Abstract
PURPOSE
To investigate in vivo 1H magnetic resonance spectroscopy imaging of lactate for assessing tumor hypoxia in head and neck (HN) cancers and to determine its utility in predicting response and outcomes.
METHODS
Volume-localized Lactate-edited 1H MRS at 1.5 T was performed in vivo on involved neck nodes and control subcutaneous tissues in 36 patients with Stage IV HN cancers. Signal intensities (SI) of Lactate, Choline (Cho), Creatine (Cr) and the Cho/Cr ratios were measured. Tumor pO2 was obtained in the same lymph node prior to MRS. Patients were treated with either 2 cycles of induction chemotherapy (Tirapazamine, cisplatin, 5-Fluorouracil) followed by simultaneous chemoradiotherapy or the same regimen without Tirapazamine. Lactate SI and Cho/Cr were correlated with tumor pO2, nodal response and locoregional control.
RESULTS
Lactate SI was higher for the involved nodes (median: 0.25) than for the subcutaneous tissue (median: 0.04, p = 0.07). There was no significant correlation between Lactate SI and tumor pO2 (mean: 0.46 ± 0.10 for hypoxic nodes [pO2≤10mmHg, n = 15] versus 0.36 ± 0.07 for non-hypoxic nodes [pO2>10mmHg, n = 21], p = 0.44). There was a significant correlation between Cho/Cr ratios and tumor pO2 (mean: 2.74 ± 0.34 for hypoxic nodes versus 1.78 ± 0.31 for non-hypoxic nodes, p = 0.02). There was no correlation between Lactate SI and complete nodal response (p = 0.52) or locoregional control rates.
CONCLUSIONS
Lac SI did not correlate with tumor pO2, treatment response or locoregional control. Further research is needed to refine this technique.
Keywords: Lactate, MRS, hypoxia, head and neck cancer, prognosis
Introduction
Tumor hypoxia is a common phenomenon in solid tumors and has been shown to adversely affect treatment outcomes in patients with head and neck (HN) squamous cell carcinoma (SCC) treated with conventional therapy.1–4 There has been a strong interest in targeting hypoxia over the years; however, it is unclear which is the best method for detecting hypoxia. Recently, imaging hypoxia has become an active area of investigation that yields promising results.5, 6 Most of the imaging studies have focused on the use of specialized hypoxia-specific tracers such as 18F-Misonidazole or Cu-ATSM coupled with PET (Position Emission Tomography) or SPECT (single photon emission computed tomography) imaging. 5–7 However, these technologies require expensive dedicated equipment for both imaging and tracer generation. We theorize that a different approach to image hypoxia using magnetic resonance imaging without the requirement of exogenous tracers would allow for a non-invasive and less expensive means of detecting hypoxia and for treatment monitoring.
In-vivo 1H MR spectroscopy (1H MRS) has been shown to offer a non-invasive means of detecting the presence of active tumor. The most common metabolites that have been studied and validated are choline and creatine. The choline: creatine ratio (Cho/Cr) has been shown to be helpful in the discrimination between regions of normal tissue, tumor and necrosis in several solid tumors, specifically gliomas and prostate cancers.8, 9 More recently, it has also been applied to head and neck (HN) cancers with promising preliminary results.10 Aside from choline and creatinine, very little has been published regarding the role of other metabolites in tumor detection or prognostication in HN cancers.
Lactate is a metabolite that is found at high levels under tumor hypoxia. The lack of intracellular oxygen shifts the balance of cellular energy production from oxidative phosphorylation to glycolysis, whose end product is lactate. Poor circulation in the hypoxic tumor microenvironment results in lactate accumulation, which theoretically can be detected with 1H MRS. Ex vivo measurements of lactate levels using a luciferase bioluminescence technique revealed that elevated tumor lactate concentration was associated with a higher risk of nodal and distant metastasis, local recurrence and poorer treatment outcomes in patients with HN and cervical cancers.11–14 In vitro lactate 1H-MRS showed that high lactate signal was associated with more failure and poorer survival in patients with HN and lung cancers.15, 16 To date, very little is known about in vivo lactate 1H MRS, its relationship to tumor oxygenation and its prognostic significance. We have therefore conducted a prospective study to evaluate the relationship between lactate signal intensity (SI) in the proven metastatic node to tumor pO2 and treatment outcomes defined as nodal response and locoregional control in patients with stage IV HN squamous cell carcinoma. In a preliminary report of the first 14 patients, we noted that hypoxic nodes, defined as those with median pO2 < 10 mmHg had higher lactate levels than non-hypoxic nodes.17 Here we report mature results of a larger group of 36 patients, in whom we correlated lac SI with tumor pO2 and treatment outcomes.
Materials and Methods
Patient population
Between 7/1996 and 6/2001, 62 patients with node positive, resectable stage IV HN SCC were enrolled on an IRB approved institutional phase II study, in which patients were randomized to receive either 2 cycles of induction chemotherapy (cisplatin and 5-Fluorouracil [5-FU]) followed by simultaneous chemoradiotherapy (cisplatin and 5-FU) or the same regimen with Tirapazamine, a hypoxic cell cytotoxin. The details of the treatment regimens and long-term treatment results of this study have been previously published.18 All patients underwent oxygen tension (pO2) measurements in the pathologically proven involved neck nodes with the Eppendorf microelectrode (“pO2 Histograph”, Eppendorf, Hamburg, Germany) as previously published.19 Forty-one patients also had in vivo 1H MRS of total choline (Cho), creatine (Cr), lipid and lactate (Lac) in the same node prior to therapy; of these 5 had un-interpretable spectra due to significant motion artifact, leaving 36 with analyzable data.
MRS Measurements and processing
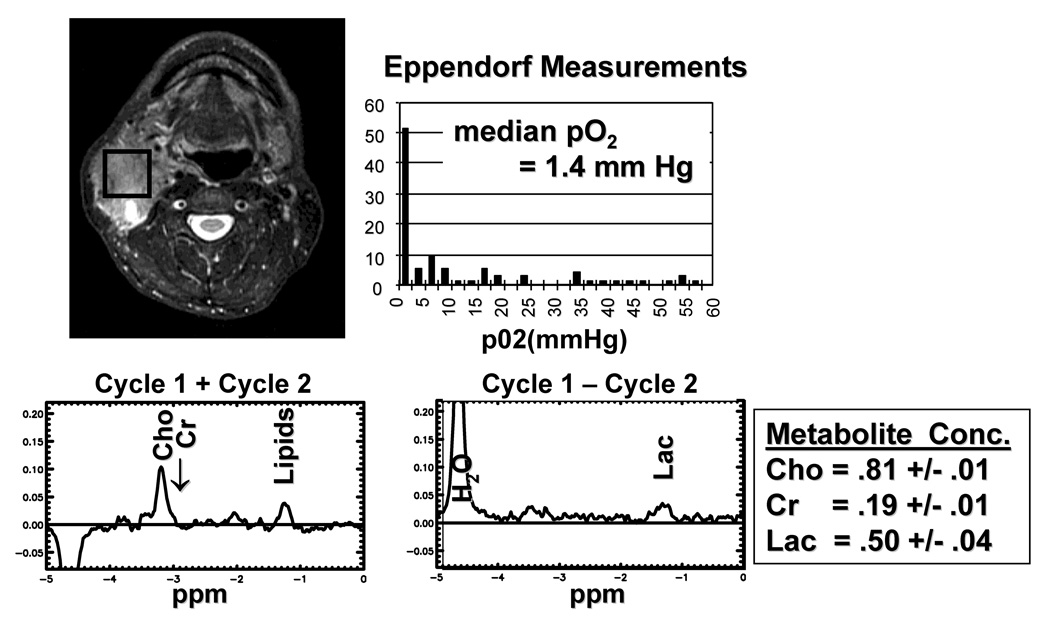
The details of the 1H MRS measurement technique and processing algorithms have been previously published.17 Briefly, data were collected at 1.5T using a General Electric Echo-Speed system (GE Medical System, Milwaukee, WI). Acquisition was performed with a four-element head, neck and cervical phased-array coil (MRI Devices Corp., Waukesha, WI) operating in receive-only mode. To help localize the lesion for MRS acquisition, T1-weighted spin-echo (500 ms / 8 ms / 2 [TR/TE/excitations]; field of view, 21cm; matrix 256X160) and dual spin-echo proton density-T2 weighted (3000 ms / 14 ms / 84 ms / 2 [TR/first TE/second TE/excitations]) images were first acquired. The point-resolved spectroscopy (PRESS) volume was prescribed to encompass as much T2-hyperintense lesion as possible. The average prescribed volume was 5.0 cc (range: 0.73 to 20.6 cc). The MRS data (2000 / 144 ms [TE/TR]) were acquired with a receiver bandwidth of 1250 Hz (512 points). Three minimum-phase CHESS pulses of 60 Hz bandwidth were used for water suppression. On the average, 256 excitations were acquired, yielding an MRS study time of 8.5 minutes. In patients with small nodes (press volume < 1cc), the MRS acquisition time was doubled. In 5 patients, an additional MRS data set was acquired from a region that comprise of contralateral neck muscle and subcutaneous tissue for comparison. Normal appearing nodes were not used for comparison as they were too small. Volume localized spectral editing was achieved using a J-different technique that incorporates two inversion (BASING) pulses into the PRESS excitation sequence as previously described.17 Briefly, separation of Lac from overlapping singlets was achieved by placing the Lac methyl quartet (4.1 ppm) within the BASING inversion band for Cycle 1 of the 2-cycle scheme. For Cycle 2, the BASING inversion band was shifted downfield of the Lac quartet. With the time separation (ΔtBAS) between the 2 BASING pulses set to ΔtBAS = 1/(2J) and for TE = 1/J, addition of Cycle 1 and Cycle 2 excitations yielded the singlets, including Cho and Cr, whereas subtraction (Cycle 1– Cycle 2) yielded the Lac methyl doublet. The bandwith of the maximum phase BASING pulses was sufficiently narrow to allow for simultaneous acquisition of the Lac doublet (1.3 ppm) and edited singlets upfield, including Cho (3.2 ppm). Figure 1 shows an example of the edited spectra in a patient.
Figure 1.
An example of the MRS data and pO2 histogram in a patient with a hypoxic node. A. T2 weighted image (3000/85/2) showing the location of the PRESS (spectroscopic) volume for spectroscopic imaging; B. Histogram pf pO2 measurement. The median pO2 is 1.4 mm Hg. C. Uncoupled spectra (cycle 1 + cycle 2) for resolution of Choline and Creatine signals. D. Coupled spectrum (cycle 1 − cycle 2) for resolution of lactate signal. The end result Choline, Creatine and Lactate SI is shown.
For MRS processing, the accumulated free induction decays were processed and displayed using software written by the authors for the Matlab (The Mathworks Inc., Natick, MA) and IDL (Research Systems Inc., Boulder, CO) platforms. To allow for comparison of absolute spectral intensity (SI), the phase-array data from each patient were normalized by the PRESS volume and receiver gain and combined in a manner designed to maximize the final signal/noise (S/N) ratio. To reduce errors resulting from motion, a phase regularization algorithm was developed to lessen the deleterious effects of shot-to-shot phase variations. Care was also taken to quantify lipid cancellation errors that remain after processing. Peak areas assigned to Cho and Cr were calculated by numerically integrating the uncoupled (summed) spectra within the 13 Hz windows of 3.3 to 3.1 ppm and 3.1 to 2.9 ppm, respectively. Lac and lipid peak areas were calculated by numerically integrating the respective edited and unedited spectra from 1.5-1.1 ppm (26 Hz).
Statistical analysis
Statistical analysis was performed using Statview (SAS Institute Inc, Carey, NY) statistical software. Mann Whitney U and ANOVA tests were use to compare the average Lac SI and Cho/Cr ratio between different patient groups. Locoregional control (LRC), freedom from distant metastasis (FFDM), freedom from relapse (FFR) and overall survival (OS) rates were calculated using the Kaplan-Meier product-limit method.20,21 Significance levels among curves were determined using the log-rank test on univariate analysis. Only relapses above the clavicles were considered locoregional failures and relapses below the clavicle or brain or any bony metastases were considered distant failures. All sites of recurrence were coded as failures for FFR and all deaths, regardless of causes, were recorded for OS.
Results
Between 7/1996 and 6/2001, 62 patients with resectable stage IV HN SCC were randomized to 2 cycles of induction chemotherapy (TPZ, cisplatin and 5-Fluorouracil [5-FU]) followed by simultaneous chemoradiotherapy (TPZ, cisplatin and 5-FU) or the same regimen without TPZ. The results of this phase II randomized study has been previously published and showed no difference in outcome with the addition of TPZ.18 Of these 62 patients, 41 patients underwent lactate and Cho/Cr MRS in the involved nodes as part of the correlative study to assess the role of MRS in detecting tumor hypoxia. Five patients had uninterpretable data due to significant motion artifact and 36 patients formed the cohort of this study. Figure 1 shows an example of the MRS data and pO2 histogram in a patient with a hypoxic node. Table 1 shows the patient characteristics for the MRS patients and all randomized patients. There were no significant differences among any of the characteristics between the two populations.
Table1.
Comparison of patient, tumor and treatment characteristics between the randomized patients and patients with Lactate MRS
| Characteristics | All Patients (N = 62) | MRS Patients (n = 36) | P - value |
|---|---|---|---|
| Median age (range) | 58 (39–81) | 57 (41–75) | NS |
| Male: Female (%) | 54:8 | 35:1 | |
| Primary Tumor Site | |||
| Oropharynx | 39 | 25 | NS |
| Oral cavity | 4* | 1 | |
| Larynx | 6 | 2 | |
| Hypopharynx | 11* | 8 | |
| Others | 3 | 0 | |
| T-stage | NS | ||
| 0 | 2 | 0 | |
| 1 | 8 | 7 | |
| 2 | 16 | 10 | |
| 3 | 12 | 5 | |
| 4 | 24 | 14 | |
| N-stage | NS | ||
| 1 | 5 | 5 | |
| 2 | 43 (2a:7, 2b:23, 2c:13) | 22 (2a:4, 2b:10; 2c:8) | |
| 3 | 14 | 9 | |
| Median Tumor pO2 | 12.13 (0.35–45.17) | 11.82 (0.43–37.20) | |
| ≤ 10 mm Hg | 25 | 15 | NS |
| >10 mm Hg | 37 | 21 | |
| Treatment | NS | ||
| CRT alone | 29 | 16 | |
| CRT + TPZ | 33 | 20 | |
| Neck dissection | NS | ||
| No | 40 | 25 | |
| Yes | 22 | 11 | |
NS: not statistically significant (p > 0.05) by χ2, Fisher’s exact or T-test.
One patient with 2 primary tumor sites (1 in the hypopharynx and 1 in the oral cavity)
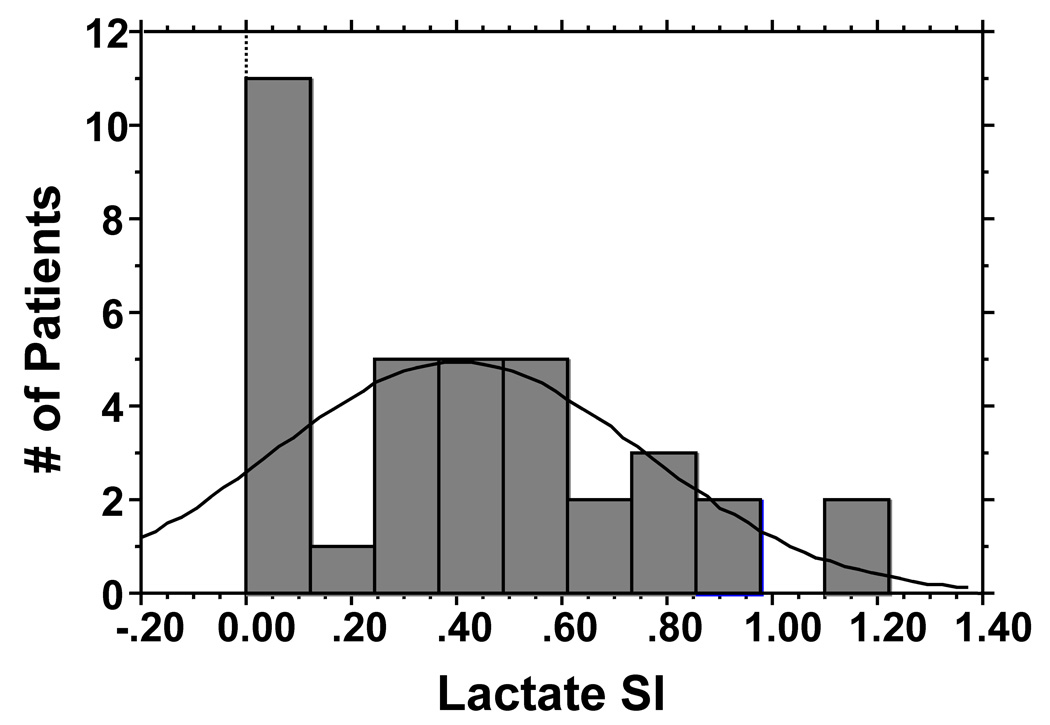
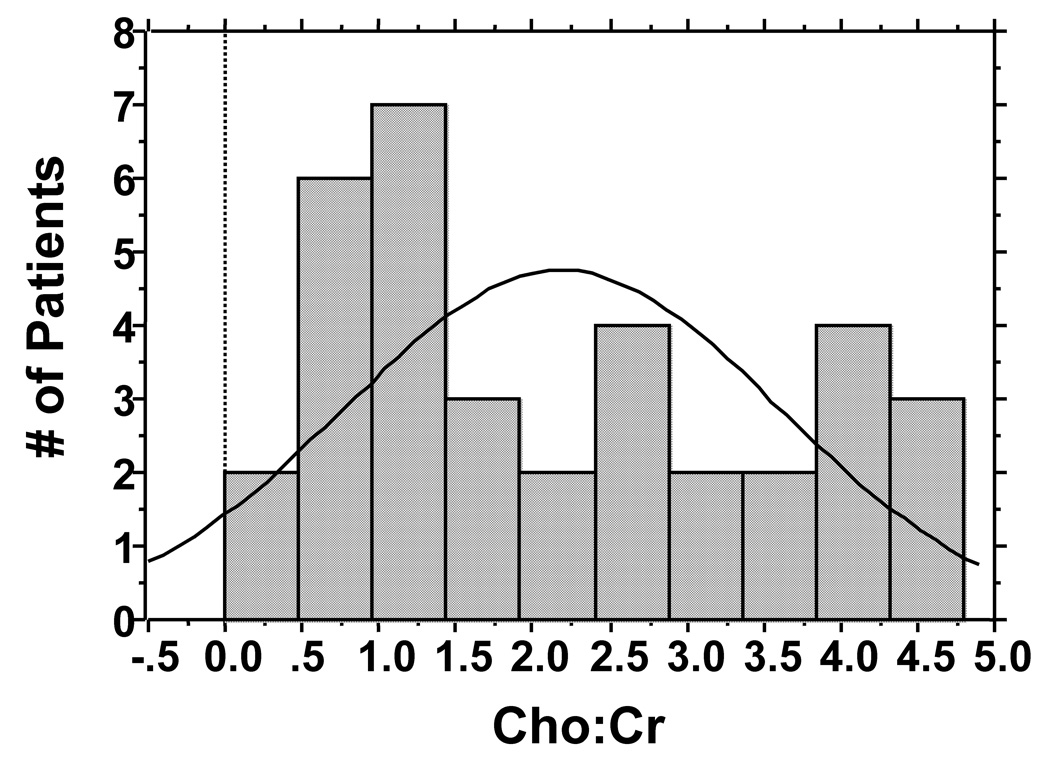
Of 36 patients with Lactate MRS, 8 patients had lactate signals at or below the baseline noise levels, all of which were interpreted as yielding as SI of 0. Figure 2a shows the histogram for lactate SI. The median Lac SI was 0.38 and the mean was 0.40 (range 0–1.22, Table 2). The Cho/Cr was available for 35 patients and missing in 1 patient due to zero creatine reading. Figure 2b shows the histogram for Cho/Cr ratios. The median value was 1.88 and the mean was 2.19 (range 0–4.8, Table 2). The first 5 patients also had lactate and Cho/Cr MRS assessments of their contralateral subcutaneous tissues. Table 2 shows that normal subcutaneous tissues had lower Lac and Cho/Cr values than the involved nodes.
Figure 2.
Figure 2a: Histogram showing distribution of Lac SI for patients who had MRS studies.
Figure 2b: Histogram showing distribution of Cho:Cr ratio for patients who had MRS studies.
Table 2.
Distribution of spectroscopic data
| Site | N | Lactate SI | Choline |
|---|---|---|---|
| Subcutaneous | 5 | 0.04 +/− 0.04 | 0.66 +/− 0.10 |
| Node* | 5 | 0.25 +/− 0.12 | 3.53 +/− 1.48 |
| p = 0.07 | p = 0.11 | ||
| All Evaluable | 36 | 0.40 +/− 0.06 | 2.19 +/− 0.24 |
| Node | (0–1.22) | (0–4.80) | |
For 5 patients with paired control subcutaneous tissue measurements
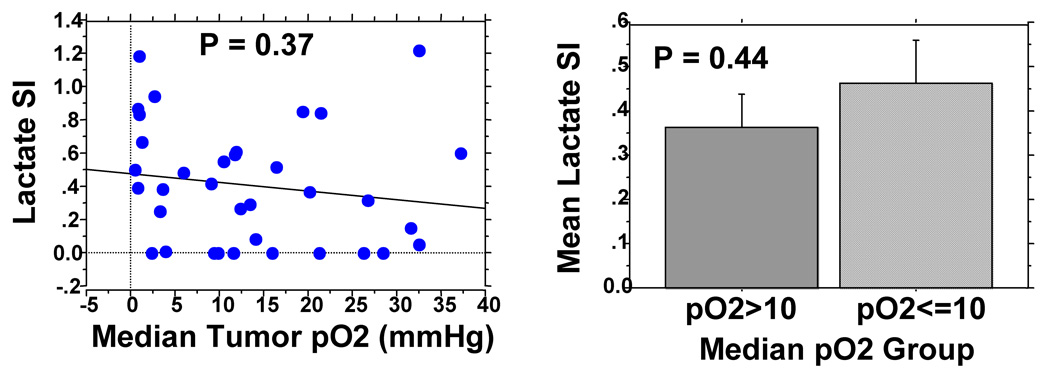
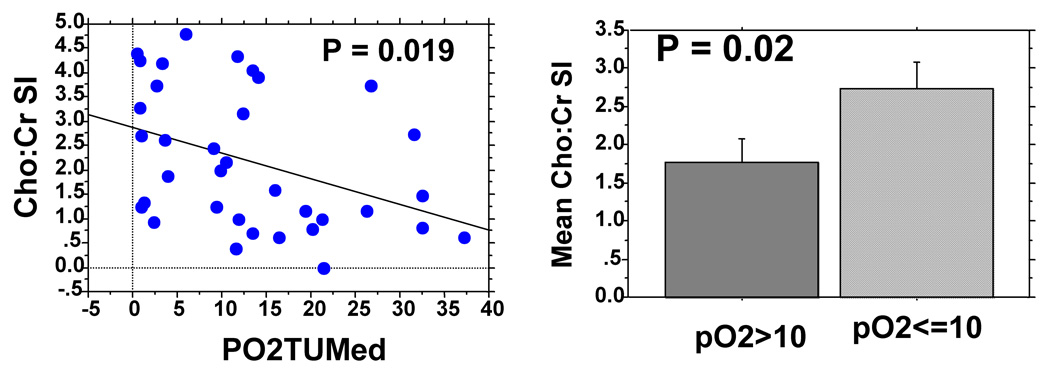
All patients had tumor pO2 assessment of the same cervical nodes with the Eppendorf histograph. Figure 3 shows no significant correlation between tumor pO2 and Lac SI. The mean Lac SI for hypoxic nodes (pO2≤10mmHg, n = 15) was 0.46 ± 0.10 [standard error] and it was 0.36 ± 0.07 for non-hypoxic nodes (pO2>10mmHg, n = 21, p = 0.44). There was, however, a significant correlation between Cho/Cr ratios and tumor pO2 (Figure 4a, p = 0.019) but the correlation coefficient was relatively low (R = 0.39). The mean Cho/Cr was 2.74 ± 0.34 for hypoxic nodes and 1.78 ± 0.31 for non-hypoxic nodes (p = 0.02).
Figure 3.
Figure 3a: Lack of correlation between Lac SI and tumor pO2.
Figure 3b: Correlation between Cho:Cr ratio and tumor pO2.
Figure 4.
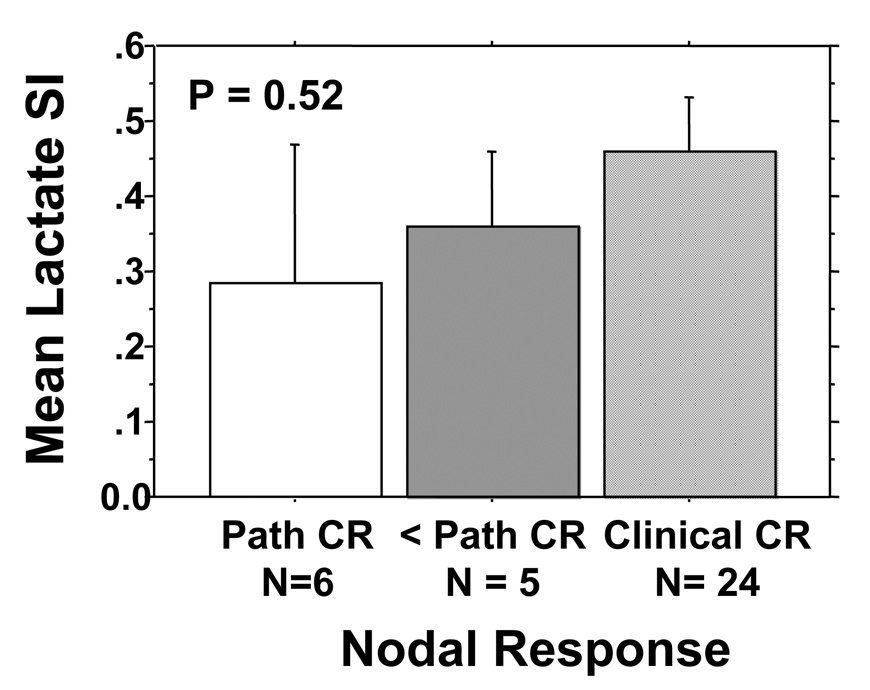
Mean Lac SI (+/− Standard error) by clinical and pathologic cervical nodal response to treatment. One patient dropped out of protocol early and was not assessable for pathologic response.
There was no correlation between Lac SI and nodal stage. In addition, there was no correlation between Lac SI and nodal response that was assessed both clinically and pathologically. As specified in the prospective study, patients who had a clinical complete response (N= 24) as evaluated by both physical examination and imaging studies (CT or MRI) at 50 Gy of radiation were followed clinically. One patient dropped out of the study after induction chemotherapy and was not assessable for nodal response. Eleven patients had less than a complete nodal response at 50 Gy and underwent a planned neck dissection as specified in the study; of these, 5 had residual nodal cancer and classified as incomplete pathologic responders and 6 had no residual cancer and classified as complete pathologic responders. Figure 4 shows the relationship between cervical nodal response and Lac SI. Although pathologic non-responders had higher Lac SI than pathologic responders, the clinical complete responders had the highest Lac SI; though none of these differences were significant.
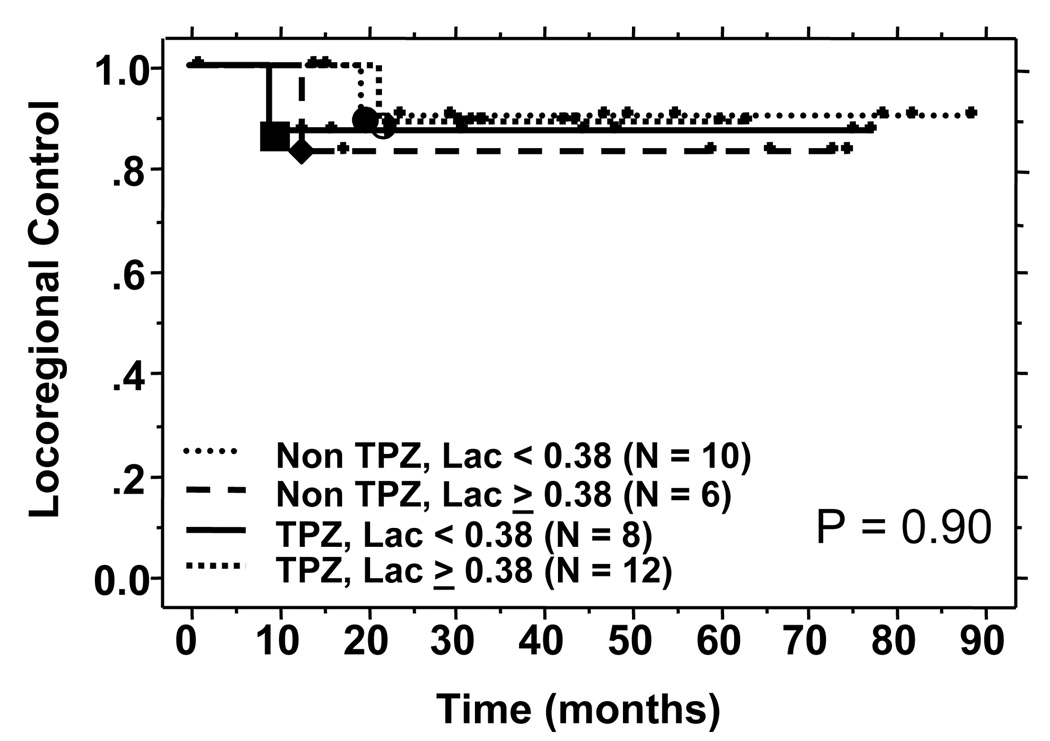
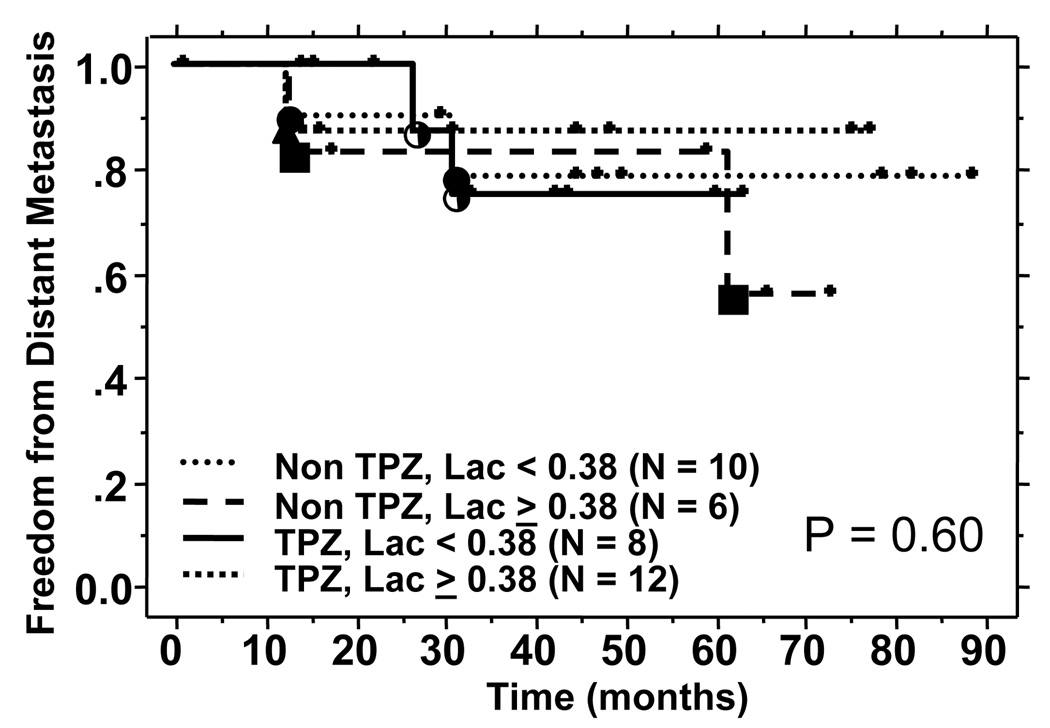
At a median follow up of 61 months, a total of 4 patients have either uncontrolled locoregional disease or locoregional failures. There was no correlation between Lac SI and locoregional failure in either the control group or the TPZ treated group. The results are shown in Figure 5a. Similarly, there was no difference in the freedom from metastasis in the high and low Lac SI group when stratified for treatment (Figure 5b). A non-correlation was also noted for overall survival (data not shown).
Figure 5.
Figure 5a: Locoregional control by Lac SI and treatment groups.
Figure 5b: Freedom from distant metastasis by Lac SI and treatment groups.
Discussion
The prognostic significance of lactate was first described by Schwickert et al who showed that elevated tumoral lactate levels, as measured by bioluminescence imaging from tumor biopsies, correlated with the presence of nodal metastasis in 11 patients with cervical tumors.11 Since then, the same group has reported similar findings for HN cancers and for a larger group of cervical cancer patients.12, 13 Brizel et al, using the same technologies, showed that elevated tumoral lactate concentration from tumor biopsies were associated with the subsequent development of nodal and/or distant metastasis in HN cancer patients.14 This approach, though promising, requires fresh frozen tumor tissues for analysis as well as technical expertise and specialized equipments. In addition, it does not provide in vivo spatial distribution of lactate rich regions.
In addition to bioluminescent approach, MR spectroscopy has also been used to quantify lactate levels in solid tumors. In most reported studies for tumor sites outside of the brain, lactate MRS was mainly performed on fresh frozen tumor specimens that had previously been removed surgically from the patients. Yokota et al reported that elevated lactate levels by ex-vivo MRS correlated with a higher risk of recurrence and a poorer overall survival in 21 non-small cell lung cancer patients treated surgically.16 Similarly, Bezabeh et al found that the ex vivo tumor spectral intensity ratio of 1.3/0.9 ppm, which corresponded to either lipid or lactate signal, significantly correlated with treatment failures in HN cancers.15 However, none of these studies have attempted to measure lactate levels in vivo, while the tumors were still in the patients. The only reported in vivo studies have focused on central nervous system tumors where motion could be minimized and specialized head coils could be used.22
To our knowledge, this is the first prospective study to perform in vivo lactate MRS in HN cancers and to correlate the signal intensities to tumor pO2 and treatment outcomes. Our patients were different from the previously reported populations in several aspects. All patients had to have nodal metastasis for lactate and pO2 assessment; therefore, we could not determine the relationship between Lac SI and nodal metastasis as previously reported for the bioluminescent technique. In addition, all patients were treated in a uniform manner on a prospective study with chemoradiation +/− TPZ, using an aggressive regimen with induction and concurrent chemotherapy, which may have affected the results of the study. Similar to other studies, we found lactate signals to be much higher in the involved nodes than in the control subcutaneous tissues in the first 10 patients. In the first 14 patients, we also noted an encouraging direct correlation between Lac SI and tumor pO2.17 However, with more patient accrual, the correlation disappeared. This discrepancy could be explained by the substantial technical difficulties associated with in vivo lactate MRS measurements in human HN cancers. These include motion artifacts from respiration, swallowing and carotid pulsation, which leads to poor magnetic field homogeneity and compromised localization. This is compounded by magnetic susceptibility problems arising from tissue-bone-air interfaces. Such technical difficulties can be surmounted for metabolites that exist at high concentration and whose spectra can be easily resolved from noise. However, for low-level metabolites such as lactate, these technical difficulties can become formidable, especially with the 1.5T magnet. A higher strength magnet such as a 3T magnet may help to improve the signal to noise ratio.
For our studies, lactate and lipid peak areas were calculated by numerically integrating the respective spectra from 1.5-1.1 ppm (26 Hz) based on known chemical shifts of lactate. However, we did not perform independent validation studies in xenograft models to confirm that these peaks are indeed unrelated. The close vicinity of the lactate peak and the very large overlapping lipid peak can easily corrupt the lactate signal, causing zero or negative lactate readings. As a result, 8 of 36 patients had Lac SI of zero, which significantly affected the sensitivity of the method. Another source of error in Lac measurements may be due to contamination by the neighboring alanine resonance (1.5 ppm), which has been detected in in vitro extracts of HN cancers. Finally, the presence of tumor necrosis, which is common in HN cancers, can also confound both Lac and tumor pO2 readings. While tumor necrosis can cause spuriously low pO2 measurements, Lac levels have been shown to be low in necrotic areas in the bioluminescent studies.11, 12 Hence, a tumor with large amount of necrosis may appear hypoxic on Eppendorf measurements but have low Lac SI on MRS.
Interestingly, we noted a correlation between Cho:Cr ratio and tumor pO2. Choline signal, which includes contributions from choline, phosphocholine, glycerol-phosphocholine and other trimethylamines, reflects cell membrane synthesis and breakdown and is characteristically elevated in tumors, including HN cancers.10 It has also been correlated with increased cell density, tumor grade and proliferation in gliomas.23, 24 Creatine signal, which includes creatine and phosphocreatine, reflects energy metabolism and the levels can be low in hypoxic and necrotic regions.17, 25 The increased Cho:Cr ratios in our series was more a reflection of a declining creatine signal rather than of an increasing choline signal. This suggested that the correlation between Cho:Cr and tumor pO2 may be due to the presence of necrosis in the involved nodes since necrosis can result in low pO2 readings and elevated Cho:Cr ratio due to decreased creatine signal. However, we cannot directly rule out a potential real correlation between Cho:Cr signal and tumor oxygenation.
Lactate SI, as measured with this approach, did not correlate with treatment response or long-term outcomes as measured by locoregional control and freedom from distant metastasis. The low sensitivity of the method is probably the major reason for this lack of correlation. In addition, the small number of patients and the low number of events may be another explanation for this lack of correlation. Interestingly, patients with high Lac SI who received chemoradiation alone had the highest risk of distant metastasis compared to the other groups, though the difference was small and not statistically significant. Another reason for lack of correlation could be due to potential contamination of the resolved peaks by other metabolites such as alanine and lipids.
In summary, we have attempted to perform in vivo lactate MRS on the involved cervical nodes of patients with stage IV HN cancers. Our results suggest that the measurements as they currently stand, do not correlate with tumor pO2 or treatment outcomes. Further technical improvements in in vivo Lac SI measurements in HN cancer patients are warranted. Future investigations will focus on improved acquisition and processing strategies, implementing higher order shimming techniques and imaging at higher fields (>= 3T) to achieve finer chemical resolutions and measurement of both water and metabolite relaxation time to improve absolute metabolite quantification.
Acknowledgement
The authors would like to thank Derek Huang for his assistance in submitting the manuscript.
Acknowledgment of Research Support: This study was supported by the USPHS Grant 1 R01 CA118582-01 (QTL,) and CA-67166 (EG, HP, JMB, DS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Poster discussion presentation at the American Society of Therapeutic Radiology and Oncology, Nov 2007, Los Angeles, CA.
Conflicts of Interest Notification: No actual or potential conflicts of interest exist.
Bibliography
- 1.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Brizel DM, Scully SP, Harrelson JM, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 3.Rudat V, Vanselow B, Wollensack P, et al. Repeatability and prognostic impact of the pretreatment pO(2) histography in patients with advanced head and neck cancer. Radiother Oncol. 2000;57:31–37. doi: 10.1016/s0167-8140(00)00200-0. [DOI] [PubMed] [Google Scholar]
- 4.Le QT, Giaccia AJ. Therapeutic exploitation of the physiological and molecular genetic alterations in head and neck cancer. Clin Cancer Res. 2003;9:4287–4295. [PubMed] [Google Scholar]
- 5.Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–2104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 6.Rajendran JG, Schwartz DL, O'Sullivan J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–5441. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Yu J, Xing L, et al. Serial hypoxia imaging with 99mTc-HL91 SPECT to predict radiotherapy response in nonsmall cell lung cancer. Am J Clin Oncol. 2006;29:628–633. doi: 10.1097/01.coc.0000242345.71582.e0. [DOI] [PubMed] [Google Scholar]
- 8.Mountford C, Lean C, Malycha P, et al. Proton spectroscopy provides accurate pathology on biopsy and in vivo. J Magn Reson Imaging. 2006;24:459–477. doi: 10.1002/jmri.20668. [DOI] [PubMed] [Google Scholar]
- 9.Kwock L, Smith JK, Castillo M, et al. Clinical applications of proton MR spectroscopy in oncology. Technol Cancer Res Treat. 2002;1:17–28. doi: 10.1177/153303460200100103. [DOI] [PubMed] [Google Scholar]
- 10.Mukherji SK, Schiro S, Castillo M, et al. Proton MR spectroscopy of squamous cell carcinoma of the extracranial head and neck: in vitro and in vivo studies. AJNR Am J Neuroradiol. 1997;18:1057–1072. [PMC free article] [PubMed] [Google Scholar]
- 11.Schwickert G, Walenta S, Sundfor K, et al. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. 1995;55:4757–4759. [PubMed] [Google Scholar]
- 12.Walenta S, Wetterling M, Lehrke M, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–921. [PubMed] [Google Scholar]
- 13.Walenta S, Salameh A, Lyng H, et al. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997;150:409–415. [PMC free article] [PubMed] [Google Scholar]
- 14.Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 15.Bezabeh T, Odlum O, Nason R, et al. Prediction of treatment response in head and neck cancer by magnetic resonance spectroscopy. AJNR Am J Neuroradiol. 2005;26:2108–2113. [PMC free article] [PubMed] [Google Scholar]
- 16.Yokota H, Guo J, Matoba M, et al. Lactate, choline, and creatine levels measured by vitro 1H-MRS as prognostic parameters in patients with non-small-cell lung cancer. J Magn Reson Imaging. 2007;25:992–999. doi: 10.1002/jmri.20902. [DOI] [PubMed] [Google Scholar]
- 17.Star-Lack JM, Adalsteinsson E, Adam MF, et al. In vivo 1H MR spectroscopy of human head and neck lymph node metastasis and comparison with oxygen tension measurements. AJNR Am J Neuroradiol. 2000;21:183–193. [PMC free article] [PubMed] [Google Scholar]
- 18.Le QT, Taira A, Budenz S, et al. Mature results from a randomized Phase II trial of cisplatin plus 5-fluorouracil and radiotherapy with or without tirapazamine in patients with resectable Stage IV head and neck squamous cell carcinomas. Cancer. 2006;106:1940–1949. doi: 10.1002/cncr.21785. [DOI] [PubMed] [Google Scholar]
- 19.Terris DJ, Dunphy EP. Oxygen tension measurements of head and neck cancers. Arch Otolaryngol Head Neck Surg. 1994;120:283–287. doi: 10.1001/archotol.1994.01880270031006. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan ES, Meier P. Non-parametric estimations from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;34:187–229. [Google Scholar]
- 22.Tarnawski R, Sokol M, Pieniazek P, et al. 1H-MRS in vivo predicts the early treatment outcome of postoperative radiotherapy for malignant gliomas. Int J Radiat Oncol Biol Phys. 2002;52:1271–1276. doi: 10.1016/s0360-3016(01)02769-9. [DOI] [PubMed] [Google Scholar]
- 23.Girard N, Wang ZJ, Erbetta A, et al. Prognostic value of proton MR spectroscopy of cerebral hemisphere tumors in children. Neuroradiology. 1998;40:121–125. doi: 10.1007/s002340050551. [DOI] [PubMed] [Google Scholar]
- 24.Tamiya T, Kinoshita K, Ono Y, et al. Proton magnetic resonance spectroscopy reflects cellular proliferative activity in astrocytomas. Neuroradiology. 2000;42:333–338. doi: 10.1007/s002340050894. [DOI] [PubMed] [Google Scholar]
- 25.Payne GS, Leach MO. Applications of magnetic resonance spectroscopy in radiotherapy treatment planning. Br J Radiol. 2006;79(Spec No 1):S16–S26. doi: 10.1259/bjr/84072695. [DOI] [PubMed] [Google Scholar]