Summary
Objective
We have previously demonstrated that the disruptions of nontypeable Haemophilus influenzae (NTHi) lipooligosaccharide (LOS) htrB and rfaD genes may play a role in the pathogenesis of otitis media (OM). The purpose of this study was to determine whether NTHi LOS gene disruptions influence the induction of gene expression for proinflammatory mediators in vivo using the rat model of acute OM.
Methods
At 3, 6, 12, 24, 48 and 72 h after transbullar inoculation with nonviable NTHi, expression of genes for the cytokines and chemolines; tumor necrosis factor alpha (TNF-α), interleukin -l β (IL-1β), and IL-6, IL-1α, IL-8, IL-10, and inducible nitric oxide synthase (iNOS) were quantitated by real-time PCR. Enzyme-linked immunosorbent assay was performed to confirm the gene expression data as determined by real-time PCR. The middle ear inflammatory responses were also evaluated.
Results
The NTHi 2019 parent and its isogenic LOS htrB (B29) and rfaD (DK-1) mutant strains induced a significant up-regulation in gene expression for the cytokines examined compared to the sham-inoculated controls at 3, 6 and 12 h post inoculation (P < 0.05 in all cases). However, the NTHi 2019 cohort demonstrated a significant increase in gene expression for TNFα (up to 6 h), IL-1α and IL-8 (up to 24 h), IL-1β and IL-6 (up to 48 h), and IL-10 and iNOS (up to 72 h) relative to the animals inoculated with NTHi B29 (P < 0.05, in all cases), Moreover, the concentrations of inflammatory cells in the middle ear lavage fluid samples from the NTHi 2019 cohort were 2.8–5.3 fold higher than those of the B29 cohort. There were no significant differences in mRNA expression of the cytokines between the NTHi 2019 and the DK-1-treated groups.
Conclusions
Data from this study indicate that the disruption of the NTHi htrB gene may impact the temporal mRNA expression of inflammatory mediators and inflammation within the middle ear.
Introduction
Nontypeable Haemophilus influenzae (NTHi) is one of the major otitis media (OM) pathogens and accounts for 25 to 30% of all OM cases, 53% of recurrent OM [1] and is the primary pathogen isolated from 62% of cases of chronic OM with effusion [2]. NTHi lipooligosaccharide (LOS) is a potent inducer of inflammation, as well as a modulator of the immune response. A previous study with a vaccine comprised of detoxified NTHi LOS conjugated to protein suggests that it confers protection against OM in the chinchilla model [3]. NTHi LOS not only has been isolated from middle ear effusions of patients during the acute inflammatory phase of the disease, but also perpetuates inflammation in the middle ear after the acute phase has resolved and viable bacteria have been eradicated from the middle ear by antibiotic therapy and host immune mechanisms [4–5].
The middle ear epithelium has been considered not only as a physical barrier against OM pathogens but also as a source of a variety of proinflammatory mediators during OM. Various inflammatory mediators are produced and involved in inflammation of middle ear against invading pathogens. A number of previous investigations have attempted to delineate the kinetics of cytokine gene expression in the middle ear during experimental OM induced in various animal species by either Streptococcus pneumoniae or NTHi, the major bacterial pathogens associated with this disease [6–10]. NTHi LOS plays an important role in activating middle ear epithelium, resulting in early production of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) that are thought to be of central importance during the pathogenesis and regulation of proliferation, chemotaxis, and activation of inflammatory cells during the course of middle ear infections [6,8].
Considerable evidence has further implicated the roles of NTHi LOS in the pathogenesis of OM. Using the NTHi htrB mutant strain (B29) expressing an LOS subunit with decreased acylation with phenotypic changes in both the lipid A moiety and the core oligosaccharides and the rfaD mutant strain (DK-1) expressing a truncated LOS with only lipid A and three deoxy-d-manno-octulosonic acid (KDO) residues as tools [17, 23], we analyzed the role of disruptions of the NTHi htrb and rfaD genes during the entire spectrum of OM beginning with NTHi colonization of the nasopharynx, their ascension of the eustachian tube, as well as NTHi multiplication in and subsequent clearance from the middle ear. Results from our previous studies indicated that alteration of the LOS phenotype impacts on the virulence of NTHi during experimental OM in the chinchilla and modifies some but not all of the progressive stages of the disease course and its inner ear sequelae. Disruption of the NTHi htrB or rfaD LOS genes does not impact on the ability of NTHi to colonize the nasopharynx, however, it induces marked differences in the ability of the two LOS mutants to induce OM, and survive in the middle ear [11]. In addition, Disruption of NTHi htrB gene has been shown to play a major role in the induction of cytokine and chemokine genes in our cultured human middle ear epithelial (HMEE) cells in vitro [12]. However, to our knowledge, no study has been reported to examine how the alteration of NTHi LOS phenotype impacts the initial stages of the host-parasite interaction within the middle ear. Specifically, does NTHi htrB or rfaD mutant strain differentially influence the temporal expression of inflammatory mediators in the middle ear? In order to define the relative contributions of the oligosaccharide and lipid A portions of LOS to this disease at the molecular level, we used these mutant strains differing in virulence to assess the role of NTHi LOS in the gene expression of the proinflammatory cytokines and to map the in vivo kinetics of induction of the inflammatory cytokine genes by NTHi LOS in the rat middle ear during the course of acute OM.
Materials and methods
Bacteria strains
The following NTHi strains were obtained from Michael A. Apicella, University of Iowa College of Medicine and were used in this study. All have been previously described [17, 23, 24]
NTHi strain 2019 is the parental isolate (wild type) and grows on chocolate agar as well as brain heart infusion agar supplemented with Fildes enrichment (sBHI) at 37°C.
Strain B29 is an isogenic htrB mutant generated by shuttle mutagenesis using a mini-Tn3. The biological consequences of mutation in the htrB locus of Strain B29 include: 1) modification of the oligosaccharide core reflected, in part, by a net loss in phosphoethanolamine, 2) loss of myristic acid substitutions of the lipid A. B29 contains a chloramphenicol resistance gene and can be differentiated from the parent and DK-1 by its ability to grow on sBHI containing 100 μg of chloramphenicol/ml.
Strain DK-1 is an isogenic rfaD gene mutant. This strain’s LOS is truncated and consists only of KDO and lipid A. It is devoid of other core oligosaccharides. This particular mutant could be used to investigate the contribution of lipid A and KDO, exclusive of oligosaccharide, in the pathogenesis of OM. DK-1 contains a kanamycin resistance cassette and can thus be differentiated from the parent 2019 or B29 by its ability to grow on sBHI containing 15 μg of kanamycin/ml.
Preparation of formalin-killed NTHI strains
The use of whole, formalin-killed NTHi as a source of LOS was developed in our laboratory and described in detail previously [25]. NTHi strains were grown on sBHI agar, with or without antibiotics as indicated above, overnight in a CO2 incubator at 37°C. The bacteria were killed by incubation with 0.3% formalin at room temperature for 24 hours. Killed NTHi were washed and suspended in sterile phosphate buffered saline (PBS) as described previously [12].
Study design
Eighty male Sprague-Dawley rats (225 to 250 g) were randomly assigned to four cohorts and anesthetized by intramuscular injection with ketamine hydrochloride (80 mg/kg of body weight) and xylazine (8 mg/kg). OM was then induced by the direct bilateral inoculation of the middle ears, with 30 μl of a suspension containing 108 CFU of formalin-inactivated NTHi in sterile PBS as previously described [10]. Inoculations were made through the bony wall of the cephalid bullae, which was accessed through a neutral midline incision and blunt dissection. A control cohort was sham inoculated with 30 μl of diluent alone (PBS), and an additional six rats were used as normal controls without injections. All animal experiments were approved by the Institutional Animal Care and Use Committee at the Ohio State University. At 3, 6, 12, 24, 48 and 72 h postinoculation, three rats, preselected and randomized were sacrificed at each time point. The rats were anesthetized and then sacrificed by the intracardiac injection of an overdose of xylazine, and the middle ear mucosa were harvested by in situ lysis as previously described by our laboratory [10]. The bullae were exposed and the middle ear space was rinsed three times with 50 μl of sterile PBS, the washings were aspirated and pooled, and the inflammatory cell concentration (in cells per cubic millimeter) for each sample was determined by use of a hemocytometer. Following lavage, the middle ear epithelium was harvested by in situ lysis with 50 μl of lysis buffer from an RNeasy Mini kit (Qiagen, Valencia, Calif.). This process was repeated three times, and the lysates were aspirated, pooled, and stored at −70°C. Total RNA was isolated by using an RNeasy Mini kit according to the manufacturer’s instructions (Qiagen). The purity of the isolated RNA was estimated by spectrophotometric determination of the 260- to 280-nm absorption ratio, and the RNAs were stored at −80°C until analyzed by real-time PCR. The study was repeated once.
Quantitation of cytokine transcripts from middle ear epithelium by real-time PCR
Real-time PCR assays were performed to specifically quantitate IL-1α, IL-1β, IL-6, IL-10, iNOS, and TNFα transcripts as we have described previously [10]. Briefly, total cellular RNA was extracted using an RNeasy Mini kit (Qiagen), and cDNAs were synthesized using the Superscript preamplification system (Gibco BRL). Each cDNA sample was used as a template for a real-time PCR amplification mixture containing forward and reverse primers and probes for the target cytokine and chemokine genes and for 18S rRNA (internal control) and 2× TaqMan Universal PCR Master Mix obtained from Applied Biosystems (Foster City, Calif.). For rat IL-8, GROCINC-1 (rat equivalent of IL-8) primers were selected according to the published cDNA sequence (5′ primer: CATTAATATTTAACGATGTGGATGCGTTTCA; 3′ primer: GCCTACCATCTTTAAACTGCACAAT). Real time PCR was performed with SYBR green PCR Master mix (Applied Biosystems) according to the manufacturer’s protocol. Real-time PCR amplifications were performed on an Applied Biosystems Prism 7900 sequence detector according to the manufacturer’s instructions. Relative changes in gene expression were determined using the 2-CT method as described elsewhere [10] and reported as the fold difference relative to a calibrator cDNA (normal control rats, uninoculated) prepared in parallel with the experimental cDNAs.
Quantitation of cytokine proteins in the middle ear lavage samples by ELISA
Middle ear lavage samples were centrifuged at 500 × g and frozen at −70°C. Concentrations of IL-1β, IL-6, and TNFα In middle ear lavage samples were measured by use of commercial ELISA kits (Quantikine; R & D Systems, Minneapolis, Minn.), according to the manufacturer’s instructions. Middle ear lavage samples from the sham-inoculated animals served as the control.
Histology
Two rats from each cohort were sacrificed at 24 h post T.B. challenge. The temporal bones were fixed in 10% neutral-buffered formalin, decalcified with ethylenediaminetetraacetic acid (EDTA) and the specimens were further processed for conventional paraffin embedding. Serial sections were cut to a thickness of 4 μm and stained with hematoxilin and eosin (H & E).
Statistical analysis
Data are expressed as the arithmetic mean ± the standard error of the mean (SEM). One-way ANOVA and Scheff’s test were used for the statistical analysis and pair-wise comparisons. Dennett’s procedure was used for multiple comparisons with a common control. In all cases, a P level of <0.05 was set as the measure of significance.
Results
Effect of NTHi LOS gene disruptions on middle ear inflammatory cell response
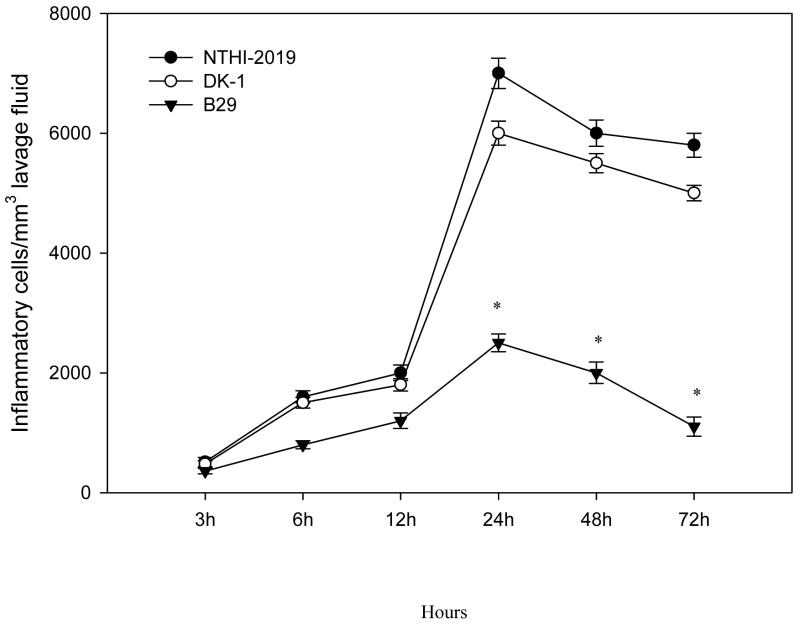
To determine whether the NTHi LOS mutant strains differentially induce inflammatory cell recruitment, cell counts were performed on the middle ear lavage samples. There were no significant differences in the inflammatory cells from the lavage samples between the parent NTHi 2019 and DK-1 treated rats. Whereas there was little difference at 3, 6 and 12 h between the NTHi 2019 and B29 treated rats, by 24 h post inoculation there were significantly more inflammatory cells in the middle ear lavage samples from rats inoculated with the parent NTHi 2019 compared to that in the animals inoculated with B29 (P < 0.05) (Fig. 1). Also, at each time point following inoculation, lavage samples collected from rats inoculated with NTHi 2019, B29 and DK-1 strains had significantly more inflammatory cells compared to those from the sham-inoculated controls (P < 0.001 in all cases). All the middle ear lavage samples from the sham-inoculated controls had fewer than 10 cells per mm3 for all the samples collected at each time point.
Fig. 1.
Accumulation of inflammatory cells in the middle ears of rats following transbullar inoculation of nonviable parent strain NTHi 2019, the htrB mutant (B29), or the rfaD mutant (DK-1). Each data point represents the mean concentration of inflammatory cells (± SEM) per microliter of middle ear lavage fluid. These results are from a total of six middle ear lavage samples obtained from three animals for each cohort at each time point. *, P < 0.05 for the comparison (B29 versus NTHi 2019).
Histopathology evaluation
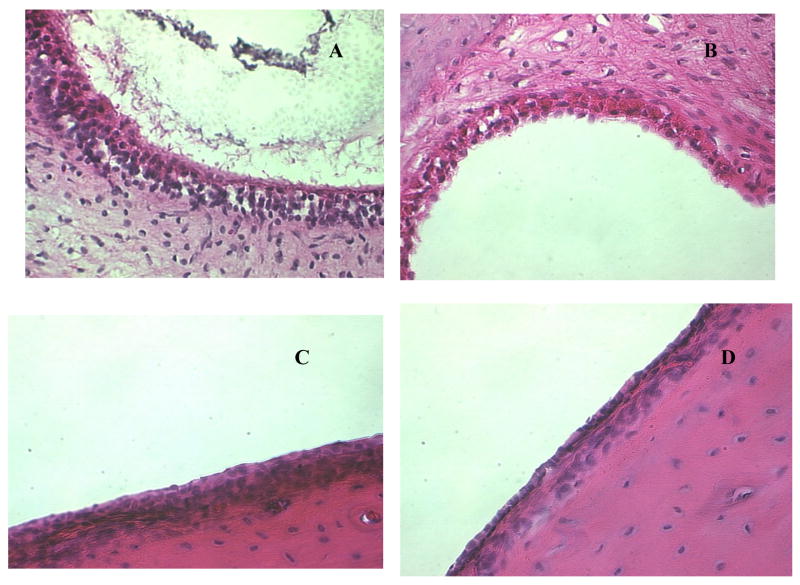
Histopathological examination of the middle ear sections showed a minimal cellular inflammatory response within the epithelium or subepithelium of the sham control at 24 h post inoculation. The changes in the middle ear mucosa of rats inoculated with NTHi 2019 were much more pronounced than that in the rats treated with both LOS deficient mutants, which showed a marked thickening of the epithelium or subepithelium with an increased number of inflammatory cells at 24 hour post inoculation. A marked influx of inflammatory cells confined to the lumen of the lateral middle ear spaces was indicative of an acute, localized inflammation (Fig. 2). The influx of these cells correlated with inflammatory cells in the middle ear lavage samples as described above.
Fig. 2.
H & E-stained middle ear sections of the rats at 24h after transbullar inoculation with nonviable NTHi 2019 (A), DK-1 (B), B29 (C), and PBS control (D). The inflammatory infiltrates in the middle ear epithelium was significant in the NTHi 2019 treated rats. Magnification, ×400.
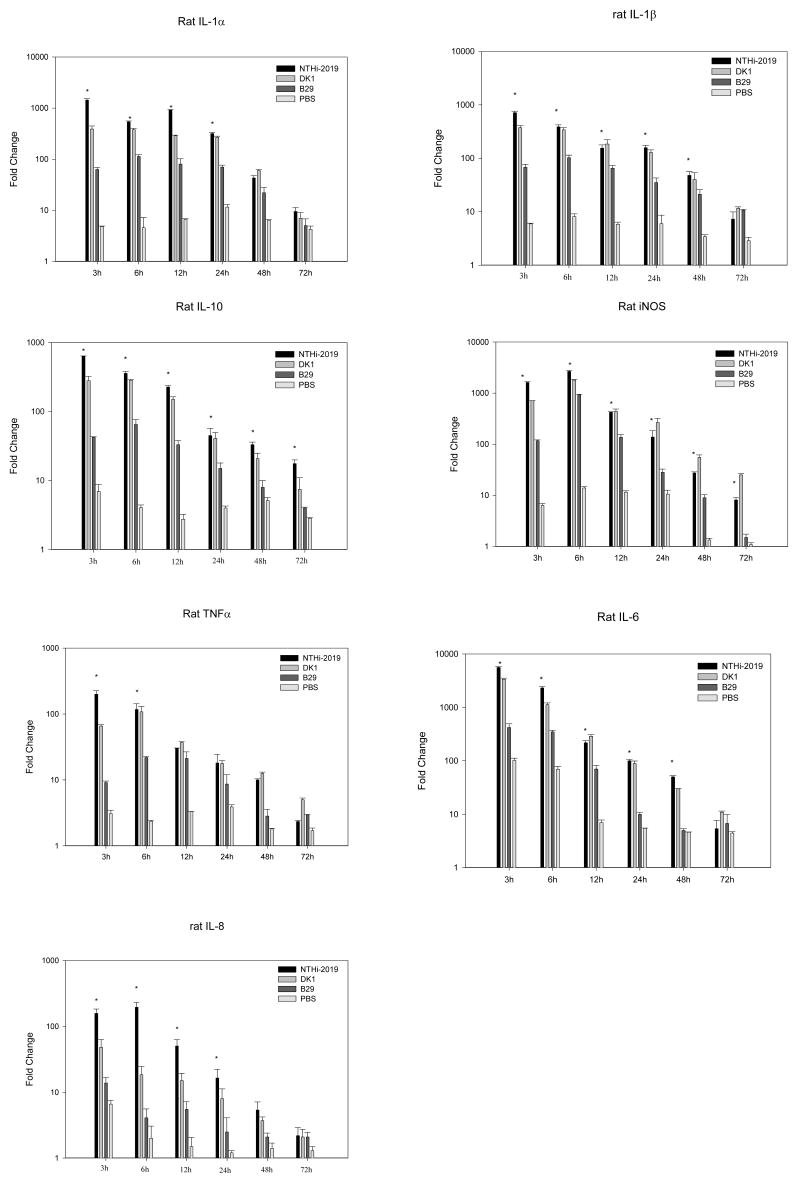
Effect of LOS gene disruption on the kinetics of cytokine and inducible nitric oxide synthase (iNOS) gene expression in the rat middle ear subsequent to direct transbullar (T.B.) inoculation
Real-time PCR was used to examine the role of the disruption of NTHi htrB or rfaD gene in the induction of a panel of proinflammatory cytokine genes in the rat middle ear epithelium in vivo during the course of experimental OM. As expected, the parent strain, NTHi 2019, induced a significant up-regulation of these genes relative to the sham control at 3, 6, 12, 24 h and 48 h post inoculation (P < 0.001 in all cases) (Fig. 3). The kinetics of cytokine gene expression showed maximum gene expressions at 3 h post inoculation with the exception of iNOS and IL-8 at 6 h. When comparing gene expression levels between the NTHi 2019 and B29 cohorts at this time point, the amount of mRNA transcripts for IL-1α, TNF-α, IL-10, IL-6, iNOS, IL-1β and IL-8 were approximately 23, 22, 15, 14, 14, 11 and 11 fold higher, respectively, in the NTHi 2019 cohort compared to levels in the B29 cohort (P <0.05 in all cases) The mRNA expression was more sustained in the NTHi 2019 inoculated rats compared to that in the B29 treated rats. The NTHi 2019 cohort demonstrated a significant increase in gene expression for TNFα (up to 6 h), IL-1α and IL-8 (up to 24 h), IL-1β and IL-6 (up to 48 h) and IL-10 and iNOS (up to 72 h) relative to the animals inoculated with NTHi B29 (P < 0.05in all cases). The induction of iNOS, IL-10, IL-1α, IL-1β genes at 72 h and that of IL-6, IL-8 and TNF-α genes at 48 h by NTHi 2019 remained at a significantly higher level compared to that of the sham controls
Fig. 3.
Induction of gene expression as measured by real-time PCR on total RNA samples prepared by the direct in situ lysis of the middle ear space at 3, 6, 12, 24, 48, and 72 h following inoculation of NTHi. Results are the mean fold increase in IL-1α, IL-1β, IL-6, IL-10, iNOS, TNFα and IL-8 transcript levels (± SEM) from duplicate samples from two separate experiments. *, P < 0.05 for the comparison (NTHi 2019 versus B29).
Inoculation of DK-1 induced a pattern of cytokine genes expression similar to that observed with NTHi 2019, although the levels of gene expression of IL-1α. IL-8 and TNFα were lower in the DK-1 inoculated rats than that in the rats inoculated with NTHi 2019 at 3 h post inoculation. (3.7, 3.3 and 3.0 fold decrease respectively).
In the rats inoculated with B29, the time course analysis revealed an early and transit cytokine mRNA expressions in the middle ear epithelium. However, the maximal levels of gene expression of these cytokines induced by B29 were significantly lower than that in the cohort inoculated with NTHi 2019 (the approximately 3.0 –11.4 fold decline in IL-1α, IL-1β, IL-6, IL-8, IL-10, TNF-α and iNOS, (P <0.05 in all cases). In addition, with the exception of IL-1β, the upregulations of TNFα, IL-8 and IL-6 were only sustained up to 12 h post inoculation, IL-1α and IL-10 for 24 h and iNOS for 48 h. These results indicate that disruption of the NTHi htrB gene may play a significant role in the induction of these genes.
It should be noted that comparisons of gene expression levels for the sham-inoculated controls versus noninoculated normal controls were included for these studies. Fold increases in gene expression levels following inoculation with PBS alone served as the inoculation control, providing information on how the physical trauma of the inoculation procedure impacted upon gene expression. Increases in gene expression for the sham controls (greater than a 10-fold increase in gene expression relative to the noninoculated control) were observed for IL-6 at 3 h and IL-1β and iNOS at 24 h.
Cytokine production in the rat middle ear following T.B. inoculation with nonviable NTHi
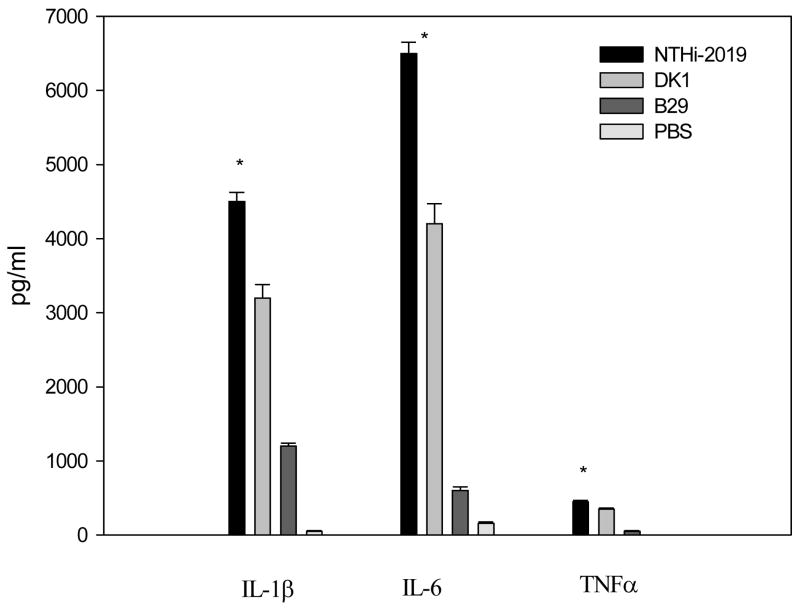
The significant induction of cytokine gene expression in the rat middle ear in vivo during the course of experimental OM was confirmed by quantitating the secretion of TNF-α, IL-1β, and IL-6 in middle ear lavage samples by use of enzyme-linked immunosorbent assays (ELISAs). At 24 h post inoculation, the concentration of cytokines in the middle ear lavage samples for controls was 50 and 160 pg/ml for IL-1β and IL-6, respectively (Fig. 4). No secreted TNFα was detected in middle ear lavage samples at this time point. The minimum detectable dose of TNF-α using the Quantikine kit was 5 pg/ml.
Fig 4.
Concentrations of cytokines in the middle ear lavage samples at 24 h post infection. Results are the mean concentrations of IL-1β, IL-6, and TNFα (± SEM) in middle ear lavage samples from two duplicate wells from a single experiment. *, P < 0.05 for the comparison (NTHi 2019 versus B29).
By comparison, at 24 h after T.B. inoculation, both NTHi 2019 and DK-1 induced a significant increase in the production of these cytokines relative to the sham-inoculated controls (P < 0.05 in all cases) (Fig. 4). Furthermore, the rats inoculated with NTHi 2019 had significantly higher levels of TNF-α, IL-1β, and IL-6 in the middle ear lavage samples (nine-, four-, and eleven fold more, respectively) than animals inoculated with B29 (P < 0.05 for all comparisons). There were no significant differences between the concentrations of IL-1β, IL-6, and TNFα induced by DK-1 and NTHI 2019. These results corroborate the gene expression data (see above) and indicate that secretion of IL- β, IL-6 and TNFα is more strongly induced by NTHI 2019 or DK-1 than that induced by B29.
Discussion
These data demonstrate that nonviable NTHi 2019 parent strain induced a significant up-regulation of cytokine and iNOS gene expression within the middle ear in the rat OM model. Our findings expand on the earlier investigations indicating the role of NTHi LOS in the production of TNFα and IL-1β in the middle ear mucosa in the guinea pig OM model [8]. Moreover, we report, for the first time, a differential induction of cytokines and iNOS genes mediated by two NTHi LOS mutant strains. Our results corroborate the earlier findings of Nichols et al. who originally described the reduced ability of NTHi B29 to induce TNFα gene expression and production in vitro [13]. These data are also consistent with our previous report, which demonstrated that expression of TNFα, IL-6 and IL-1β genes was significantly induced in HMEE cells stimulated with formalin-killed NTHi 2019 in vitro compared to that induced by NTHi B29 strain [12]. However, There is a difference in the potency of B29 in the induction of cytokine genes in HMEE cells in vitro and in rat middle ear epithelium in vivo, which may reflect the complex of environment in middle ear and the orchestrated interactions between the host inflammatory response and pathogens. It is noteworthy that the ability of NTHi 2019 and each LOS mutant strain to induce cytokine gene expression seems closely related to the local inflammatory response in the middle ear. Histological examination revealed significant differences in influxes of inflammatory cells in the middle ear between the rats infected with NTHi 2019 and B29. Our data strongly suggest that the induction of proinflammatory cytokine gene expression in the rat middle ear epithelium and the middle ear inflammation are attenuated in response to NTHi htrB mutant..
The factors that might account for the observed differential ability of NTHI 2019 and B29 in the induction of cytokine gene expression are not fully elucidated. LOS from wild-type NTHi 2019 was a potent inducer of IL-1β, IL-6 and TNFα secretion. The levels of IL-1β, IL-6 and TNFα secretion induced by B29 were at least four-fold less than the wild-type strain. This reduction in potency is similar to that described for htrB mutants in other bacteria [14–16]. NTHi 2019 htrB mutants have a predominantly tetraacyl lipid A compared to the hexaacylated parent strain (17). The level of lipid A acylation is critical to the immunostimulatory effects of LPS. A previous study demonstrated that penta-acylated LPS from a WaaN mutant of E. coli is much less potent than wild-type LPS in eliciting a proinflammatory cytokine response from cultured uroepithelial cells [18]. In addition, NTHi htrB mutants are more sensitive to bile and inducible human beta-defensins, and elicit lesser degrees of cytoskeletal rearrangement and less stimulation of host cell signaling in vitro [19, 20]. A recent report suggested that the proinflammatory action of NTHi LOS is thought to be mediated through Toll-like receptor 4 (TLR-4), however, the decrease in NTHi LOS acylation in NTHi B29 mutant strain exhibited TLR-2 dependent neutrophil influx and bacterial clearance in a mouse pneumonia model [21]. These results suggest that alterations of NTHI LOS subunit might alter recognition by TLRs and therefore impact the signaling pathways for the inflammatory response.
TNFα, IL-1β, IL-6, IL-8, IL-10 and iNOS are key proinflammatory cytokines and chemokines expressed during OM both clinically and experimentally. IL-1β, IL-6, and iNOS are proinflammatory mediators associated with monocyte/macrophage and lymphocyte activation. IL-8 belongs to the CXC chemokine subfamily, is chemotactic primarily for neutrophils (and for T cells, NK cells, endothelial cells, basophils, and eosinophils), and stimulates neutrophil degranulation, adhesion, and microbicidal activity. IL-10 is primarily a product of mononuclear cells and inhibits inflammatory and immunologic responses and plays a central role in autoregulation of the host response. These mediators act in concert. A previous report from our laboratory indicates that there is a correlation between endotoxin, TNFα and IL-1β concentrations in the middle ear effusions from children with OM [22]. Our data suggested that the most strongly induced cytokine by NTHi 2019 is IL-6, which has been shown to be essentially protective in infection and linked to its capacity to induce the release of acute-phase protein. Other cytokine and chemokine genes highly induced by NTHi 2019 were TNFα, IL-1β and IL-8. TNFα contributes to the acute phase of the inflammatory response, primes the immune system for rapid activity, and promotes the release of other cytokines, It is conceivable that the early induction of cytokine and chemokine genes drives the influx of neutrophils into the middle ear as evidenced by the enhanced accumulation of more inflammatory cells within the middle ear in the rats treated with NTHi 2019. However, there was no significant difference in the cytokine and chemokine gene expression at 72 h among the cohorts infected with the NTHi parent and LOS mutant strains. Nonviable NTHi 2019 induced a severe middle ear inflammatory response at 24h, which was reduced at 72h (data not shown). The resolution of NTHi middle ear infection at 72 h might account for the observed patterns of cytokine and chemokine gene expression.
It is believed that activation of middle ear mucosal epithelium and inflammatory cells contribute the overall gene expression levels observed within the middle ear following inoculation of nonviable NTHi. As the middle ear is relatively devoid of resident lymphocytes and has no lymphoid tissue, the early induction of genes in the middle ear epithelium that peaked at 3–6 h after infection and proceeded the accumulation of inflammatory cells suggested that the middle ear epithelium itself appears to play a key role in up-regulation of the host immune defense by recognizing and subsequently responding to invading pathogenic threats. IL-6, IL-1β, and TNF-α were detected in the middle ear lavage sample by 24 h post T.B. inoculation suggesting that the inflammatory cells contribute to the higher cytokine products in the middle ear lavage samples as the inflammatory cells accumulate in the middle ear. However, the significant alteration of the middle ear mucosal epithelium induced by NTHi 2019 compared to that by B29 also implicates the mucosa surface as one of the major contributors to the significant difference in gene transcript levels between these two cohorts.
In conclusions, the results from this study indicate that the disruption of the NTHi htrB gene that alters the phenotype of NTHi LOS may play a major role in the induction of these particular inflammatory mediators. These data will contribute to our understanding of the role of various LOS gene disruptions on OM pathogenesis and may thus provide potential new targets for future protection and intervention strategies.
Acknowledgments
This study was supported by NIH grant 5 R01 DC 00090-24 awarded to T.F. DeMaria.
Footnotes
Conflict of interest statement
All the authors declared there is no financial conflict interest with other people or organizations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spinola SM, Peacock J, Denny FW, Smith DL, Cannon JG. Epidemiology of colonization by nontypeable Haemophilus influenzae. J Infect Dis. 1986;154:100–109. doi: 10.1093/infdis/154.1.100. [DOI] [PubMed] [Google Scholar]
- 2.Faden H, Brodsky L, Bernstein J, Stanievich J, Krystofik D, Shuff C, Hong JJ, Ogra PL. Otitis media in children: Local immune response to nontypeable Haemophilus influenzae. Infect Immun. 1989;57(11):3555–3559. doi: 10.1128/iai.57.11.3555-3559.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, Chen J, Cheng Z, Robbins JB, Battery JF, Gu XX. Biological activities of antibodies elicited by lipooligosaccharide based-conjugate vaccines of nontypeable Haemophilus influenzae in an otitis media model. Vaccine. 2000;18:1264–1272. doi: 10.1016/s0264-410x(99)00381-3. [DOI] [PubMed] [Google Scholar]
- 4.DeMaria TF, Prior RB, Briggs BR, Lim DJ, Birck HG. Endotoxin in middle ear effusions from patients with chronic otitis media with effusion. J Clin Microbial. 1984;20(1):15–17. doi: 10.1128/jcm.20.1.15-17.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lino Y, Kaneko Y, Takasaka J. Endotoxin in middle ear effusions tested with Limulus assay. Acta Otolaryngol (Stockh) 1985;100:42–50. doi: 10.3109/00016488509108586. [DOI] [PubMed] [Google Scholar]
- 6.Melhus A, Ryan AF. Expression of cytokine genes during pneumococcal and nontypeable H. influenzae acute otitis media in the rat. Infect Immun. 2000;68(7):4024–4031. doi: 10.1128/iai.68.7.4024-4031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato K, Liebeler CL, Quartey MK, Le CT, Giebink GS. Middle ear fluid cytokine and inflammatory cell kinetics in the chinchilla otitis media model. Infect Immun. 1999;67(4):1943–1946. doi: 10.1128/iai.67.4.1943-1946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato K, Kwana M, Nonomura N, Nakano Y. Course of IL-1β, IL-6, IL-8, and TNF-α in the middle ear fluid of guinea pig otitis media model induced by nonviable H. influenzae. Ann Otol Rhinol Laryngol. 1999;108:599–563. doi: 10.1177/000348949910800606. [DOI] [PubMed] [Google Scholar]
- 9.Hebda PA, Burckart GJ, Alper CM, Diven WF, Doyle WJ, Zeevi A. Upregulation of messenger RNA for inflammatory cytokines in middle ear mucosal in a rat model of acute otitis media. Ann Otol Rhinol Laryngol. 1998;107:501–507. doi: 10.1177/000348949810700608. [DOI] [PubMed] [Google Scholar]
- 10.Long JP, Tong HH, Shannon PA, DeMaria TF. Differential Expression of Cytokine Genes and Inducible Nitric Oxide Synthase Induced by Opacity Phenotype Variants of Streptococcus pneumoniae during Acute Otitis Media in the Rat. Infect Immun. 2003;71:5531–5540. doi: 10.1128/IAI.71.10.5531-5540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMaria TF, Apicella MA, Nichols WA, Leake ER. Evaluation of the virulence of nontypeable H. influenzae lipooligosaccharide htrB and rfaD mutants in the chinchilla model of otitis media. Infect Immun. 1997;5(11):4431–4435. doi: 10.1128/iai.65.11.4431-4435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong HH, Chen Y, James MA, Van Deusen J, Welling DB, DeMaria TF. Expression of cytokine and chemokine genes by human middle ear epithelial cells induced by formalin-killed H. influenzae or its LOS htrB and rfaD mutants. Infect Immun. 2001;69(6):3678–3684. doi: 10.1128/IAI.69.6.3678-3684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols WA, Raetz CRH, Clementz T, Smith AI, Hanson JA, Ketterer MR, Sunshine M, Apicella MA. HtrB of Haemophilus influenzae: determination of biochemical activity and effects on virulence and lipooligosaccharide toxicity. J Endotoxin Res. 1997;4:163–172. [Google Scholar]
- 14.Jones BD, Nichols WA, Gibson BW, Sunshine MG, Apicella MA. Study of the role of the htrB gene in Salmonella typhimurium virulence. Infect Immun. 1997;65:4778–4783. doi: 10.1128/iai.65.11.4778-4783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis CD, Lindner B, Anjam Khan CM, Zahringer U, Demarco de Hormaeche R. The Neisseria gonorrhoeae lpxLII gene encodes for a late-functioning lauroyl acyl transferase, and a null mutation within the gene has a significant effect on the induction of acute inflammatory responses. Mol Microbiol. 2001;42:167–181. doi: 10.1046/j.1365-2958.2001.02619.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Ley P, Steeghs L, Hamstra HJ, ten Hove J, Zomer B, van Alphen L. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun. 2001;69:5981–5990. doi: 10.1128/IAI.69.10.5981-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee NG, Sunshine MG, Engstrom JJ, Gibson BW, Apicella MA. Mutation of the htrB locus of H. influenzae nontypeable strain 2019 is associated with modifications of lipid A and phosphorylation of the lipo-oligosaccharide. J Biol Chem. 1995;270(45):27151–27159. [PubMed] [Google Scholar]
- 18.Backhed F, Soderhall M, Ekman P, Normark S, Richter-Dahlfors A. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell Microbiol. 2001;3:153–8. doi: 10.1046/j.1462-5822.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- 19.Starner TD, Swords WE, Apicella MA, McCray PB., Jr Susceptibility of Nontypeable Haemophilus influenzae to Human {beta}-Defensins Is Influenced by Lipooligosaccharide Acylation. Infect Immun. 2002;70:5287–5289. doi: 10.1128/IAI.70.9.5287-5289.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swords WE, Chance DL, Cohn LA, Shao J, Apicella MA, Smith AL. Acylation of the lipooligosaccharide of Haemophilus influenzae and colonization: an htrB mutation diminishes the colonization of human airway epithelial cells. Infect Immun. 2002;70(8):4661–8. doi: 10.1128/IAI.70.8.4661-4668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenz E, Chemotti DC, Jiang AL, McDougal LD. Differential involvement of Toll-like receptors 2 and 4 in the host response to acute respiratory infections with wild-type and mutant Haemophilus influenzae strains. Infect Immun. 2005;73:2075–2082. doi: 10.1128/IAI.73.4.2075-2082.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willet DN, Rezaee RP, Billy JM, Tighe MB, DeMaria TF. Relationship of endotoxin to tumor necrosis factor-α and interleukin-1β in children with otitis media with effusion. Ann Otol Rhinol Laryngol. 1998;7(1):28–33. doi: 10.1177/000348949810700106. [DOI] [PubMed] [Google Scholar]
- 23.Lee NG, Sunshine MG, Apicella MA. Molecular cloning and characterization of the nontypeable H. influenzae 2019 rfaE gene required for lipopolysaccharide biosynthesis. Infect Immun. 1995;63(3):818–824. doi: 10.1128/iai.63.3.818-824.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols WA, Gibson BW, Melaugh W, Lee NG, Sunshine M, Apicella MA. Identification of the ADP-L-glycero-D-manno-heptose-6-epimerase (rfaD) and heptosyltransferase II (rfaF) biosynthesis genes from nontypeable Haemophilus influenzae 2019. Infect Immun. 1997;65:1377–1386. doi: 10.1128/iai.65.4.1377-1386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okazaki N, DeMaria TF, Briggs BR, Lim DJ. Experimental otitis media induced by formalin-killed H. influenzae: cytological and histological study. Amer J Otolaryngol. 1984;5:80–92. doi: 10.1016/s0196-0709(84)80026-5. [DOI] [PubMed] [Google Scholar]