Mechanical circulatory support devices (MCSD) are a major form of therapy for congestive heart failure. However, MCSD have been associated with a high incidence of infections. The most common of these infections involves the transcutaneous driveline 1–4. Here, we describe a murine driveline model developed to study MCSD-related infections.
This study was reviewed and approved by the IACUC. The clinical driveline outer surface is composed of Dacron™, a highly textured material designed to promote tissue integration. In our model, drivelines were constructed in the following manner: Dacron™ was glued onto a solid 2mm silicon tube using liquid silicon (Figure1A). Drivelines (15 × 3mm) were washed in 0.1% Tween, deionized water followed by 70% alcohol, and sterilized under ultraviolet light. The drivelines were implanted transcutaneously into the neck of C57BL/6 mice as follows: Following anesthesia, the area was shaved and cleaned with betadine chlorhexidine followed by alcohol. Two medial transverse incisions, a 5mm incision between the ears and a 1.5mm incision 30mm along the spine were made. A tunnel was created between the incisions and the driveline was pulled through. Five millimeters of the driveline remained exposed (Figure1B/C). The incision between the ears was sutured (6.0 Nylon) and the driveline was covered with a 20mm round sterile bandage.
Figure 1. Murine driveline model.
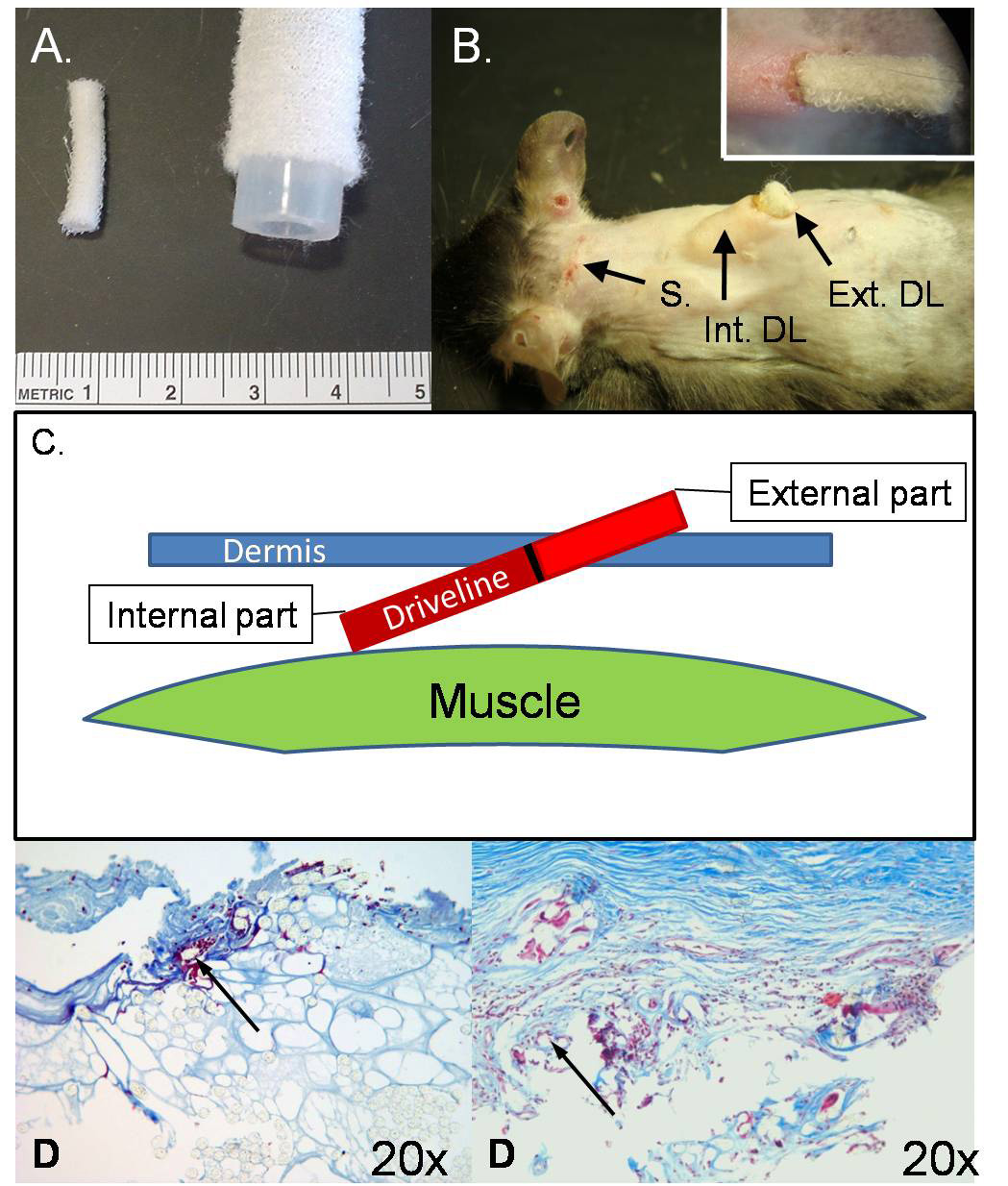
Panel A, Size comparison between murine drivelines (left) and clinical Heartmate I drivelines (right). Panel B, Implanted murine driveline. S, medial lateral incision used to tunnel the driveline under the dermis; Int. DL, internal part of the implanted driveline; Ext. DL, external part of the driveline. The small panel in the upper right hand corner shows a detailed view of the implanted driveline. Panel C, Schematic illustration of a lateral view of a driveline in the back of a mouse. Figure 1D: Comparison of the histology (Trichrome stain) of the mouse model on the left (implanted 14 days) and the Human driveline on the right (implanted 90 days). The arrows are pointing toward a Dacron fiber imbedded in collagen surrounded by mononuclear cells and collagen.
Mice were sacrificed 2,4,7, or 14 days after driveline implantation (n=5 per group). Following sacrifice, the driveline exit site was disinfected with 70% alcohol and the driveline explanted. The skin was dissected free from the driveline. The external part of the driveline was removed and the internal part divided into two pieces, fixed in 10% formalin PBS for 30 minutes and paraffin-embedded. Human explanted drivelines (Heartmate I, Thoratec Corporation, Pleasenton, CA) were prepared in the same way for comparison.
Paraffin sections (6µm) from the proximal and distal ends of the internal (10mm) driveline were stained with H&E (n=10 sections) for morphological studies, with trichrome (n=30) to assess for collagen deposition and with an elastin stain (n=10) (data not shown). We examined 45 sections for collagen deposition at the timepoints outlined above.
The mouse survival rate was 92%. By postoperative day 4, the driveline surface was covered with a thin layer of collagen and other extracellular matrix components. Mononuclear cells were distributed between the fibers of the driveline (Figure2A). Mononuclear cells were quantified and characterized with H&E (pictures not shown). On day 7 the surface material on the driveline was more distinctly organized, and both, extracellular matrix and collagen layers, were more dense (Figure2B). By day 14 fewer mononuclear cells were present and the driveline showed a thicker and more homogenous coating composed mainly of collagen (Figure2C). Collagen deposition was confirmed with trichrome and elastin stain at all time points. No elastin was present. While a greater amount of collagen was detected on the explanted human driveline (90 days post implantation), the pattern of collagen deposition in the murine model was comparable (Figure1D).
Figure 2. Trichrome stain of the implanted driveline.
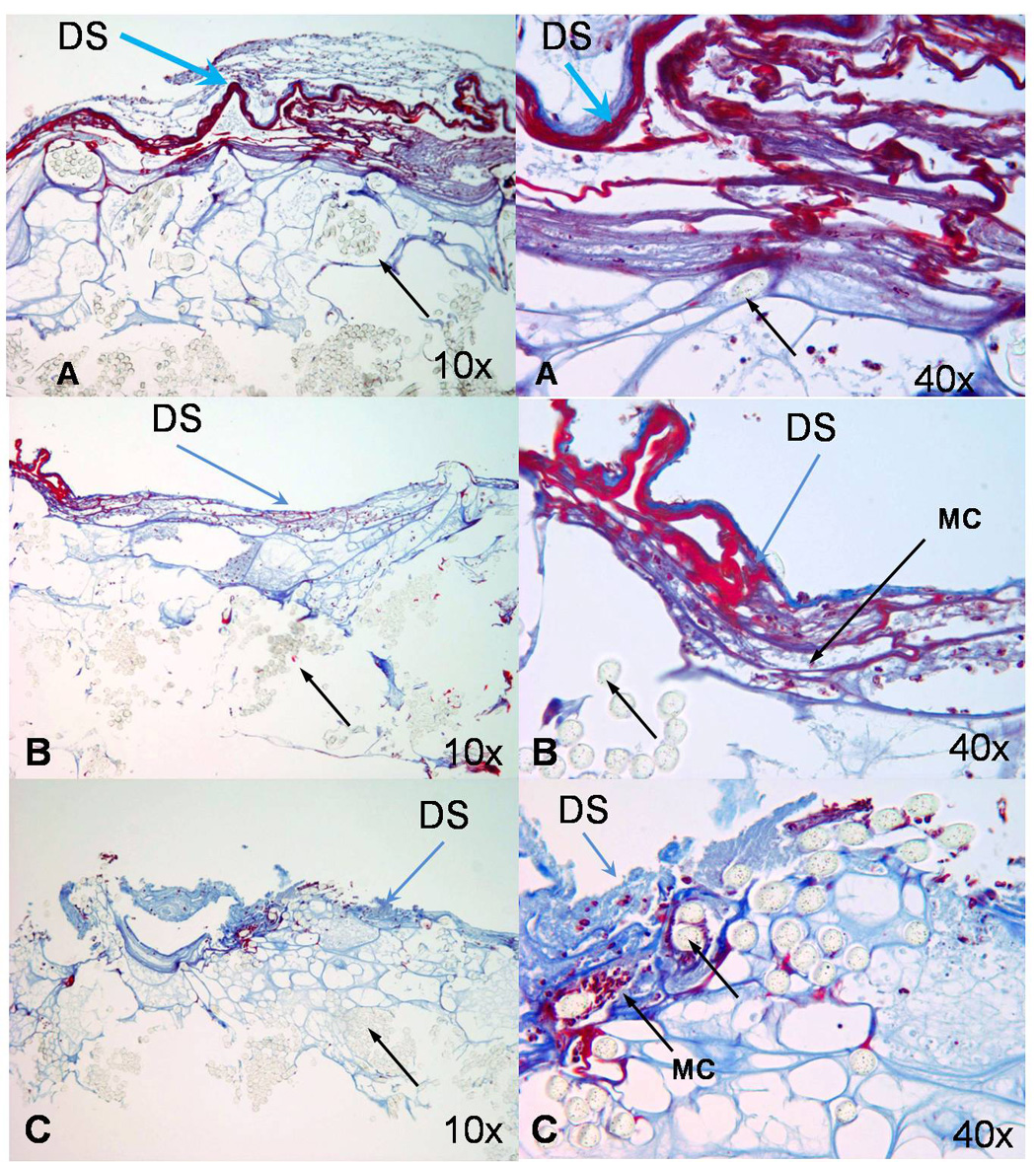
The collagen layer is stained blue, mononuclear cells are visible between the fibers and the collagen. Black arrow shows the fibers of the Dacron cloth, DS indicates the driveline surface and MC indicate Mononuclear cells. Figure 2A: 4 days, Figure 2B: 7 days, Figure 2C: 14 days.
This murine driveline model is currently being used to define mechanisms of bacterial driveline infection in heart failure patients on MCSD-support.
Acknowledgments
Supported by NHLBI grant HL 077096-01 named “The Biology of Human Long-Term Mechanical Circulatory Support” and Philipp Geier Philanthropic gift to “Advanced Heart Failure Research”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 2.Holman WL, Murrah CP, Ferguson ER, Bourge RC, McGiffin DC, Kirklin JK. Infections during extended circulatory support: University of Alabama at Birmingham experience 1989 to 1994. Ann Thorac Surg. 1996;61:366–371. doi: 10.1016/0003-4975(95)01021-1. [DOI] [PubMed] [Google Scholar]
- 3.Deng MC, Edwards LB, Hertz MI, et al. Mechanical Circulatory Support Device Database of the International Society for Heart and Lung Transplantation: Third annual report--2005. J Heart Lung Transplant. 2005;23:1027–1034. doi: 10.1016/j.healun.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]


