Summary
IL-17 is a cytokine that plays an important role in orchestrating innate immune function. In addition, IL-17 has been shown to exacerbate autoimmune diseases. CD4+ αβ T cells, γδ T cells, and NK cells all produce IL-17. Th17 cells are a newly defined αβ+ T cell lineage characterized by IL-17 production. However, γδ T cells are often the major source of this cytokine. Their response can be very rapid during bacterial infections and has been shown to be protective, but IL-17 producing γδ T cells have also been found to exacerbate collagen-induced arthritis. Interestingly, some γδ T cells produce IL-17 in response to IL-23 alone, even in naïve animals, suggesting they are already differentiated and may develop differently than CD4+ αβ Th17 cells.
Introduction
Effector CD4+ αβ T cells can be divided into 3 separate lineages: Th1, Th2, and the more recently identified Th17 lineage. Th1 cells are characterized by production of IL-2 and IFNγ. These cells develop when local antigen presenting cells secrete IL-12, especially in the presence of IFNγ, and are important in promoting a cell-mediated immune response to intracellular pathogens [1,2]. Th2 cells produce IL-4, IL-5, and IL-13 and develop when IL-4 is present. These cells promote the humoral response to extracellular pathogens [3,4]. Th17 cells are characterized by production of IL-17 (or IL-17A) and IL-17F. They also can produce IL-21, IL-22, tumor necrosis factor (TNFα), IL-6, and granulocyte-macrophage colony stimulating factor (GM-CSF), but usually not IFNγ or IL-4 [5–13]. Th17 cells are thought to increase inflammation by recruiting cells, particularly neutrophils, to the peripheral tissues for pathogen clearance. In addition, Th17 cells have been associated with pathology in autoimmune disease.
The IL-17 family
There are six members in the IL-17 family: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (IL-25), and IL-17F. Most studies have focused on IL-17A and IL-17F and these 2 family members are the most closely related [14]. Multiple cell types including CD4+ αβ T cells, γδ T cells, NK cells, and neutrophils have been shown to produce IL-17A and IL-17F. Among CD4+ T cells, co-expression of IL-17 A and IL-17 F has been noted, suggesting that these related cytokines may work together to mediate a distinct function. In fact, these two family members have been shown to form a heterodimeric protein that induces airway neutrophil recruitment [15]. IL-17A has been found to play a central role in autoimmune inflammation (reviewed in [16]). Similarly, other IL-17 family members may also play a role in autoimmune diseases, such as collagen-induced arthritis (CIA). Recently, IL-17B and IL-17C were found associated with TNF-α production, and adoptive transfer of IL-17B and IL-17C transduced CD4+ T cells exacerbated arthritis in mice [17].
Th17 cell differentiation
Many reviews have focused on how Th17 cells differentiate [18–20]. Briefly, TGFβ and IL-6 are necessary for the differentiation of naïve CD4+ αβ T cells into Th17 cells [21–23]. When IL-6 is not available, IL-21, in combination with TGFβ, can also induce Th17 cells using an IL-6 independent pathway. IL-21 is also highly expressed by Th17 cells, and the IL-21 production creates a positive feedback loop to further amplify Th17 responses in vivo (reviewed in [19]). IL-21 signaling additionally upregulates expression of RORγt, a key transcription factor in the differentiation program of Th17 cells [24]. In addition, IL-21 activates expression of IL-23R and this process is also dependent on RORγt and STAT3 [8,25]. Once the IL-23R is expressed in T cells, IL-23 can bind to this receptor and bypass the requirement for IL-6 and TGFβ, to induce IL-17 on its own [8]. IL-23, which is produced by activated dendritic cells, is necessary for the maintenance of the Th17 response and induces previously differentiated Th17 cells to expand [21].
IL-17 producing γδ T cells
The development of IL-17 producing γδ T cells is less well understood. In RORγt deficient animals, γδ T cells that express IL-17 in the intestinal lamina propria were absent suggesting that γδ T cells also require RORγt for their development [24]. Similarly, in IL-21−/− animals, lamina propria and splenic γδ T cells showed a ten-fold decrease in IL-17 expressing cells compared to wild type mice, indicating a role for IL-21 in the differentiation of IL-17 producing γδ T cells as well [26]. In IL-6−/− animals, the mRNA levels of RORγt, IL-23R, IL-17, and IL-17F from sorted lamina propria CD4+ αβ T cells were greatly diminished as compared to wild type mice and there was a 10-fold decrease in the percent of IL-17+ CD4+ αβ+ lamina propria cells. However, the percent of non-CD4+ IL-17+ cells, which includes IL-17 producing γδ T cells, was only decreased 2 fold [24]. This may indicate that when IL-6 is absent, IL-21 in combination with TGF-β is largely sufficient to differentiate non-CD4+ cells into IL-17 producing cells.
γδ T cells can dominate the IL-17 response
While the focus of most studies on IL-17 production has been on CD4+ αβ T cells, γδ T cells have also been shown to be a potent source of IL-17, and in some cases, more dominant than Th17 αβ T cells. For example, in a model of Fas-ligand induced inflammation, Umemura, et al. found that injection of a FasL-expressing tumor cell line into the peritoneum of mice induced a marked increase in proinflammatory cytokines, including IL-17. CD4−CD8− cells were identified as the major producers of IL-17 in this system. Many of these cells were γδ TCR+, and IL-17 producing γδ T cells represented a much higher proportion of the total γδ T cell population than IL-17 producing αβ T cells did of total αβ T cells [27]. In Mycobacterium tuberculosis (M. tb.) infection, T cells isolated from the lung were found to produce IL-17 when stimulated in vitro with M. tb-infected dendritic cells. Again, γδ T cells and other non-CD4+ or non-CD8+ cells were found to be the main producers of IL-17 [28]. Moreover, γδ T cells from the lung and spleen of M. tb-infected mice produced more IL-17 than did CD4+ T cells as measured by ELISPOT. In addition, γδ T cells were found to be the main producers of IL-17 after Escherichia coli (E. coli) infection, and antibody depletion of γδ T cells during the infection led to decreased IL-17 production and less neutrophil infiltration into the peritoneum [29]. Similarly, in pulmonary Mycobacterium bovis Bacille Calmette-Guerin infection, γδ T cells in the lung were identified as the main source of IL-17, rather than CD4+ αβ T cells [30]. We have recently found that in a mouse model of hypersensitivity pneumonitis, Vγ6+ cells dominate the response to Bacillus subtilis, and a large proportion of those Vγ6+ γδ T cells produce IL-17 (P. Simonian, et al. in preparation). Finally, in a model of experimental sepsis, γδ T cells, but not αβ T cells, were identified as the source of elevated plasma IL-17A that is induced after cecal ligation and puncture [31].
Some γδ T cells appear to have an inherent ability to produce IL-17
In bacterial infections, γδ T cells can be the dominant source of IL-17, and rapid production of IL-17 was observed in many of the models described above. Interestingly, unlike naïve CD4+ αβ+ cells, which must be induced, γδ T cells from naïve animals often also produce IL-17. This was discovered by Stark, et al. while investigating neutrophil homeostasis in mice with various defects in leukocyte adhesion molecules. IL-17 is important in regulating granulopoeisis through G-CSF induction. In mice that lack leukocyte adhesion molecules, neutrophilia in the blood is observed and trafficking by neutrophils is disturbed. αβ and γδ T cells were identified as the cellular sources of IL-17 in these mice and surprisingly, in wild type naïve C57BL/6 (B6) mice, splenic γδ T cells, stimulated with PMA/ionomycin, accounted for 63% of all the IL-17 producing cells [32]. Moreover, the IL-17 producing cells were shown to have characteristics of effector memory cells: CD44 high, CD45RB low, and CD62L low. Similarly, Romani et al. have shown that γδ T cells from naïve wild type B6 animals produce IL-17, after overnight stimulation with anti-CD3 antibody and lipopolysaccharide [33]. Moreover, naïve γδ T cells have been shown to produce IL-17 in response to IL-23 alone, a cytokine known to expand and/or stabilize Th17 cells [28,29]. These findings suggest that some γδ T cells may be already differentiated in the periphery, poised to produce IL-17 in a rapid manner. For example, IL-17 production peaked in the peritoneum of E. coli infected animals 6 hours post-infection and was found to be produced by Vγ1+ γδ T cells in response to IL-23 [29]. In contrast, when these γδ T cells were stimulated in vitro with TGFβ and IL-6, no IL-17 was produced, suggesting γδ T cells behave like IL-17 producing memory αβ T cells.
We find that γδ T cells taken from the lymph nodes, spleen, and even thymus of naïve mice can produce IL-17 after in vitro stimulation with PMA/ionomycin. We have compared Vγ1+ and Vγ4+ subsets in the thymus and found this potential occurs within a proportion of the Vγ4+ cells, while the Vγ1+ cells essentially do not produce IL-17. Similarly, in the spleen of naïve C57BL/6 mice, 10% of the Vγ4+ cells produce IL-17 in response to PMA/ionomycin (C. Roark, unpublished observation). This observation was not limited to C57BL/6 mice, for in studies on collagen-induced arthritis (CIA), a mouse model of rheumatoid arthritis that requires the DBA/1 mouse strain, 20% of the naïve Vγ4+ cells in the lymph nodes were also capable of producing IL-17. Again, the Vγ1+ population produced virtually no IL-17 [34]. In contrast, Romani, et al. found that in the lungs of uninfected wild type C57BL/6 mice, Vγ1+ cells produced IL-17 after overnight stimulation with anti-CD3 and bacterial lipopolysaccharide, whereas the Vγ4+ cells produced IL-10 [33]. This may indicate that the cytokine bias of particular γδ T cell subsets can be altered by subsequent stimuli.
IL-17 producing γδ T cells can exacerbate autoimmune disease
In addition to infections, Th17 cells and/or IL-17 have been shown to play a very important role in the induction and propagation of autoimmune disease. Studies in CIA have shown that the disease is markedly suppressed in IL-17 deficient animals and IL-17 has been shown to be responsible for priming collagen-specific T cells and for collagen-specific IgG2a autoantibody production [35]. We have recently shown that during CIA, the Vγ4/Vδ4+ subset of γδ T cells is expanded and produces IL-17 [34]. These cells are a potent source of IL-17 and appear to be present in numbers similar to those of the CD4+ αβ+ Th-17 cells. Sequence analysis of this expanded Vγ4/Vδ4+ cell population suggested that these cells are selected, because most also expressed a particular motif for both the TCR-γ and the TCR-δ junction. Antibody depletion of the Vγ4+ subset in mice with CIA resulted in lower disease incidence as well as less severe disease, revealing a pathogenic role for this subset of γδ T cells. However, neither collagen nor arthritis was necessary to induce this specific Vγ4/Vδ4+ T cell expansion. Instead, the γδ T cell response was due to the adjuvant used in the system to break tolerance to collagen. Therefore, the Vγ4/Vδ4 IL-17 producing γδ T cells appear to be driven by self-molecules that arise during inflammation.
Are Vγ6/Vδ1+ invariant T cells specialized to produce IL-17 rapidly?
Vγ6/Vδ1+ γδ T cells are a subset of γδ T cells that reside primarily in mucosal areas in the mouse [36]. These cells are produced during fetal development and they express an invariant TCR [37]. Vγ6/Vδ1+ cells are often expanded during bacterial infection, but also can be induced in other inflammatory conditions [38–43]. Some evidence suggests that Vγ6/Vδ1+ cells are specially primed for IL-17 production. Though very low in number in a normal mouse, within 6 hours after E. coli infection, this subset appeared to be responsible for most of the IL-17 measured in the peritoneum [29]. Days later, the Vγ6/Vδ1+ cells were still present in expanded numbers, and continued to produce IL-17. Similarly, Hamada, et al. found that during Listeria infection, Vγ4 and Vγ6 cells in the liver produce IL-17, and the peak mRNA expression of IL-17 occurred on day 1 of the infection. In addition, this early IL-17 response was found to be protective (Hamada, et al., submitted). In our studies on mice infected with Listeria intraperitoneally, we also found that Vγ6/Vδ1+ cells are increased in the peritoneum, and by day 6, the majority of the Vγ6/Vδ1+ cells produce IL-17. Interestingly, an overlapping population of these cells produces IFNγ suggesting that dual expression of these two cytokines occurs in at least some of the cells (Roark, et al. in preparation). Despite our finding, most studies describing IL-17 production by γδ T cells report that similar to CD4+ αβ Th17, IL-17 producing γδ T cells do not express both of these cytokines [27,28,34]. Our finding of dual production was unexpected because both Th1 and Th2 cytokines, including IFNγ, have been shown to antagonize the development of Th17 cells [5,6].
In conclusion, several lines of evidence suggest that IL-17 producing γδ T cells probably develop differently from CD4+ αβ Th17 cells. They may require the same developmental pathways when differentiating from naïve cells to cells committed to IL-17 production, but in the periphery, many γδ T cells already seem to exist as effector memory cells, ready to produce IL-17 quickly, and possibly other Th17 cytokines such as IL-21 and IL-22. For instance, when γδ T cells were stimulated in vitro with anti-CD3 and anti-CD28, they did not produce IL-17, but IL-17 was readily produced in response to PMA/ionomycin or IL-23 [28]. Therefore, Th17 like γδ T cells may differentiate early in the life of the mouse in response to environmental, inflammatory, or stress-related stimuli and position themselves in the peripheral tissues in order to mount a quick response, when triggered by IL-23 produced by dendritic cells, following activation by external pathogens (Fig. 1). Rapid production of IL-17 can then quickly activate and recruit neutrophils to the site of inflammation. In addition, IL-17 and/or other Th17 cell products may influence the subsequent αβ T cell response. Umemura, et al. found that IFNγ production by mycobacterial Ag-specific T cells was impaired in IL-17 deficient mice infected with M. bovis BCG, suggesting that IL-17 is important for an optimal Th1 response [30]. Further studies will help elucidate the functional roles of IL-17 producing γδ T cells.
Figure 1.
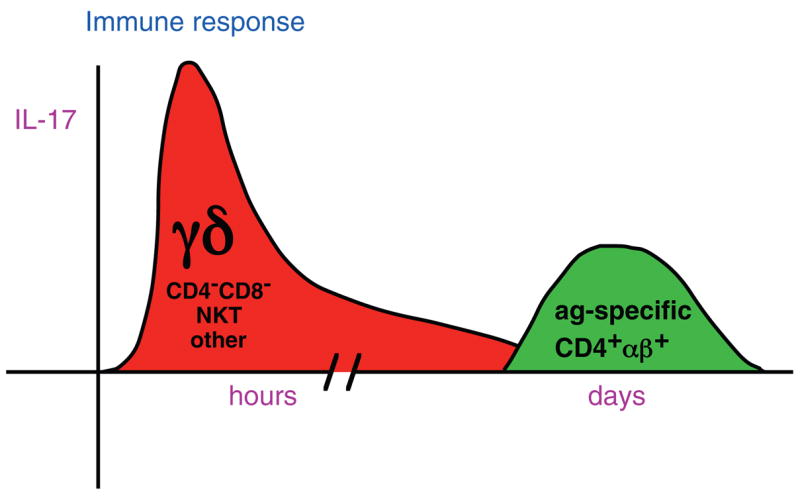
γδ T cells appear to be the dominant early source of IL-17. During an immune response, γδ T cells rapidly produce IL-17 in response to IL-23 and/or other dendritic cell products. Later, antigen-specific CD4+ αβ+ Th17 cells may also develop and contribute to the response.
Acknowledgments
Work cited as in preparation was supported by an Arthritis Investigator Award from the Arthritis Foundation (C.L.R.), and NIH grants R01 AI044920 (R.L.O.), 2RO1 HL65410 (W.K.B.), HL089766 (P.L.S.) and HL062410 (A.P.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 2.Scharton TM, Scott P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 5.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 7.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 9.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 14.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 16.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi Y, Fujio K, Shoda H, Okamoto A, Tsuno NH, Takahashi K, Yamamoto K. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007;179:7128–7136. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 18.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007 doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 23.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 26.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 27.Umemura M, Kawabe T, Shudo K, Kidoya H, Fukui M, Asano M, Iwakura Y, Matsuzaki G, Imamura R, Suda T. Involvement of IL-17 in Fas ligand-induced inflammation. Int Immunol. 2004;16:1099–1108. doi: 10.1093/intimm/dxh111. [DOI] [PubMed] [Google Scholar]
- 28**.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. This study identified in naïve mice, as well as in mice infected with Mycobacterium tuberculosis, that IL-17 production is primarily from γδ T cells and other non-CD4+CD8+ cells, rather than from αβ+ CD4+ cells. In addition, they showed that IL-23 alone could stimulate IL-17 production from these cells. [DOI] [PubMed] [Google Scholar]
- 29**.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident V{delta}1+ {gamma}{delta} T Cells Control Early Infiltration of Neutrophils after Escherichia coli Infection via IL-17 Production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. This study revealed that γδ T cells in the peritoneal cavity of naïve mice produced IL-17 in response to IL-23 and this response was rapid. It also demonstrated that Vδ1+ γδ T cells were the source of IL-17, and that neutralization of IL-17 resulted in reduced numbers of neutrophils and impaired bacterial clearance. [DOI] [PubMed] [Google Scholar]
- 30**.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. This study identified γδ T cells as the primary source of IL-17 in M. bovis infected lungs, and IL-17 expression was shown to be dependent on IL-23. The data also suggested that induction of optimal Th1 responses and protective immunity against mycobacterial infections is dependent on IL-17. [DOI] [PubMed] [Google Scholar]
- 31*.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL, et al. Adverse functions of IL-17A in experimental sepsis. Faseb J. 2008 doi: 10.1096/fj.07-105221. This study showed that γδ T cells are the source of IL-17A that is induced after cecal ligation and puncture. Protective effects were observed when IL-17A was blocked. [DOI] [PubMed] [Google Scholar]
- 32.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 33*.Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. This study demonstrated that Vγ1+ cells produce high levels of IL-17 in an unrestrained manner in a mouse model of chronic granulomatous disease. It also showed that Vγ1+ cells taken from the lungs of untreated wild type animals produce IL-17 while Vγ4+ cells produce IL-10. [DOI] [PubMed] [Google Scholar]
- 34**.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. This study identified a specific γδ T cell subset, Vγ4/Vδ4+ cells, that becomes activated and produces IL-17 in the collagen-induced arthritis model. When CIA mice were depleted of this subset, the mice developed less severe disease and a lower disease incidence, suggesting that these cells are pathogenic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 36.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, Tonegawa A. Homing of a γδ thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 37.Havran W, Allison JP. Developmentally ordered appearance of thymocytes expressing different T cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 38.Roark CE, Vollmer M, Campbell P, Born WK, O’Brien RL. Response of a γδ-TCR monomorphic subset during bacterial infection. J Immunol. 1996;156:2214–2220. [PubMed] [Google Scholar]
- 39.Mukasa A, Lahn M, Pflum EK, Born W, O’Brien RL. Evidence that the same γδ T cells respond during infection-induced and autoimmune inflammation. J Immunol. 1997;159:5787–5794. [PubMed] [Google Scholar]
- 40.Ikebe H, Yamada H, Nomoto M, Takimoto H, Nakamura T, Sonoda KH, Nomoto K. Persistent infection with Listeria monocytogenes in the kidney induces anti-inflammatory invariant fetal-type gammadelta T cells. Immunology. 2001;102:94–102. doi: 10.1046/j.1365-2567.2001.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heyborne KD, Cranfill RL, Carding SR, Born WK, O’Brien RL. Characterization of γδ T lymphocytes at the maternal-fetal interface. J Immunol. 1992;149:2872–2878. [PubMed] [Google Scholar]
- 42.Olive C. Modulation of experimental allergic encephalomyelitis in mice by immunization with peptide specific for the γδ T cell receptor. Immunol and Cell Biol. 1997;75:102–106. doi: 10.1038/icb.1997.14. [DOI] [PubMed] [Google Scholar]
- 43.Mukasa A, Born WK, O’Brien RL. Inflammation alone evokes the response of a TCR-invariant mouse γδ T cell subset. J Immunol. 1998;162:4910–4913. [PubMed] [Google Scholar]


