disturbances of the monoamine function occur when people are ill with ED, and persist after recovery from AN and BN. (45, 56-59). For example, ill AN subjects have a significant reduction in CSF 5-hydroxyindoleacetic acid (5-HIAA) compared to healthy control women (CW) whereas CSF 5-HIAA levels are normal in ill BN subjects (Figure 1; ((33, 60-64) Kaye, unpublished data). In comparison, REC AN and BN subjects have higher than normal concentrations of CSF 5–HIAA, that is about 50% greater than CSF 5-HIAA levels found in the ill state (Figure 1). In addition, REC AN and BN show altered behavioral responses to 5-HT challenges (65-68) or other measures, such as platelet binding of paroxetine (69). As noted above, determining cause and effect is a major methodological confound in this illness. Thus this review will focus on studies that have been done in people who have REC from AN and BN.
Diet and brain 5-HT neurotransmission
Tryptophan (TRP), an essential amino acid only available in the diet, is the precursor of 5-HT. Meal consumption, depending on the proportion of carbohydrate and protein, can enhance brain 5-HT release (70, 71); thereby affecting appetite regulation. In brief, carbohydrate consumption causes an insulin-mediated fall in plasma levels of the large neutral amino acids (LNAA; tyrosine; phenylalanine; valine; leucine; isoleucine) which compete with TRP for uptake into the brain. This elevates the plasma TRP/large neutral amino acid ratio (TRP/LNAA), and thus brain TRP, which rapidly accelerates brain 5-HT synthesis and release. Dietary proteins tend to block these effects by contributing large amounts of LNAA to the blood stream. Considerable amounts of evidence in animals and healthy humans (70-76) show that a restricted diet significantly lowers plasma TRP, resulting in a decreased plasma ratio of TRP to neutral amino acids, and, in turn, a reduction in the availability of TRP to the brain. Thus, restricted diet (and experimentally reduced TRP) decreases brain 5-HT synthesis, down-regulates the density of 5-HT transporters (77), and produces a compensatory supersensitivity of postsynaptic receptors in response to reduced 5-HT turnover (78, 79) Limited data show that malnourished and emaciated AN women have a reduction of plasma TRP availability (80). In addition, these alterations in postmeal amino acid metabolism are only partly reversed by nutritional rehabilitation (80). It has been speculated (1, 12) that there is an anxiety-reducing character to dietary restraint in people with AN. In fact, we have found that the anxiolytic effects of dieting in AN were related to a reduction in 5-HT neurotransmission. (66). Moreover administration of meta-chlorophenylpiperazine (m-CPP), a relatively selective 5-HT drug (81-85) is associated with a significant reduction in dysphoric mood states in one study(68) but not in another(86).
Implications for medication
While BN individuals show a response to higher doses of fluoxetine (87), the efficacy of such medication has been questioned since relatively few individuals abstain from binge and purge behaviors, and relapse during treatment is common (88). Despite the abundance of data implicating 5-HT dysregulation in AN, it remains controversial whether SSRIs are effective in restricting type AN (RAN) individuals (89, 90). Our clinical experience (91) suggests that RAN respond better to fluoxetine than do binge eating-purging type AN (BAN) and that some BN individuals can be relatively insensitive to high doses of SSRIs. Few control trials of any therapy have been performed, in part, because it has been difficult to enlist cooperation of individuals with AN, and in part because psychological and pharmacological strategies that have been successful in other disorders appear to be less effective in this illness. For severely emaciated patients, hospitalization for supportive medical care and weight restoration may be useful or necessary. Still, relapse is common after discharge.
Dopamine
Altered striatal DA function may contribute to symptoms in AN. Reduced CSF DA metabolites occur in malnourished individuals with AN (60) and persist after recovery (92). Individuals with AN have altered frequency of functional polymorphisms of DA D2 receptor genes that might affect receptor transcription and translation efficiency (93). The anteroventral striatum (AVS) and dorsal caudate are components of limbic and executive-associative pathways (94-96). Thus striatal DA dysfunction might contribute to altered reward and affect, decision-making, and executive control, as well as to stereotypic motor activity (95) and decreased food ingestion (97) in AN.
Brain Imaging
The past decade has seen the introduction of tools, such as brain imaging, which hold the promise of being better able to characterize complex neurocircuits and their relationship to behavior in living humans. In fact, these tools have rapidly advanced knowledge to the point where we can begin to make educated guesses about the pathophysiology of AN and BN and start to model mechanisms that may be used to test hypotheses.
Brain imaging studies in AN and BN can be divided into several categories. First, there has been substantial literature, using computerized tomograph (CT) and more recently magnetic resonance imaging (MRI) that seeks to determine whether there are brain structural alterations in individuals with ED. Second, are imaging studies, such as positron emission tomography (PET) and single photon emission computed tomography (SPECT, that employ a radioligand). These studies, which may use flurodeoxyglucose (FDG) to study glucose metabolism, or a ligand that is specific for a serotonin receptor, provide information that is specific for the system being studied, such as the 5-HT2A receptor. Third, more recent studies have used fMRI or other technologies to assess blood flow responses to some stimuli, such as pictures of food. Overall, imaging studies have been relatively consistent, in that many studies show differences in ill and REC ED individuals in frontal, cingulate, temporal, and/or parietal regions compared to controls. However, it should be noted that these studies have not consistently identified regions, pathways, or behavioral correlates. Sample sizes have been small, and imaging technologies and methods vary widely. Moreover, investigations have tended to assess relatively large regions of brain that vary widely between studies. Papers to date indicate gross alterations of brain function. Because brain pathways are highly complex, the neuroanatomy of AN and BN have only begun to be characterized.
Brain structure
Neuroimaging studies with CT reported cerebral atrophy and enlarged ventricles in ill AN (98-106). In BN similar but less pronounced structural brain abnormalities were reported (107) and may have been related to a chronic dietary restriction. Similarly, MRI studies in AN showed larger CSF volumes in association with deficits in both total grey matter (GM) and total white matter (WM) volumes (108) as well as enlarged ventricles (109-111). Fewer neuroimaging studies have been conducted in BN, and those have found decreased cortical mass (112-114). Whether these abnormalities persist to a lesser degree after weight restoration is less certain, since some studies show persistent alterations (108) but other studies show normalization of grey and white matter after recovery in AN and BN {Wagner, 2006 #2829 As noted above, in order to avoid the confounding effects of malnutrition, extremes of food ingestion and/or weight loss, the review of other imaging studies will focus mostly on studies of individuals after recovery from an ED.
Body image distortion
A most puzzling symptom of AN is the severe and intense body image distortion in which emaciated individuals perceive themselves as fat. Theoretically, body image distortion might be related to the syndrome of neglect, (217) which may be coded in parietal, frontal, and cingulate regions that assign motivational relevance to sensory events. It is well known that lesions in the right parietal cortex may not only result in denial of illness or anosognosia, somatoparaphrenia, the numerous misidentification syndromes, but may also produce experiences of disorientation of body parts and body image distortion. Wagner and colleagues confronted AN patients and age-matched healthy controls with their own digitally distorted body images as well as images of a different person using a computer-based video-technique (115). These studies reported a hyper-responsiveness in brain areas belonging to the frontal visual system and the attention network (BA 9) as well as inferior parietal lobule (BA 40), including the anterior part of the intraparietal (IPL) sulcus. Bailer (116) reported negative relationships between 5-HT2A receptor activity and the Eating Disorder Inventory Drive for Thinness scale in the left parietal cortex and other regions. It is intriguing to raise the possibility that left hemisphere disturbances of this pathway may contribute to body image distortion. It has long been recognized that parietal cortex mediates perceptions of the body and its activity in physical space (117). Recent work extends this concept to suggest that the parietal lobe contributes to the experience of being an ‘agent’ of one’s own actions (118). The well-known distortion of body image in individuals with AN may suggest abnormalities of circuits through the postulated ‘self’ networks. In line with this, left parietal cortical activation has previously been linked to AN womens’ evaluation of body image (115, 116).
Appetitive regulation
Individuals with AN and those who have had lifetime diagnoses of both AN and BN (AN-BN) tend to have negative mood states and dysphoric temperament. There is evidence that there is a dysphoria reducing character to dietary restraint (1, 12, 66) and binge-purge behaviors (119-121). This would suggest some interaction between pathways regulating appetitive behaviors and emotions. In fact, functional magnetic resonance imaging (fMRI) studies support this hypothesis. When emaciated and malnourished AN individuals are shown pictures of food, they display abnormal activity in the insula and orbitofrontal cortex (OFC) as well as in mesial temporal, parietal, and the anterior cingulate cortex (122-127). Studies using SPECT, PET-O15, or fMRI, found that when subjects ill with AN ate food, or were exposed to food, they had activated temporal regions, and often increased anxiety (122, 124-126). Those results could be consistent with anxiety provocation and related amygdala activation, and the notion that the emotional value of an experience is stored in the amygdale (128). Uher (129) used pictures of food and non-food aversive emotional stimuli and fMRI to assess ill and REC AN subjects. Food stimulated medial prefrontal and anterior cingulate cortex in both REC and ill AN subjects but lateral prefrontal regions only in the REC group. In REC AN subjects, prefrontal cortex, ACC and cerebellum were more highly activated after food compared to both control subjects and those chronically ill with AN. This finding suggested that higher ACC and medial prefrontal cortex activity in both ill and REC AN women compared to CW may be a trait marker for AN.
Imaging studies of 5-HT and DA function
The development of selective tracers for the 5-HT system has made in vivo study of 5-HT receptor function possible using PET brain imaging. In turn, this offers the possibility of better understanding of 5-HT neurotransmitter activity and dynamic relationships to behavior.
5-HT1A receptor
Our group used PET imaging with the radioligand [11C]WAY100635 to assess the binding potential (BP) of the 5-HT1A receptor. The 5-HT1A autoreceptor is located presynaptically on 5-HT somatodendritic cell bodies in the raphe nucleus, where it functions to decrease 5-HT neurotransmission (130). High densities of postsynaptic 5-HT1A exist in the hippocampus, septum, amygdala, and entorhinal and frontal cortex, where they serve to mediate the effects of released 5-HT. Although the molecular organization for the receptor transduction seems to be identical in all of the areas where 5-HT1A receptors are expressed, some differences in both functional and regulatory properties have been reported from area to area (131). Studies in animals and humans implicate the 5-HT1A receptor in anxiety (132-134) and depression and/or suicide (55, 135, 136).
Bailer (137) reported that ill AN individuals had a 50 to 70% increase in [11C]WAY100635 BP in subgenual, mesial temporal, orbital frontal, and raphe brain regions as well as prefrontal, lateral temporal, anterior cingulate, and parietal regions. Similarly, REC BAN and BN subjects (138) (Kaye unpublished data) (Figure 2) had a significant 20 to 40% increase in [11C]WAY100635 BP in these same regions compared to CW (138). In contrast, REC RAN women showed no difference in [11C]WAY100635 BP compared to controls (138). Increased 5-HT1A postsynaptic activity has been reported in ill BN subjects (139).
Figure 2.
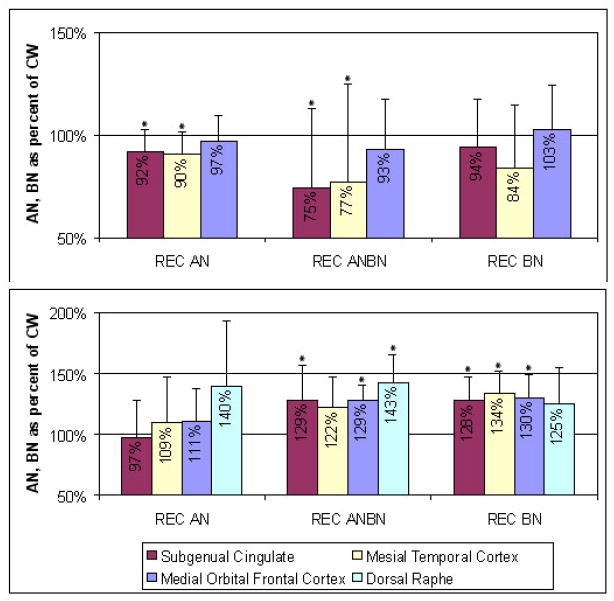
Compared to CW, values for recovered anorexia and bulimia subjects expressed as a percent of the control woman values. * indicate difference at P< .05
The role of the 5-HT1A receptor in behavior is not certain. 5-HT1A receptor activity has been reported to play a role in anxiety (133). For example, some, but not all studies, show that 5-HT1A knockout mice have increased anxiety (140). REC RAN subjects showed positive relationships between harm avoidance, a trait characterized by anticipatory worry and fear of uncertainty, and postsynaptic [11C]WAY100635 BP in subgenual cingulate, mesial temporal, lateral temporal, medial orbital frontal, and parietal cortex. It is not clear why this animal model and human phenomena appear to be opposite.
As noted above, EDs are frequently comorbid with depression and anxiety disorders. Reduced [11C]WAY100635 BP has been found in ill (141, 142) and REC (143) depressed subjects, as well as in a primate model for depresion (144). Parsey (145) found no difference in carbonyl-11C]WAY100635 BP in MDD, although a subgroup of never medicated subjects had elevated carbonyl-11C]WAY100635 BP. Recent studies have found reduced [11C]WAY100635 BP in social phobia (146) and panic disorder (147). These findings suggest ED, mood, and depression share disturbances of common systems but are etiologically different.
There is an extensive literature associating the serotonergic systems and fundamental aspects of behavioral inhibition (51, 148). Reduced CSF 5-HIAA levels are associated with increased impulsivity and aggression in humans and non-human primates, whereas increased CSF 5-HIAA levels are related to behavioral inhibition (149, 150). Brainstem 5-HT1A receptors inhibit stress-induced sympathetic activity and inhibit fight-or-flight behavioral responses, supporting a role for this receptor in behavioral inhibition and self-control (151). Furthermore, recent animal studies also support 5-HT1A receptors’ modulation of impulse control through effects on catecholamine systems (152). Other studies have shown that blunted 5-HT1A receptor number or function is associated with increased aggression (153, 154). A recent study (155) found a significant inverse relationship between dorsal raphe 5-HT1A autoreceptor BP and bilateral amygdala reactivity. 5-HT1A receptor function could contribute to behavioral inhibition in BN, although there is no direct evidence for this conjecture. Still other studies show that various measures of 5-HT activity are related to measures of affective instability and impulsivity in ill BN subjects (59, 156, 157).
5-HT2A receptor
Our group used PET imaging with the radioligand [18F]altanserin to assess BP of 5-HT2A receptors. (Figure 2) Post-synaptic 5-HT2A receptors are in high densities in the cerebral cortex and other regions of rodents and humans (158, 159). The 5-HT2A receptor is of interest in ED because it has been implicated in the modulation of feeding and mood, as well as SSRI response (116, 160-163).
Ill AN subjects had normal [18F]altanserin BP values (137). In comparison, REC RAN individuals (164) had reduced [18F]altanserin BP in mesial temporal and parietal cortical areas as well as in subgenual and pregenual cingulate cortex. Similarly, REC BAN (116) women had reduced [18F]altanserin BP relative to controls in left subgenual cingulate, left parietal, and right occipital cortex. And REC BN women only had reduced [18F]altanserin BP relative to controls in the orbital frontal region. Audenaert et al (165) used SPECT and 123I-5-I-R91159 and found that ill AN subjectshad reduced binding of postsynaptic 5-HT2A receptors in the left frontal, bilateral parietal and occipital cortex.
Brain regions/pathways enervated by 5HT1A/2A receptors
In REC subjects, altered 5-HT1A and 5-HT2A receptor BP shows persistent alterations in frontal, subgenual cingulate and mesial temporal regions that are part of the ventral limbic system. The subcaudal cingulate regions play a role in emotion (‘affect component’) and have extensive connections with the amygdala, periaqueductal grey, frontal lobes, ventral striatum, etc. They are involved in conditioned emotional learning, vocalizations associated with expressing internal states and assigning emotional valence to internal and external stimuli (166). Mesial temporal regions include the amygdala and related regions that play a pivotal role in anxiety and fear (167) as well in the modulation and integration of cognition and mood. The amygdala may enable the individual to initiate adaptive behaviors to threat based upon the nature of the threat and prior experience. Together these findings raise the possibility that mesial temporal (amygdala) - cingulate 5-HT2A receptor alterations may be a trait shared by AN subgroups related to anticipatory anxiety and integration of cognition and mood.
Several lines of evidence show that 5-HT1A and 5-HT2A receptors interact in the brain. In rats, 5-HT1A and 5-HT2A receptors interact robustly to regulate the inhibition of exploration of novel environments produced by either 5-HT1A or 5-HT2A receptor agonists (168). 5-HT2A and 5-HT1A receptors are highly co-localized in rodent frontal cortex (169). Postsynaptic 5-HT1A and 5-HT2A receptors mediate, respectively, the direct hyperpolarizing and depolarizing actions of 5-HT on prefrontal neurons (170), which in turn project to numerous cortical and subcortical areas. Thus a balance between postsynaptic 5-HT1A and 5-HT2A receptor activity on neurons may modulate the descending excitatory input into limbic and motor structures. These data raise the speculation that postsynaptic 5-HT1A and 5-HT2A receptors fine tune cortical systems that modulate behavioral inhibition and self-control. Mixed 5-HT2A/1A agonists, e.g. psilocybin, seem to disrupt the 5-HT1A/2A balance (171) by driving 5-HT2A activity, thus resulting in excessive neuronal output that contributes to extremes of disinhibition, disorganization, and loss of self-control. In our studies, REC ED subjects had a relative increase in 5-HT1A receptor activity compared to 5-HT2A receptor binding. While speculative, this possible imbalance could contribute to behavioral inhibition and over control commonly seen in ED. As discussed below, we found considerable correlations between binding of these 2 receptors and harm avoidance. Together these findings raise the possibility that mesial temporal (amygdala) - cingulate 5HT1A/2A imbalance may also be a trait shared by AN subgroups related to behavioral inhibition, anticipatory anxiety, or integration of cognition and mood.
5-HT transporter (5-HTT)
Our group (172) used PET imaging with [11C]McN5652 to determine if alterations of 5-HTT persist after recovery from AN and BN. We compared 11 subjects recoveed (> 1 year normal weight, regular menstrual cycles, no bingeing or purging) from restricting type AN (REC RAN), 7 REC from bulimia-type AN (REC BAN), and 10 healthy CW. After correction for multiple comparisons, we found that the REC RAN had significantly increased [11C]McN5652 BP compared to REC BAN for the dorsal raphe and antero-ventral striatum. It remains controversial whether SSRIs are effective in RAN individuals. Our clinical experience and data (89-91) suggest that individuals with RAN respond better to fluoxetine than do those with BAN. While highly speculative, our findings raise the provocative possibility that decreased 5-HTT function may be related to poor response to SSRI medication whereas individuals with increased 5-HTT activity may respond to higher SSRI doses. In general, the REC RAN individuals had elevated 5-HTT binding, suggesting they have relatively greater 5-HT uptake, and reduced extracellular 5-HT, compared to REC BAN. In support of this possibility, the REC BAN individuals tend to have higher binding of 5-HT1A post-synaptic receptors and autoreceptors (138), which may be a compensatory means of downregulating raphe activity (48, 173). Moreover, reduced 5-HTT activity, in terms of genotypes (174), has been associated with affect dysregulation, which tends to be more common in the bulimic subgroups. Furthermore, in people with impulsive aggressivity reduced 5-HTT binding was found in the anterior cingulate cortex, a region involved in affect regulation (175).
DA D2/D3 receptor
A recent study from our group, (176) found that REC AN had increased binding of D2/D3 receptors in the anteroventral striatum (AVS), a region that contributes to optimal responses to reward stimuli (177-179). In addition, there were positive correlations between DA D2/D3 binding in the dorsal caudate/dorsal putamen and anxiety measures in REC AN (176). The AVS and dorsal caudate are components of limbic and executive-associative pathways (94-96). Thus striatal DA dysfunction might contribute to altered reward and affect, decision-making, and executive control, as well as stereotypic motor activity (95) and decreased food ingestion (97) in AN.
5-HT, DA, and harm avoidance
The PET imaging studies in ill and REC AN and BN subjects described above have found significant correlations between harm avoidance and binding for the 5-HT1A, 5-HT2A, DA D2/D3 receptors in mesial temporal and other limbic regions. Bailer(116) found that REC AN-BN subjects showed a positive relationship between [18F]altanserin BP in the left subgenual cingulate and mesial temporal cortex and harm avoidance. For ill AN subjects, [18F]altanserin BP was positively related to harm avoidance in the suprapragenual cingulate, frontal, and parietal regions. 5-HT2A receptor binding and harm avoidance were shown to be negatively correlated in the frontal cortex in healthy subjects (180) and in the prefrontal cortex in patients that attempted suicide (181).
Clinical and epidemiological studies have consistently shown that one or more anxiety disorders occur in the majority of people with AN or BN (15, 16, 182, 183). Silberg and Bulik (184), using twins, found a unique genetic effect that influences liability to early anxiety and eating disorder symptoms. When a lifetime anxiety disorder is present, the anxiety most commonly occurs first in childhood, preceding the onset of AN or BN (25, 26, 28). Anxiety and harm avoidance remain elevated after recovery from AN, AN-BN, and BN (185), even if individuals never had a lifetime anxiety disorder diagnosis (15). Finally, anxiety (186) and Harm Avoidance from the Cloninger (TCI) (187) Temperament and Character Inventory have been a robust signal in our (WK PI) genetic studies (188). In summary, the premorbid onset and the persistence of anxiety and harm avoidance symptoms after recovery suggest these are traits that contribute to the pathogenesis of AN and BN. The PET imaging data suggest that such behaviors are related to disturbances of 5-HT and DA neurotransmitter function in limbic and executive pathways.
Implications
Phillips (94) has described a ventral limbic system, which includes the amygdale, insula, ventral striatum, and ventral regions of the anterior cingulate gyrus and prefrontal cortex, which identifies the emotional significance of a stimulus and the production of an affective state in response to that stimulus. In addition, these regions are important for automatic regulation and mediation of autonomic responses to emotional stimuli and contexts accompanying the production of affective states.
The findings described above offer evidence that individuals with ED have 5-HT and DA dysregulation within brain regions that constitute limbic circuits. In general, such alterations tend to be present in the ill state and persist after recovery. Patterns of 5-HT receptor binding vary by subtype in the REC subjects, raising the possibility that each ED subtype has a unique pathophysiology. (Table 3) Similar patterns of binding (elevated 5-HT1A and reduced 5-HT2A) were also found in other temporal, cingulate, and parietal regions suggesting a widespread distribution involving more that just limbic function. We also found that REC AN had increased binding of the DA D2/D3 receptors in the AVS.
Table 3.
Compared to normal control values of receptor and transport binding potential in subjects recovered from anorexia and bulimia nervosa
| AN | AN-BN | BN | |
|---|---|---|---|
| ROI | Medial orbital frontal, subgenual cingulate, mesial temporal | ||
| 5-HT1A BP | - | ↑ | ↑ |
| 5-HT2A BP | ↓ | ↓ | - |
| ROI | Anterio ventral striatum | ||
| 5-HTT BP | ↑ | ↓ | - |
| DA D2/D3 BP | ↑ | ↑ | - |
Drawing inferences about behavior from these 5-HT and DA findings is speculative. Moreover, no receptor works in isolation in the brain. Monoamine enervation of limbic pathways is complex, and involves many pathways, receptors, enzymes, intracellular transcription messengers, other neurotransmitters and molecules. It can be hypothesized that these receptor findings might reflect the “health” of the 5-HT and DA function within limbic circuits, but not necessarily be the specific cause of these disorders. The genetic literature suggests that behavioral disorders are complex, and consist of multiple factors, each of small effect. Finally, altered 5-HT and DA receptor binding is often found in depression and anxiety, although patterns of binding tend to be different. Disturbances of affect and impulse regulation may involve similar pathways, but with very different patterns of molecular disturbances.
Feeding behavior
Within the limbic system are brain regions that contribute to rewarding and sensory aspects of feeding behavior. That is, a primary taste cortex resides in the rostral insula and adjoining frontal operculum (189-192). Some studies argue that these regions provide a representation of food in the mouth that is independent of hunger, and thus is of reward value (193). The responsiveness of taste neurons in secondary regions, such as the OFC, computes the hedonic value of food (193-195). Other studies (196) raise the possibility that the insula and OFC contribute to feeding behavior by encoding changes in the value of food reward in addition to sensory processing, suggesting overlapping representations of sensory and affective processing of taste. As described above, previous brain imaging studies have shown pictures of food to emaciated and malnourished AN individuals. These studies found altered activity not only in the insula and OFC, but in broad regions including mesial temporal, parietal, and the anterior cingulate cortex when ill AN subjects were compared to controls (122, 125-127, 129, 197).
Our group (198) is the first to use fMRI to investigate the effect of administration of nutrients to REC RAN individuals. Compared to CW, the remitted AN subjects had a significantly reduced fMRI signal response to the blind administration of sucrose or water in the insula, anterior cingulate and striatal regions (Figure 3). Importantly, REC AN and CW also showed differences in correlations between experience of pleasant taste and brain activation. For CW, self-ratings of pleasantness of the sugar taste were positively correlated to the signal response in the insula, anterior cingulate, and ventral and dorsal putamen. In comparison, REC AN individuals failed to show any relationship in these regions to self-ratings of pleasant response to a sucrose taste.
Figure 3.
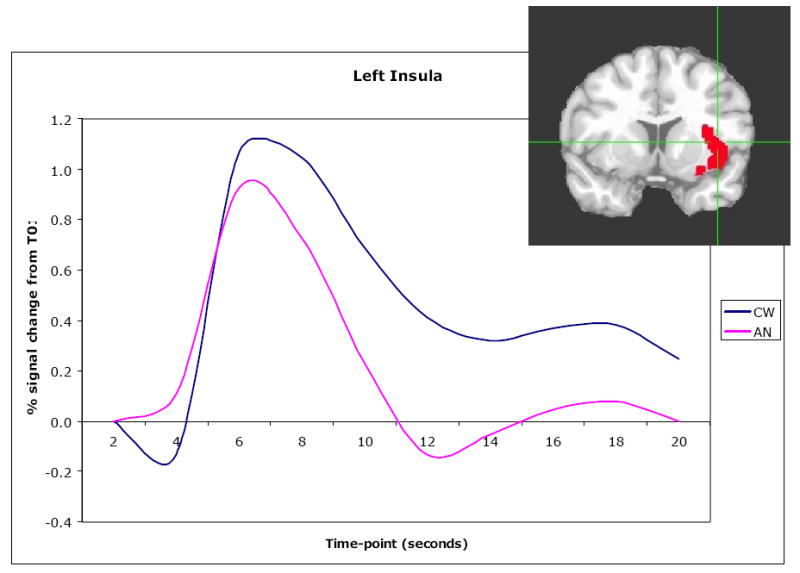
Coronal view of left insula ROI (x=-41, y=5, z=5). Time course of BOLD signal as a mean of all 16 recovered restricting-type anorexia nervosa and 16 control women for taste-related (sucrose and water) response in the left insula.
In general the anterior, less differentiated half of the insula (199-202) receives the majority of projections from the amygdala and thalamic taste centers, making it an ideal site for the formulation of hedonic representations of taste. Consistent with this, anterior portions of the insula have been shown to be differentially activated to the intensity and valence of pleasant and unpleasant tastes (196), and are thus important to examine in the eating disordered population. A large literature shows that the anterior insula and associated gustatory cortex responds not only to the taste and physical properties of food, but also to its rewarding properties (196, 203, 204). Animal models show that fine-tuning of feeding responses to salient food items is lost after insula lesions. For example, the natural devaluation of food after feeding to satiety is attenuated in lesioned animals, suggesting that the insula encodes representations of the incentive value of taste under specific conditions (205). Similarly, appropriate avoidance of foods previously associated to sickening agents is lost after insula lesions, providing further evidence that the insula encodes the incentive value of taste in particular circumstances (206).
Our results in the insula are reinforced by parallel findings in basal ganglia subregions that receive insular inputs. While the entire striatum receives inputs from the insula as a whole, the ventral putamen is a specific recipient of inputs from anterior insular regions encompassing the gustatory cortex (207). The ventral putamen in turn projects to the globus pallidus, another region with significant alterations in signal. The pattern of relatively decreased signal in these interconnected structures suggests a circuit-wide disturbance in REC AN. Insular inputs to the striatum are hypothesized to mediate behaviors involving eating, particularly of highly palatable, high energy foods. The ventrolateral striatal subregions, including the ventral putamen, have been especially implicated (208). AN subjects tend to avoid high calorie, palatable food. In theory, this is consistent with abnormal responses of insula-striatal circuits that are hypothesized to mediate behavioral responses to the incentive value of food.
The insula and interoceptive awareness
Do individuals with AN have an insular disturbance specifically related to gustatory modulation, or a more generalized disturbance related to the integration of interoceptive stimuli? The insula is thought to play an important role in processing interoceptive information, which can be defined as the sense of the physiological condition of the entire body (209). Aside from taste, interoceptive information includes sensations such as temperature, touch, muscular and visceral sensations, vasomotor flush, airhunger and others (210). The role of the insula is thus focused on how the value of stimuli might affect the body state. Interoception has long been thought to be critical for self-awareness because it provides the link between cognitive and affective processes and the current body state. The insula has bidirectional connections to limbic regions, including the amygdale, nucleus accumbens (211) and OFC (212). Thus the insular cortex is centrally placed to receive information about the salience (both appetitive and aversive) and relative value of the stimulus environment and integrate this information with the effect that these stimuli may have on the body state. The insula plays a key role in interoceptive monitoring of the sensations that are important for the integrity of the internal body state and connecting to systems, through dorsolateral striatal pathways that are important for allocating attention, evaluating context, and planning actions (209, 210, 213).
Individuals with ED have a complex of puzzling symptoms, for which there has been no neurobiological explanation. Many of the symptoms commonly found in AN, such as distorted body image, lack of recognition of the symptoms of malnutrition, could be related to disturbed interoceptive awareness. In support of this possibility, only the controls, but not the REC RAN, showed a positive relationship between self-ratings of pleasantness and the intensity of the signal for sugar in the insula, ventral and dorsal putamen as well as anterior cingulate cortex. In addition, for example, studies have consistently found that AN and BN individuals have elevated pain thresholds (214), which persists after recovery (215), and is potentially a marker of altered interoceptive awareness. While more speculative, it is possible that other symptoms, such as diminished insight and motivation to change, and altered central coherence, could be related to disturbed interoceptive awareness. Those with AN fail to accurately recognize and incorporate affective and social stimuli in the environment, as confirmed by laboratory tests (19, 20). Furthermore, individuals with AN have enhanced ability to pay attention to detail or use a logical/analytic approach, but exhibit worse performance with global strategies (19, 22).
Conclusion
We hypothesize that a trait-related disturbance of 5-HT neuronal modulation predates the onset of AN and contributes to premorbid symptoms of anxiety and inhibition. This dysphoric temperament may involve an inherent dysregulation of emotional and reward pathways (216) which also mediate the hedonic aspects of feeding, thus making these individuals vulnerable to disturbed appetitive behaviors. This 5-HT disturbance contributes to a vulnerability for restricted eating and dysphoric mood states such as increased harm avoidance. Most importantly, we think that restricting food intake is powerfully reinforcing because it provides a temporary respite from dysphoric mood. Several factors may act on these vulnerabilities to cause AN to start in adolescence. First, puberty-related female gonadal steroids or age-related changes may exacerbate 5-HT dysregulation. Second, stress and/or cultural and societal pressures may contribute by increasing anxious and obsessional temperament. We hypothesize that people with AN may discover that reduced dietary intake, by reducing plasma TRP availability (80), is a means by which they can crudely modulate brain 5-HT functional activity and anxious mood (66). People with AN enter a vicious cycle which accounts for the chronicity of this disorder because caloric restriction results in a brief respite from dysphoric mood. However, malnutrition and weight loss, in turn, produce alterations in many neuropeptides and monoamine function, perhaps in the service of conserving energy, but which also exaggerates dysphoric mood. Thus those with AN pursue starvation in an attempt to avoid the dysphoric consequences of eating. SSRI administration does not appear to be effective in counteracting 5-HT disturbances in patients will with AN, perhaps because of the extreme changes induced by malnutrition in the 5-HT1A receptor and extracellular 5-HT concentrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strober M. Family-genetic perspectives on anorexia nervosa and bulimia nervosa. In: Brownell K, Fairburn C, editors. Eating Disorders and Obesity-A Comprehensive Handbook. New York: The Guilford Press; 1995. pp. 212–218. [Google Scholar]
- 2.Hoek HW, Bartelds AI, Bosveld JJ, van der Graaf Y, Limpens VE, Maiwald M, Spaaij CJ. Impact of urbanization on detection rates of eating disorders. Am J Psychiatry. 1995;152(9):1272–8. doi: 10.1176/ajp.152.9.1272. [DOI] [PubMed] [Google Scholar]
- 3.Treasure J, Campbell I. The case for biology in the aetiology of anorexia nervosa. Psychol Med. 1994;24(1):3–8. doi: 10.1017/s0033291700026775. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, MacLean C, Neale M, Kessler R, Heath A, Eaves L. The genetic epidemiology of bulimia nervosa. Am J Psychiatry. 1991;148(12):1627–37. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]
- 5.Schweiger U, Fichter M. Eating Disorders: Clinical presentation, classification and etiologic models. In: Jimerson DC, Kaye WH, editors. Balliere’s Clinical Psychiatry. London: Balliere’s Tindall; 1997. pp. 199–216. [Google Scholar]
- 6.Lilenfeld LR, Kaye WH, Greeno CG, Merikangas KR, Plotnicov K, Pollice C, Rao R, Strober M, Bulik CM, Nagy L. A controlled family study of anorexia nervosa and bulimia nervosa: psychiatric disorders in first-degree relatives and effects of proband comorbidity. Arch Gen Psychiatry. 1998;55(7):603–610. doi: 10.1001/archpsyc.55.7.603. [DOI] [PubMed] [Google Scholar]
- 7.Strober M, Freeman R, Lampert C, Diamond J, Kaye W. Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. Am J Psychiatry. 2000;157(3):393–401. doi: 10.1176/appi.ajp.157.3.393. [DOI] [PubMed] [Google Scholar]
- 8.Klump KL, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based sample of twins. Psychol Med. 2001;31:737–740. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- 9.Wade TD, Bulik CM, Neale M, Kendler KS. Anorexia nervosa and major depression: Shared genetic and environmental risk factors. American Journal of Psychiatry. 2000;157(3):469–471. doi: 10.1176/appi.ajp.157.3.469. [DOI] [PubMed] [Google Scholar]
- 10.Bulik C, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability and prospective risk factors for anorexia nervosa. Archives of General Psychiatry. 2006;63(3):305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 11.Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biol Psychiatry. 1998;44(12):1210–8. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- 12.Vitousek K, Manke F. Personality variables and disorders in anorexia nervosa and bulimia nervosa. J Abnorm Psychol. 1994;103(1):137–147. doi: 10.1037//0021-843x.103.1.137. [DOI] [PubMed] [Google Scholar]
- 13.Shroff H, Reba L, Thornton L, Tozzi F, Klump K, Berrettini W, Brandt H, Crawford S, Crow S, Fichter M, Goldman D, Halmi K, Johnson C, Kaplan A, Keel P, LaVia M, Mitchell J, Rotondo A, Strober M, Treasure J, woodside D, Kaye WH, Bulik C. Features associated with excessive exercise in women with eating disorders. Int J Eat Disord. 2006;39:454–461. doi: 10.1002/eat.20247. [DOI] [PubMed] [Google Scholar]
- 14.Halmi K, Agras WS, Crow S, Mitchell J, Wilson G, Bryson S, Kraemer HC. Predictors of treatment acceptance and completion in anorexia nervosa. Arch Gen Psychiatry. 2005;62:776–781. doi: 10.1001/archpsyc.62.7.776. [DOI] [PubMed] [Google Scholar]
- 15.Kaye W, Bulik C, Thornton L, Barbarich N, Masters K, Fichter M, Halmi K, Kaplan A, Strober M, Woodside DB, Bergen A, Crow S, Mitchell J, Rotondo A, Mauri M, Cassano G, Keel PK, Plotnicov K, Pollice C, Klump K, Lilenfeld LR, Devlin B, Quadflieg R, Berrettini WH. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 16.Godart NT, Flament MF, Perdereau F, Jeammet P. Comorbidity between eating disorders and anxiety disorders: a review. Int J Eat Disord. 2002;32(3):253–270. doi: 10.1002/eat.10096. [DOI] [PubMed] [Google Scholar]
- 17.Godart N, Perdereau F, Rein Z, Berthoz S, Wallier J, Jeammet P, Flament M. Comorbidity studies of eating disorders and mood disorders. Critical review of the literature. J Affect Disord. 2007;97(13):37–49. doi: 10.1016/j.jad.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Cassin S, von Ranson K. Personality and eating disorders: a decade in review. Clin Psychol Rev. 2005;25(7):895–916. doi: 10.1016/j.cpr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Strupp BJ, Weingartner H, Kaye W, Gwirtsman H. Cognitive processing in anorexia nervosa. A disturbance in automatic information processing. Neuropsychobiology. 1986;15(2):89–94. doi: 10.1159/000118248. [DOI] [PubMed] [Google Scholar]
- 20.Kingston K, Szmukler G, Andrewes D, Tress B, Desmond P. Neuropsychological and structural brain changes in anorexia nervosa before and after refeeding. Psychol Med. 1996;26(1):15–28. doi: 10.1017/s0033291700033687. [DOI] [PubMed] [Google Scholar]
- 21.Tchanturia K, Campbell I, Morris R, Treasure J. Neuropsychological studies in anorexia nervosa. Int J Eat Disord. 2005;37:S72–S76. doi: 10.1002/eat.20119. [DOI] [PubMed] [Google Scholar]
- 22.Lopez C, Tchanturia K, Stahl D, Booth R, Holliday J, Treasure J. An examination of central coherence in women with anorexia nervosa. doi: 10.1002/eat.20478. Submitted. [DOI] [PubMed] [Google Scholar]
- 23.Fairburn CG, Welch SL, Doll HA, Davies BA, O’Connor ME. Risk factors for bulimia nervosa. A community-based case-control study. Arch Gen Psychiatry. 1997;54(6):509–17. doi: 10.1001/archpsyc.1997.01830180015003. [DOI] [PubMed] [Google Scholar]
- 24.Anderluh MB, Tchanturia K, Rabe-Hesketh S, Treasure J. Childhood obsessive-compulsive personalitiy traits in adult women with eating disorders: defining a broader eating disorder phenotype. Am J Psychiatry. 2003;160(2):242–247. doi: 10.1176/appi.ajp.160.2.242. [DOI] [PubMed] [Google Scholar]
- 25.Deep AL, Nagy LM, Weltzin TE, Rao R, Kaye WH. Premorbid onset of psychopathology in long-term recovered anorexia nervosa. Int J Eat Disord. 1995;17(3):291–297. [PubMed] [Google Scholar]
- 26.Godart NT, Flament MF, Lecrubier Y, Jeammet P. Anxiety disorders in anorexia nervosa and bulimia nervosa: co-morbidity and chronology of appearance. Eur Psychiatry. 2000;15(1):38–45. doi: 10.1016/s0924-9338(00)00212-1. [DOI] [PubMed] [Google Scholar]
- 27.Bulik CM, Sullivan PF, Fear J, Pickering A. Predictors of the development of bulimia nervosa in women with anorexia nervosa. J Nerv Ment Dis. 1997;185(11):704–7. doi: 10.1097/00005053-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Bulik CM, Sullivan PF, Fear JL, Joyce PR. Eating disorders and antecedent anxiety disorders: a controlled study. Acta Psychiatr Scand. 1997;96(2):101–7. doi: 10.1111/j.1600-0447.1997.tb09913.x. [DOI] [PubMed] [Google Scholar]
- 29.Pollice C, Kaye WH, Greeno CG, Weltzin TE. Relationship of depression, anxiety, and obsessionality to state of illness in anorexia nervosa. Int J Eat Disord. 1997;21(4):367–76. doi: 10.1002/(sici)1098-108x(1997)21:4<367::aid-eat10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Casper RC. Personality features of women with good outcome from restricting anorexia nervosa. Psychosom Med. 1990;52(2):156–170. doi: 10.1097/00006842-199003000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasagam NM, Kaye WH, Plotnicov KH, Greeno C, Weltzin TE, Rao R. Persistent perfectionism, symmetry, and exactness after long-term recovery from anorexia nervosa. Am J Psychiatry. 1995;152(11):1630–1634. doi: 10.1176/ajp.152.11.1630. [DOI] [PubMed] [Google Scholar]
- 32.Strober M. Personality and symptomatological features in young, nonchronic anorexia nervosa patients. J Psychosom Res. 1980;24(6):353–9. doi: 10.1016/0022-3999(80)90027-6. [DOI] [PubMed] [Google Scholar]
- 33.Kaye WH, Greeno CG, Moss H, Fernstrom J, Fernstrom M, Lilenfeld LR, Weltzin TE, Mann JJ. Alterations in serotonin activity and psychiatric symptomatology after recovery from bulimia nervosa. Arch Gen Psychiatry. 1998;55(10):927–935. doi: 10.1001/archpsyc.55.10.927. [DOI] [PubMed] [Google Scholar]
- 34.Klein D, Walsh B. Eating disorders. Int Rev Psychiatry. 2003;15:205–216. doi: 10.1080/0954026031000136839. [DOI] [PubMed] [Google Scholar]
- 35.Connan F, Campbell I, Katzman M, Lightman S, Treasure J. A neurodevelopmental model for anorexia nervosa. Physiol Behav. 2003;79(1):13–24. doi: 10.1016/s0031-9384(03)00101-x. [DOI] [PubMed] [Google Scholar]
- 36.Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in preadolescent and adolescent female twins. Journal of Abnormal Psychology. 2000;109(2):239–251. [PubMed] [Google Scholar]
- 37.Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839–50. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 38.Torpy D, Papanicolaou D, Chrousos G. Sexual dismorphism of the human stress response may be due to estradiol-mediated stimulation of hypothalamic corticotropin-releasing hormone synthesis. J Clin Endocrinol Metab. 1997;82:982. doi: 10.1210/jcem.82.3.3824-1. [DOI] [PubMed] [Google Scholar]
- 39.Benes F. Brain development, VII: Human brain growth spans decades. Am J Psychiatry. 1998;155:1489. doi: 10.1176/ajp.155.11.1489. [DOI] [PubMed] [Google Scholar]
- 40.Jimerson DC, Wolfe BE, Naab S. Anorexia nervosa and bulimia nervosa. In: Coffee CE, Brumback RA, editors. Textbook of Pediatric Neuropsychiatry. Washington, D.C.: American Psychiatric Press; 1998. pp. 563–578. [Google Scholar]
- 41.Stoving RK, Hangaard J, Hansen-Nord M, Hagen C. A review of endocrine changes in anorexia nervosa. J Psychiatr Res. 1999;33:139–152. doi: 10.1016/s0022-3956(98)00049-1. [DOI] [PubMed] [Google Scholar]
- 42.Morton G, Cummings D, Baskin D, Barsh G, Schwartz M. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 43.Morley JE, Blundell JE. The neurobiological basis of eating disorders: some formulations. Biol Psychiatry. 1988;23(1):53–78. doi: 10.1016/0006-3223(88)90106-0. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 45.Kaye W, Strober M, Jimerson D. The neurobiology of eating disorders. In: Charney DS, Nestler EJ, editors. The Neurobiology of Mental Illness. New York: Oxford Press; 2004. pp. 1112–1128. [Google Scholar]
- 46.Vink T, Hinney A, van Elburg AA, van Goozen SH, Sandkuijl LA, Sinke RJ, Herpertz-Dahlmann BM, Hebebrand J, Remschmidt H, van Engeland H, Adan RA. Association between an agouti-related protein gene polymorphism and anorexia nervosa. Molecular Psychiatry. 2001;6(3):325–8. doi: 10.1038/sj.mp.4000854. [DOI] [PubMed] [Google Scholar]
- 47.Siegfried Z, Kanyas K, Latzer Y, Karni O, Bloch M, Lerer B, Berry E. Association Study of Cannabinoid Receptor Gene (CNR1) Alleles and Anorexia Nervosa: Differences Between Restricting and Bingeing/Purging Subtypes. Am J Med Genetics Part B (Neuropsychiatric Genetics) 2004;125B:126–130. doi: 10.1002/ajmg.b.20089. [DOI] [PubMed] [Google Scholar]
- 48.Cooper SJ. Cholecystokinin modulation of serotonergic control of feeding behavior. Ann N Y Acad Sci. 1996;780:213–222. doi: 10.1111/j.1749-6632.1996.tb15125.x. [DOI] [PubMed] [Google Scholar]
- 49.Blundell JE. Serotonin and appetite. Neuropharmacology. 1984;23(12B):1537–51. doi: 10.1016/0028-3908(84)90098-4. [DOI] [PubMed] [Google Scholar]
- 50.Leibowitz SF, Shor-Posner G. Brain serotonin and eating behavior. Appetite. 1986;7(Suppl):1–14. doi: 10.1016/s0195-6663(86)80049-6. [DOI] [PubMed] [Google Scholar]
- 51.Soubrie P. Reconciling the role of central serotonin neurons in human and animal behavior. Beh Brain Sci. 1986;9:319. [Google Scholar]
- 52.Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44(6):573–88. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- 53.Higley JD, Linnoila M. Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Ann NY Acad Sci. 1997;836:39–56. doi: 10.1111/j.1749-6632.1997.tb52354.x. [DOI] [PubMed] [Google Scholar]
- 54.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44(3):151–62. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 55.Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21(2 Suppl):99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 56.Brewerton TD, Brandt HA, Lessem MD, Murphy DL, Jimerson DC. Serotonin in eating disorders. In: Coccaro EF, Murphy DL, editors. Serotonin in major psychiatric disorders. Progress in psychiatry. Vol. 21. Washington, DC, US: American Psychiatric Press, Inc; 1990. pp. 155–84. [Google Scholar]
- 57.Jimerson DC, Wolfe BE, Metzger ED, Finkelstein DM, Cooper TB, Levine JM. Decreased serotonin function in bulimia nervosa. Arch Gen Psychiatry. 1997;54(6):529–34. doi: 10.1001/archpsyc.1997.01830180043005. [DOI] [PubMed] [Google Scholar]
- 58.Walsh BT, Devlin MJ. Eating disorders: progress and problems. Science. 1998;280(5368):1387–1390. doi: 10.1126/science.280.5368.1387. [DOI] [PubMed] [Google Scholar]
- 59.Steiger H, Young SN, Kin NM, Koerner N, Israel M, Lageix P, Paris J. Implications of impulsive and affective symptoms for serotonin function in bulimia nervosa. Psychol Med. 2001;31(1):85–95. doi: 10.1017/s003329179900313x. [DOI] [PubMed] [Google Scholar]
- 60.Kaye WH, Ebert MH, Raleigh M, Lake R. Abnormalities in CNS monoamine metabolism in anorexia nervosa. Archives of General Psychiatry. 1984;41(4):350–5. doi: 10.1001/archpsyc.1984.01790150040007. [DOI] [PubMed] [Google Scholar]
- 61.Kaye WH, Gwirtsman HE, George DT, Jimerson DC, Ebert MH. CSF 5-HIAA concentrations in anorexia nervosa: reduced values in underweight subjects normalize after weight gain. Biol Psychiatry. 1988;23(1):102–5. doi: 10.1016/0006-3223(88)90113-8. [DOI] [PubMed] [Google Scholar]
- 62.Kaye WH, Gwirtsman HE, George DT, Jimerson DC, Ebert MH, Lake CR. Disturbances of noradrenergic systems in normal weight bulimia: relationship to diet and menses. Biol Psychiatry. 1990;27(1):4–21. doi: 10.1016/0006-3223(90)90015-t. [DOI] [PubMed] [Google Scholar]
- 63.Kaye WH, Gwirtsman HE, George DT, Ebert MH. Altered serotonin activity in anorexia nervosa after long-term weight restoration. Does elevated cerebrospinal fluid 5-hydroxyindoleacetic acid level correlate with rigid and obsessive behavior? Arch Gen Psychiatry. 1991;48(6):556–62. doi: 10.1001/archpsyc.1991.01810300068010. [DOI] [PubMed] [Google Scholar]
- 64.Jimerson DC, Lesem MD, Kaye WH, Brewerton TD. Low serotonin and dopamine metabolite concentrations in cerebrospinal fluid from bulimic patients with frequent binge episodes. Arch Gen Psychiatry. 1992;49(2):132–8. doi: 10.1001/archpsyc.1992.01820020052007. [DOI] [PubMed] [Google Scholar]
- 65.Ward A, Brown N, Lightman S, Campbell IC, Treasure J. Neuroendocrine, appetitive and behavioural responses to d-fenfluramine in women recovered from anorexia nervosa. Br J Psychiatry. 1998;172:351–358. doi: 10.1192/bjp.172.4.351. [DOI] [PubMed] [Google Scholar]
- 66.Kaye WH, Barbarich NC, Putnam K, Gendall KA, Fernstrom J, Fernstrom M, McConaha CW, Kishore A. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int J Eat Disord. 2003;33(3):257–267. doi: 10.1002/eat.10135. [DOI] [PubMed] [Google Scholar]
- 67.Smith KA, Morris JS, Friston KJ, Cowen PJ, Dolan RJ. Brain mechanisms associated with depressive relapse and associated cognitive impairment following acute tryptophan depletion. Brit J Psychiatry. 1999;174:525–9. doi: 10.1192/bjp.174.6.525. [DOI] [PubMed] [Google Scholar]
- 68.Frank GK, Kaye WH, Weltzin TE, Perel J, Moss H, McConaha C, Pollice C. Altered response to meta-chlorophenylpiperazine in anorexia nervosa: support for a persistent alteration of serotonin activity after short-term weight restoration. Int J Eat Disord. 2001;30(1):57–68. doi: 10.1002/eat.1054. [DOI] [PubMed] [Google Scholar]
- 69.Steiger H, Richardson J, Israel M, Ng Ying Kin NM, Bruce K, Mansour S, Marie Parent A. Reduced density of platelet-binding sites for [3H]paroxetine in remitted bulimic women. Neuropsychopharmacology. 2005;30(5):1028–1032. doi: 10.1038/sj.npp.1300693. [DOI] [PubMed] [Google Scholar]
- 70.Fernstrom JD, Wurtman RJ. Brain serotonin content: increase following ingestion of carbohydrate diet. Science. 1971;174(13):1023–5. doi: 10.1126/science.174.4013.1023. [DOI] [PubMed] [Google Scholar]
- 71.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. 1972;178(59):414–6. doi: 10.1126/science.178.4059.414. [DOI] [PubMed] [Google Scholar]
- 72.Biggio G, Fadda F, Fanni P, Tagliamonte A, Gessa GL. Rapid depletion of serum tryptophan, brain tryptophan, serotonin and 5- hydroxyindoleacetic acid by a tryptophan-free diet. Life Sci. 1974;14(7):1321–9. doi: 10.1016/0024-3205(74)90440-8. [DOI] [PubMed] [Google Scholar]
- 73.Messing RB, Fisher LA, Phebus L, Lytle LD. Interaction of diet and drugs in the regulation of brain 5- hydroxyindoles and the response to painful electric shock. Life Sci. 1976;18(7):707–14. doi: 10.1016/0024-3205(76)90182-x. [DOI] [PubMed] [Google Scholar]
- 74.Gibbons JL, Barr GA, Bridger WH, Leibowitz SF. Manipulations of dietary tryptophan: effects on mouse killing and brain serotonin in the rat. Brain Res. 1979;169(1):139–53. doi: 10.1016/0006-8993(79)90380-9. [DOI] [PubMed] [Google Scholar]
- 75.Young SN, Gauthier S. Effect of tryptophan administration on tryptophan, 5- hydroxyindoleacetic acid and indoleacetic acid in human lumbar and cisternal cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1981;44(4):323–327. doi: 10.1136/jnnp.44.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson IM, Parry-Billings M, Newsholme EA, Fairburn CG, Cowen PJ. Dieting reduces plasma tryptophan and alters brain 5-HT function in women. Psychol Med. 1990;20(4):785–91. doi: 10.1017/s0033291700036473. [DOI] [PubMed] [Google Scholar]
- 77.Huether G, Zhou D, Ruther E. Long-term modulation of presynaptic 5-HT-output: experimentally induced changes in cortical 5-HT-transporter density, tryptophan hydroxylase content and 5-HT innervation density. J Neural Transm Gen Sect. 1997;104(10):993–1004. doi: 10.1007/BF01273313. [DOI] [PubMed] [Google Scholar]
- 78.Goodwin GM, Fairburn CG, Cowen PJ. The effects of dieting and weight loss on neuroendocrine responses to tryptophan, clonidine, and apomorphine in volunteers. Important implications for neuroendocrine investigations in depression. Arch Gen Psychiatry. 1987;44(11):952–7. doi: 10.1001/archpsyc.1987.01800230032007. [DOI] [PubMed] [Google Scholar]
- 79.Goodwin GM, Fairburn CG, Cowen PJ. Dieting changes serotonergic function in women, not men: implications for the aetiology of anorexia nervosa? Psychol Med. 1987;17(4):839–42. doi: 10.1017/s0033291700000635. [DOI] [PubMed] [Google Scholar]
- 80.Schweiger U, Warnhoff M, Pahl J, Pirke KM. Effects of carbohydrate and protein meals on plasma large neutral amino acids, glucose, and insulin plasma levels of anorectic patients. Metabolism. 1986;35(10):938–43. doi: 10.1016/0026-0495(86)90058-2. [DOI] [PubMed] [Google Scholar]
- 81.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacology, Biochemistry & Behavior. 1996;54(1):129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 82.Thomas DR, Gager TL, Holland V, Brown AM, Wood MD. m-Chlorophenylpiperazine (mCPP) is an antagonist at the cloned human 5- HT2B receptor. Neuroreport. 1996;7(9):1457–1460. doi: 10.1097/00001756-199606170-00002. [DOI] [PubMed] [Google Scholar]
- 83.Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282(2):699–706. [PubMed] [Google Scholar]
- 84.Hoyer D, Neijt HC, Karpf A. Competitive interaction of agonists and antagonists with 5-HT3 recognition sites in membranes of neuroblastoma cells labelled with [3H]ICS 205-930. J Recept Res. 1989;9(1):65–79. doi: 10.3109/10799898909066045. [DOI] [PubMed] [Google Scholar]
- 85.Kahn RS, Wetzler S. m-Chlorophenylpiperazine as a probe of serotonin function. Biol Psychiatry. 1991;30(11):1139–66. doi: 10.1016/0006-3223(91)90184-n. [DOI] [PubMed] [Google Scholar]
- 86.Hadigan CM, Walsh BT, Buttinger C, Hollander E. Behavioral and neuroendocrine responses to metaCPP in anorexia nervosa. Biol Psychiatry. 1995;37(8):504–11. doi: 10.1016/0006-3223(94)00169-4. [DOI] [PubMed] [Google Scholar]
- 87.Fluoxetine Bulimia Nervosa Collaborative Study Group. Fluoxetine in the treatment of bulimia nervosa. A multicenter, placebo-controlled, double-blind trial. Arch Gen Psychiatry. 1992;49(2):139–147. [PubMed] [Google Scholar]
- 88.Walsh BT, Devlin MJ. Pharmacotherapy of bulimia nervosa and binge eating disorder. Addictive Behaviors. 1995:757–764. doi: 10.1016/0306-4603(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 89.Kaye WH, Nagata T, Weltzin TE, Hsu LK, Sokol MS, McConaha C, Plotnicov KH, Weise J, Deep D. Double-blind placebo-controlled administration of fluoxetine in restricting- and restricting-purging-type anorexia nervosa. Biol Psychiatry. 2001;49(7):644–52. doi: 10.1016/s0006-3223(00)01013-1. [DOI] [PubMed] [Google Scholar]
- 90.Walsh B, Kaplan A, Attia E, Olmsted M, Parides M, Carter J, Pike K, Devlin M, Woodside B, Roberto C, Rocket W. Fluoxetine After Weight Restoration in Anorexia Nervosa: A Randomized Controlled Trial. JAMA. 2006;295:2605–2612. doi: 10.1001/jama.295.22.2605. [DOI] [PubMed] [Google Scholar]
- 91.Kaye WH, Weltzin TE, Hsu LK, Bulik CM. An open trial of fluoxetine in patients with anorexia nervosa. Journal of Clinical Psychiatry. 1991;52(11):464–71. [PubMed] [Google Scholar]
- 92.Kaye WH, Frank GK, McConaha C. Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharm. 1999;21(4):503–6. doi: 10.1016/S0893-133X(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 93.Bergen A, Yeager M, Welch R, Haque K, Ganjei JK, Mazzanti C, Nardi I, van den Bree MBM, Fichter M, Halmi K, Kaplan A, Strober M, Treasure J, Woodside DB, Bulik C, Bacanu A, Devlin B, Berrettini WH, Goldman D, Kaye W. Association of multiple DRD2-141 polymorphism with anorexia nervosa. Neuropsychopharm. 2005;30(9):1703–1710. doi: 10.1038/sj.npp.1300719. [DOI] [PubMed] [Google Scholar]
- 94.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of Emotion Perception I: The Neural Basis of Normal Emotion Perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 95.Yin H, Knowlton B. The role of the basal ganglia in habit formation. Nature Neuroscience Rev. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 96.Haber SN, Kim K, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Halford J, Cooper G, Dovey T. The pharmacology of human appetite expression. Curr Drug Targets. 2004;5:221–240. doi: 10.2174/1389450043490541. [DOI] [PubMed] [Google Scholar]
- 98.Heinz ER, Martinez J, Haenggeli A. Reversibility of Cerebral Atrophy in Anorexia Nervosa and Cushing’s Syndrome. Journal of Computer Assisted Tomography. 1977;1(4):415–418. doi: 10.1097/00004728-197710000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Nussbaum M, Shenker IR, Marc J, Klein M. Cerebral atrophy in anorexia nervosa. Journal of Pediatrics. 1980;96(5):867–869. doi: 10.1016/s0022-3476(80)80561-0. [DOI] [PubMed] [Google Scholar]
- 100.Zeumer H, Hacke W, Hartwich P. A Quantitative Approach to Measuring the Cerebrospinal Fluid Space with CT. Neuroradiology. 1982;22:193–197. doi: 10.1007/BF00341248. [DOI] [PubMed] [Google Scholar]
- 101.Kohlmeyer K, Lehmkuhl G, Poustka F. Computed Tomography of Anorexia Nervosa. AJNR. 1983;4:437–438. [PMC free article] [PubMed] [Google Scholar]
- 102.Artmann H, Grau H, Adelmann M, Schleiffer R. Reversible and non-reversible enlargement of cerebrospinal fluid spaces in anorexia nervosa. Neuroradiology. 1985;27(4):304–312. doi: 10.1007/BF00339562. [DOI] [PubMed] [Google Scholar]
- 103.Dolan RJ, Mitchell J, Wakeling A. Structural brain changes in patients with anorexia nervosa. Psychological Medicine. 1988;18:349–353. doi: 10.1017/s0033291700007893. [DOI] [PubMed] [Google Scholar]
- 104.Krieg JC, Pirke KM, Lauer C, Backmund H. Endocrine, metabolic, and cranial computed tomographic findings in AN. Biol Psychiatry. 1988;23:377–387. doi: 10.1016/0006-3223(88)90288-0. [DOI] [PubMed] [Google Scholar]
- 105.Palazidou E, Robinson PS, Lishman WA. Neuroradiological and neuropsychological assessment in anorexia nervosa. Psychol Med. 1990;20(3):521–527. doi: 10.1017/s0033291700017037. [DOI] [PubMed] [Google Scholar]
- 106.Lankenau H, Swigar M, Bhimani S, Luchins D, Quainlan D. Cranial CT scans in eating disorder patients and controls. Compr Psychiatry. 1985;26(2):136–147. doi: 10.1016/0010-440x(85)90034-3. [DOI] [PubMed] [Google Scholar]
- 107.Krieg JC, Lauer C, Pirke KM. Structural brain abnormalities in patients with bulimia nervosa. Psychiatry Research. 1989;27:39–48. doi: 10.1016/0165-1781(89)90007-3. [DOI] [PubMed] [Google Scholar]
- 108.Katzman DK, Lambe EK, Mikulis DJ, Ridgley JN, Goldbloom DS, Zipursky RB. Cerebral gray matter and white matter volume deficits in adolescent girls with anorexia nervosa. Journal of Pediatrics. 1996;129:794–803. doi: 10.1016/s0022-3476(96)70021-5. [DOI] [PubMed] [Google Scholar]
- 109.Swayze VW, Andersen A, Arndt S, Rajarethinam R, Fleming F, Sato Y, Andreasen NC. Reversibility of brain tissue loss in anorexia nervosa assessed with a computerized Talairach 3-D proportional grid. Psychol Med. 1996;26(2):381–90. doi: 10.1017/s0033291700034772. [DOI] [PubMed] [Google Scholar]
- 110.Golden NH, Ashtari M, Kohn MR, Patel M, Jacobson MS, Fletcher A, Shenker IR. Reversibility of cerebral ventricular enlargement in anorexia nervosa, demonstrated by quantitative magnetic resonance imaging. Journal of Pediatrics. 1996;128:296–301. doi: 10.1016/s0022-3476(96)70414-6. [DOI] [PubMed] [Google Scholar]
- 111.Kornreich L, Shapira A, Horev G, Danzinger Y, Tyano S, Moimouni M. CT and MR Evaluation of the Brain in Patients with Anorexia Nervosa. American Journal of Neuroradiology. 1991;12:1213–1216. [PMC free article] [PubMed] [Google Scholar]
- 112.Laessle RG, Krieg JC, Fichter MM, Pirke KM. Cerebral atrophy and vigilance performance in patients with anorexia and bulimia nervosa. Neuropsychobiology. 1989;21(4):187–191. doi: 10.1159/000118575. [DOI] [PubMed] [Google Scholar]
- 113.Hoffman GW, Ellinwood EHJ, Rockwell WJ, Herfkens RJ, Nishita JK, Guthrie LF. Cerebral atrophy in bulimia. Biol Psychiatry. 1989;25(7):894–902. doi: 10.1016/0006-3223(89)90269-2. [DOI] [PubMed] [Google Scholar]
- 114.Husain MM, Black KJ, Doraiswamy PM, Shah SA, Rockwell WJ, Ellinwood EHJ, Krishnan KR. Subcortical Brain Anatomy in Anorexia and Bulimia. Biol Psychiatry. 1992;31:735–738. doi: 10.1016/0006-3223(92)90284-7. [DOI] [PubMed] [Google Scholar]
- 115.Wagner A, Ruf M, Braus DF, Schmidt MH. Neuronal activity changes and body image distortion in anorexia nervosa. NeuroReport. 2003;14(17):2193–2197. doi: 10.1097/00001756-200312020-00012. [DOI] [PubMed] [Google Scholar]
- 116.Bailer UF, Price JC, Meltzer CC, Mathis CA, Frank GK, Weissfeld L, McConaha CW, Henry SE, Brooks-Achenbach S, Barbarich NC, Kaye WH. Altered 5-HT2A receptor binding after recovery from bulimia-type anorexia nervosa: relationships to harm avoidance and drive for thinness. Neuropsychopharmacology. 2004;29(6):1143–1155. doi: 10.1038/sj.npp.1300430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gerstmann J. Fingeragnosie: eine umschriebene Storung der Orientierung am eigenen Korper. Wien Kinische Wochenschr. 1924;37:1010–1013. [Google Scholar]
- 118.Farrar C, Franck N, Georgieff N, Frith C, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003;18:324–333. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 119.Abraham S, Beaumont P. How patients describe bulimia or binge eating. Psychol Med. 1982;12(3):625–635. doi: 10.1017/s0033291700055732. [DOI] [PubMed] [Google Scholar]
- 120.Kaye WH, Gwirtsman HE, George DT, Weiss SR, Jimerson DC. Relationship of mood alterations to bingeing behaviour in bulimia. British Journal of Psychiatry. 1986;149:479–85. doi: 10.1192/bjp.149.4.479. [DOI] [PubMed] [Google Scholar]
- 121.Johnson C, Larson R. Bulimia: an analysis of mood and behavior. Psychosom Med. 1982;44(4):341–351. doi: 10.1097/00006842-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 122.Nozoe S, Naruo T, Nakabeppu Y, Soejima Y, Nakajo M, Tanaka H. Changes in regional cerebral blood flow in patients with anorexia nervosa detected through single photon emission tomography imaging. Biol Psychiatry. 1993;34(8):578–80. doi: 10.1016/0006-3223(93)90205-r. [DOI] [PubMed] [Google Scholar]
- 123.Nozoe S, Naruo T, Yonekura R, Nakabeppu Y, Soejima Y, Nagai N, Nakajo M, Tanaka H. Comparison of regional cerebral blood flow in patients with eating disorders. Brain Res Bull. 1995;36(3):251–5. doi: 10.1016/0361-9230(94)00199-b. [DOI] [PubMed] [Google Scholar]
- 124.Ellison Z, Foong J, Howard R, Bullmore E, Williams S, Treasure J. Functional anatomy of calorie fear in anorexia nervosa. Lancet. 1998;352(9135):1192. doi: 10.1016/S0140-6736(05)60529-6. [DOI] [PubMed] [Google Scholar]
- 125.Naruo T, Nakabeppu Y, Sagiyama K, Munemoto T, Homan N, Deguchi D, Nakajo M, Nozoe S. Characteristic regional cerebral blood flow patterns in anorexia nervosa patients with binge/purge behavior. Am J Psychiatry. 2000;157(9):1520–2. doi: 10.1176/appi.ajp.157.9.1520. [DOI] [PubMed] [Google Scholar]
- 126.Gordon CM, Dougherty DD, Fischman AJ, Emans SJ, Grace E, Lamm R, Alpert NM, Majzoub JA, Rausch SL. Neural substrates of anorexia nervosa: a behavioral challenge study with positron emission tomography. J Pediatr. 2001;139(1):51–57. doi: 10.1067/mpd.2001.114768. [DOI] [PubMed] [Google Scholar]
- 127.Uher R, Murphy T, Brammer M, Dalgleish T, Phillips M, Ng V, Andrew C, Williams S, Campbell I, Treasure J. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161(7):1238–1246. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- 128.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(45):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Uher R, Brammer M, Murphy T, Campbell I, Ng V, Williams S, Treasure J. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol Psychiatry. 2003;54:934–942. doi: 10.1016/s0006-3223(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 130.Staley J, Malison R, Innis R. Imaging of the serotonergic system: interactions of neuroanatomical and functional abnormalities of depression. Biological Psychiatry. 1998;44(7):534–549. doi: 10.1016/s0006-3223(98)00185-1. [DOI] [PubMed] [Google Scholar]
- 131.Lanfumey L, Hamon M. Central 5-HT1A receptors: regional distribution and functional characteristics. Nuclear Medicine & Biology. 2000;27(5):429–435. doi: 10.1016/s0969-8051(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 132.Cervo L, Mocaer E, Bertaglia A, Samanin R. Roles of 5-HT1A receptors in the dorsal raphe and dorsal hippocampus in anxiety assessed by the behavioral effects of 8-OH-DPAT and S 15535 in a modified Geller-Seifter conflict model. Neuropharmacology. 2000;39(6):1037–43. doi: 10.1016/s0028-3908(99)00189-6. [DOI] [PubMed] [Google Scholar]
- 133.File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000;66(1):65. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- 134.Olivier B, Pattij T, Wood S, Oosting R, Sarnyai Z, Toth M. The 5-HT1A receptor knockout mouse and anxiety. Behavioural Pharmacology. 2001;12(67):439–450. doi: 10.1097/00008877-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 135.Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. Journal of Neural Transmission - General Section. 1991;85(3):181–94. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- 136.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Research. 1995;688(12):121–33. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 137.Bailer UF, Frank G, Henry S, Price J, Meltzer C, Mathis C, Wagner A, Thornton L, Hoge J, Ziolko SK, Becker C, McConaha C, Kaye WH. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.07.018. EPub ahead of Print. [DOI] [PubMed] [Google Scholar]
- 138.Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Weissfeld L, Mathis CA, Drevets WC, Wagner A, Hoge J, Ziolko SK, McConana CW, Kaye WH. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]WAY100635. Arch Gen Psychiatry. 2005;62(2):1032. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- 139.Tiihonen J, Keski-Rahkonen A, Lopponen M, Muhonen M, Kajander J, Allonen T, Nagren K, Hietala J, Rissanen A. Brain serotonin 1A receptor binding in bulimia nervosa. Biol Psychiatry. 2004;55:871. doi: 10.1016/j.biopsych.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 140.Groenink L, van Bogaert M, van der Gugten J, Oosting R. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behavioural Pharmacology. 2003;14:369–383. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- 141.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46(10):1375–87. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 142.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57(2):174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 143.Bhagwagar Z, Rabiner E, Sargent P, Grasby P, Cowen P. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men mesured by positron emission tomography with [11C]WAY-100635. Molecular Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 144.Shively C, Friedman D, Gage H, Bounds M, Brown-Proctor C, Blair J, Henderson J, Smith M, Buchheimer N. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006;63(4):396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 145.Parsey RV, Oquendo MA, Ogden RT, Olvet D, Simpson N, Huang Y, Van Heertum R, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2005;59(2):106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 146.Lanzenberger R, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien L, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Keletter K, Kasper S, Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61(9):1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 147.Neumeister A, Brain E, Nugent A, Carson R, Bonne O, Lucnekbaugh D, Eckelman W, Herschovitch P, Charney D, Drevets W. Reduced serotinin type 1A receptor binding in panic disorder. Journal of Neuroscience. 2004;24(3):589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Geyer MA. Serotonergic functions in arousal and motor activity. Behavioural Brain Research. 1996;73:31. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 149.Fairbanks L, Melega W, Jorgensen M, Kaplan J, McGuire M. Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vermet monkeys. Neuropschopharmacology. 2001;24(4):370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- 150.Westergaard G, Suomi S, Chavanne T, Houser L, Hurley A, Cleveland A, Snoy P, Higley J. Physiological correlates of aggression and impulsivity in free-ranging female primates. Neuropsychopharmacology. 2003;28:1045–1055. doi: 10.1038/sj.npp.1300171. [DOI] [PubMed] [Google Scholar]
- 151.Johnson P, Lightman S, Lowry C. A functional subset of serotonergic neruons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann NY Acad Sci. 2004;1018:58. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- 152.Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: Therapeutic implications for impulse control disorders. Neuropschopharmacology. 2005;30:669. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- 153.Cleare A, Bond AJ. Ipaspirone challenge in aggressive men shows an inverse correlation between 5HT1A receptor function and aggression. Psychopharmacology (Berl) 2000;148:344. doi: 10.1007/s002130050061. [DOI] [PubMed] [Google Scholar]
- 154.Coccaro EF, Gabriel S, Siever LJ. Buspirone challenge: preliminary evidence for a role of 5HT1A receptors in behavioral irritability in personality disorderd patients. Psychopharmacol Bull. 1990;26:285. [PubMed] [Google Scholar]
- 155.Fischer P, Meltzer C, Ziolko S, Price J, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nature Neuroscience. 2007;9:1362. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- 156.Steiger H, Gauvin L, Israel M, LKoerner N, Ng Ying Kin NM, Paris J, Young SN. Association of serotonin and cortisol indices with childhood abuse in bulimia nervosa. Arch Gen Psychiatry. 2001;58(9):837–843. doi: 10.1001/archpsyc.58.9.837. [DOI] [PubMed] [Google Scholar]
- 157.Steiger H, Koerner N, Engelberg MJ, Israel M, Ng Ying Kin NM, Young SN. Self-destructiveness and serotonin function in bulimia nervosa. Psychiatry Res. 2001;103(1):15–26. doi: 10.1016/s0165-1781(01)00264-5. [DOI] [PubMed] [Google Scholar]
- 158.Burnet PW, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int. 1997;30(6):565–74. doi: 10.1016/s0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 159.Saudou F, Hen R. 5-Hydroxytryptamine receptor subtypes in vertebrates and invertebrates. Neurochem Int. 1994;25(6):503–32. doi: 10.1016/0197-0186(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 160.Bonhomme N, Esposito E. Involvement of serotonin and dopamine in the mechanism of action of novel antidepressant drugs: a review. J Clin Psychopharmacol. 1998;18(6):447–454. doi: 10.1097/00004714-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 161.De Vry J, Schreiber R. Effects of selected serotonin 5-HT(1) and 5-HT(2) receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev. 2000;24(3):341–53. doi: 10.1016/s0149-7634(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 162.Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73(12):37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 163.Stockmeier CA. Neurobiology of serotonin in depression and suicide. Ann N Y Acad Sci. 1997;836:220–32. doi: 10.1111/j.1749-6632.1997.tb52362.x. [DOI] [PubMed] [Google Scholar]
- 164.Frank GK, Kaye WH, Meltzer CC, Price JC, Greer P, McConaha C, Skovira K. Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biol Psychiatry. 2002;52:896–906. doi: 10.1016/s0006-3223(02)01378-1. [DOI] [PubMed] [Google Scholar]
- 165.Audenaert K, Van Laere K, Dumont F, Vervaet M, Goethals I, Slegers G, Mertens J, van Heeringen C, Dierckx R. Decreased 5-HT2a receptor binding in patients with anorexia nervosa. J Nucl Med. 2003;44(2):163–169. [PubMed] [Google Scholar]
- 166.Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. The Journal of Comparative Neurology. 2000;421:172–188. [PubMed] [Google Scholar]
- 167.Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol. 1996;10(34):419–46. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 168.Krebs-Thomson K, Geyer MA. Evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in rats. Psychopharmacology (Berl) 1998;140:69–74. doi: 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- 169.Amargos-Bosch M, Bortolozzi A, Puig M, S J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cerebral Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- 170.Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotoinin1A and serotonin2A receptor in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;1 doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- 171.Vollenweider F, Vontobel P, Hell D, Leenders K. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man--a PET study with [11C]raclopride. Neuropsychopharmacology. 1999;20(5):424–433. doi: 10.1016/S0893-133X(98)00108-0. [DOI] [PubMed] [Google Scholar]
- 172.Bailer U, Frank G, Henry S, Price J, Meltzer CC, Weissfeld L, Mathis C, Wagner A, Hoge J, Ziolko SK, McConaha C, Kaye W. Altered serotonin transporter binding after recovery from bulimia-type eating disorders measured by positron emission tomography and [11C]McN5652. Psychopharmacology (Berl) In Press. [Google Scholar]
- 173.Hajos M, Gartside SE, Varga V, Sharp T. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology. 2003;45(1):72–81. doi: 10.1016/s0028-3908(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 174.Steiger H, Joober R, Israel M, Young S, Ng Ying Kin NM, Gauvin L, Bruce K, Joncas J, Torkaman-Zehi A. The 5HTTLPR polymorphism, psychopathological symptoms, and platelet [3H-] paroxetine binding in bulimic syndromes. Int J Eat Disord. 2005;37(1):57–60. doi: 10.1002/eat.20073. [DOI] [PubMed] [Google Scholar]
- 175.Frankle W, Lombardo I, New AS, Goodman M, Talbot P, Huang Y, Hwang D, Slifstein M, Curry S, Abi-Dargham A, Lauruelle M, Siever L. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. Am J Psychiatry. 2005;162(5):915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- 176.Frank G, Bailer UF, Henry S, Drevets W, Meltzer CC, Price JC, Mathis C, Wagner A, Hoge J, Ziolko SK, Barbarich N, Weissfeld L, Kaye W. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]raclopride. Biol Psychiatry. 2005;58(11):908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 177.Montague R, Hyman S, Cohen J. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- 178.Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Science. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 179.Delgado M, Nystrom L, Fissel C, Noll D, Fiez J. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 180.Moresco FM, Dieci M, Vita A, Messa C, Gobbo C, Galli L, Rizzo G, Panzacchi A, De Peri L, Ivernizzi G, Fazio F. In vivo serotonin 5HT2A receptor binding and personality traits in healthy subjects: A positron emission tomography study. NeuroImage. 2002;17:1470–1478. doi: 10.1006/nimg.2002.1239. [DOI] [PubMed] [Google Scholar]
- 181.van Heeringen C, Audenaert K, Van Laere K, Dumont F, Slegers G, Mertens J, Dierckx RA. Prefrontal 5-HT2a receptor binding index, hopelessness and personality characteristics in attempted suicide. Journal of Affective Disorders. 2003;74:149–158. doi: 10.1016/s0165-0327(01)00482-7. [DOI] [PubMed] [Google Scholar]
- 182.Walters EE, Kendler KS. Anorexia nervosa and anorexic-like syndromes in a population-based female twin sample. American Journal of Psychiatry. 1995;152(1):64–71. doi: 10.1176/ajp.152.1.64. [DOI] [PubMed] [Google Scholar]
- 183.Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch Gen Psychiatry. 1995;52(5):374–83. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- 184.Silberg J, Bulik C. Developmental association between eating disorders symptoms and symptoms of depression and anxiety in juvenile twin girls. J Child Psychol Psychiat. 2005;46(12):1317–1326. doi: 10.1111/j.1469-7610.2005.01427.x. [DOI] [PubMed] [Google Scholar]
- 185.Wagner A, Barbarich N, Frank G, Bailer UF, Weissfeld L, Henry S, Achenbach S, Vogel V, Plotnicov K, McConaha C, Kaye W, Wonderlich S. Personality traits after recovery from eating disorders: Do subtypes differ? Int J Eat Disord. 2006;39(4):276–284. doi: 10.1002/eat.20251. [DOI] [PubMed] [Google Scholar]
- 186.Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 187.Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory (TCI): A Guide to its Development and Use. St Louis, MO: Center for Psychobiology of Personality, Washington University; 1994. [Google Scholar]
- 188.Bacanu S, Bulik C, Klump K, Fichter M, Halmi K, Keel P, Kaplan A, Strober M, Treasure J, Woodside DB, Berrettini W, Kaye W. Linkage analysis of anorexia and bulimia nervosa cohorts using selected behavioral phenotypes as quantitative traits or covariates. American Journal of Medical Genetics B, Neuropsychiatric Genetics. 2005;139(1):61–68. doi: 10.1002/ajmg.b.30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Faurion A, Cerf B, Van De Moortele PF, Lobel E, Mac Leod P, Le Bihan D. Human taste cortical areas studied with functional magnetic resonance imaging: evidence of functional lateralization related to handedness. Neurosci Lett. 1999;277(3):189–192. doi: 10.1016/s0304-3940(99)00881-2. [DOI] [PubMed] [Google Scholar]
- 190.Schoenfeld M, Neuer G, Tempelmann C, Schussler K, Noesselt T, Hopf J, Heinze H. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 2004;127(2):347–353. doi: 10.1016/j.neuroscience.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 191.Scott TR, Yaxley S, Sienkiewicz Z, Rolls E. Gustatory responses in the frontal opercular cortex of the alert cynomolgus monkey. J Neurophysiol. 1986;56:876–890. doi: 10.1152/jn.1986.56.3.876. [DOI] [PubMed] [Google Scholar]
- 192.Yaxley S, Rolls E, Sienkiewicz Z. Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol. 1990;63:689–700. doi: 10.1152/jn.1990.63.4.689. [DOI] [PubMed] [Google Scholar]
- 193.Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85(1):45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 194.Kringelbach ML, O’Doherty J, Rolls E, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 195.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11(4):893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- 196.Small D, Zatorre R, Dagher A, Evans A, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 197.Ellison AR, Fong J. Neuroimaging in Eating Disorders. In: Hoek HW, Treasure JL, Katzman MA, editors. Neurobiology in the treatment of eating disorders. Chichester: John Wiley & Sons LTd.; 1998. pp. 255–269. [Google Scholar]
- 198.Wagner A, Aizenstein H, Frank GK, Figurski J, May JC, Putnam K, Bailer UF, Fischer L, Henry SE, McConaha C, Kaye WH. Altered insula response to a taste stimulus in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301443. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 199.Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346(3):403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- 200.Kaada BR, Jansen J, Andersen P. Stimulation of the hippocampus and medial cortical areas in unanesthetized cats. Neurology. 1953;3(11):844–857. doi: 10.1212/wnl.3.11.844. [DOI] [PubMed] [Google Scholar]
- 201.Mesulam MM, Mufson EJ. Insula of the old world mokey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212(1):1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 202.Mesulam MM, Mufson EJ. Insula of the old world monkey. III Efferent cortical output and comments on function. J Comp Neurol. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 203.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- 204.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10(3):272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 205.Balleine B, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning evidence for a role in incentive memory. J Neurosci Methods. 2000;20(23):8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dunn L, Everitt B. Double dissociations of the effects of amygdala and insular cortex lesions on conditioned taste aversion, passive avoidance, and neophobia in the rat using excitotoxin ibotenic acid. Behav Neurosci. 1988;102(1):3–23. doi: 10.1037//0735-7044.102.1.3. [DOI] [PubMed] [Google Scholar]
- 207.Fudge J, Breitbart M, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005;490(2):101–118. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kelley AE, Bakshi VP, Haber S, Steininger T, Will M, Zhang M. Opioid modulation of taste hedonics within ventral striatum. Physiol Behav. 2002;76(3):365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 209.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 210.Paulus M, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 211.Reynolds S, Zahm D. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25(50):11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ongur D, Price JL. Organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 213.Chikama M, McFarland N, Amaral D, H SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17(24):9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Papezova H, Yamamotova A, Uher R. Elevated pain threshold in eating disorders: physiological and psychological factors. J Psychiatr Res. 2005;39(4):431–438. doi: 10.1016/j.jpsychires.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 215.Stein D, Kaye W, Matsunaga H, Myers D, Orbach I, Har-Even D, Frank G, Rao R. Pain perception in recoverd bulimia nervosa patients. Int J Eat Disord. 2003;34:331–336. doi: 10.1002/eat.10164. [DOI] [PubMed] [Google Scholar]
- 216.Kelley AE. Ventral striatal control of appetite motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 217.Wagner A, Greer P, Bailer U, Frank G, Henry S, Putnam K, Meltzer CC, Ziolko SK, Hoge J, McConaha C, Kaye WH. Normal brain tissue volumes after long-term recovery in anorexia and bulimia nervosa. Biological Psychiatry. 2006d;59:291–293. doi: 10.1016/j.biopsych.2005.06.014. [DOI] [PubMed] [Google Scholar]


