Abstract
We previously conducted a randomized, double-blind, placebo-controlled study conducted from 2000–2003 of palifermin, a recombinant human keratinocyte growth factor, dosed from 240 mcg/kg to 720 mcg/kg, in 100 allogeneic hematopoietic stem cell transplantation (HCT) recipients. Treatment with palifermin showed beneficial effects on mucositis, but no significant effect on engraftment, acute GVHD, or early survival. In addition to the effect of palifermin on mucosa, other pleotrophic effects, including more rapid immune reconstitution, have been seen in experimental transplant models. Therefore, we investigated whether with longer follow-up we could detect additional differences between the palifermin treated and placebo cohorts. We found no differences in CMV or invasive fungal infections, chronic GVHD, or long-term survival between cohorts. We conclude that the benefits of palifermin appear to primarily be limited to ameliorating mucotoxicity when given to allogeneic HCT recipients.
Keywords: palifermin, GVHD, hematopoietic stem cell transplantation
Introduction
Allogeneic hematopoietic cell transplantation represents the only curative therapy for large numbers of patients with hematologic malignancies. A major advantage of allogeneic HCT is the potential for graft-versus-leukemia effects which can reduce the risk of relapse and improve survival. However, there is a tight association between GVL and graft-versus-host disease (GVHD) and GVHD continues to be the major contributor to post-transplant morbidity and mortality. The pathophysiology of GVHD is complex but involves inflammatory cytokine effectors, amplified by translocation of lipopolysaccharide (LPS) across damaged gastrointestinal epithelium (1, 2). Therefore, protection of the gastrointestinal tract from HCT conditioning induced injury has the potential to ameliorate the GI tract contribution to GVHD.
Palifermin (keratinocyte growth factor, KGF) has proven to be effective at preventing chemotherapeutic or radiation gastrointestinal injury in the setting of high-dose autologous HCT (3). Furthermore, preclinical animal models showed that palifermin reduced the severity of acute GVHD arising after allogeneic HCT (4–6) An additional potential benefit of palifermin may be improved thymopoiesis and peripheral immune reconstitution as has been seen in experimental models of GVHD (7, 8).
In order to determine the potential benefits of palifermin in the allogeneic HCT setting, we conducted a phase I/II randomized, placebo-controlled trial of palifermin in 100 patients undergoing matched, related donor, HCT following myeloablative therapy at the University of Minnesota and the University of Michigan from 2000 to 2003. The results of this trial have been reported (9), and contrary to expectations, palifermin administration before and after HCT had no significant effect on the incidence and severity of acute GVHD, survival to day 100, or relapse rates. We now update this data with longer follow-up and report infection rates observed during this study.
Patients, Materials, and Methods
Patient and transplant characteristics
The patients characteristics have been previously reported (9). Briefly, one hundred patients were enrolled on the study, 31 patients randomly assigned to receive placebo and 69 to receive one of four doses and schedules of palifermin. Two patients who were randomized to palifermin did not undergo transplant (death and patient decision) and were not further analyzed. Baseline demographic and disease characteristics were balanced between the placebo and palifermin cohort with respect to center (Michigan or Minnesota), gender, age, weight, year of transplant, and disease treated (Table 1). The median age for placebo patients was 46 (7–63) years and for palifermin patients was 46 (7–65) years. Median follow-up of 365 days is the same between placebo and palifermin treated patients.
Table 1.
Patient Characteristics
| N | Placebo | Palifermin | P | |
|---|---|---|---|---|
| Total | 100 | 31 | 60 | |
| Center | 0.91 | |||
| Michigan | 46 | 14 | 32 | |
| Minnesota | 54 | 17 | 37 | |
| Gender | 0.99 | |||
| Male | 58 | 18 | 40 | |
| Female | 42 | 13 | 29 | |
| Median Age, y (range) | 46 (7–63) | 46 (7–65) | 0.58 | |
| Disease | 0.08 | |||
| ALL | 9 | 1 | 8 | |
| AML | 36 | 12 | 24 | |
| CML | 15 | 8 | 7 | |
| MDS | 12 | 6 | 6 | |
| NHL | 14 | 1 | 13 | |
| Hodgkin | 1 | 0 | 1 | |
| Other malignancy | 13 | 3 | 10 | |
AML indicates acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma.
All patients received a myeloablative allogeneic HCT from a HLA-genotypically identical - sibling donor. Conditioning regimens consisted of either cyclophosphamide (120 mg/kg) and fractionated total body irradiation (1320 cGy) (University of Minnesota) or oral busulfan (16 mg/kg) and cyclophosphamide (120 mg/kg) (University of Michigan).
GVHD prophylaxis
GVHD prophylaxis consisted of a calcineurin inhibitor (tacrolimus or cyclosporine) and short-course methotrexate (15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11).
Study Design
The study utilized a randomized, double-blind, placebo-controlled, dose-escalation design. Three palifermin cohorts were sequentially enrolled with dose escalation of palifermin for each cohort. All cohorts received 3 days of palifermin at 40 mcg/kg (8 patients) or 60 mcg/kg (61 patients) prior to the start of conditioning therapy and then received the same dose of palifermin on 3 consecutive days weekly starting day 0 for one (18 patients), two (14 patients), or three (37 patients) weeks. Thus the total palifermin dose ranged from 240 mcg/kg for the lowest dose cohort to 720 mcg/kg for the highest dose cohort. Patients were randomized to palifermin or placebo using a 1:2 scheme and structured to achieve balance within each study site and in each cohort with stratification based on conditioning regimen and patient age.
Follow-up design
Patient study charts and transplant center database records were reviewed. Chronic GVHD was recorded by date of onset and organs involved. CMV detection by antigenemia or blood DNA PCR was considered evidence as CMV reactivation if there was more than one positive result with no intervening negative studies. CMV infections were categorized as viremia, in the absence of organ involvement, or as disease, if there was end organ involvement with CMV as demonstrated by biopsy or organ dysfunction in the setting of documented viremia. Invasive fungal infections (proven or probable) required documentation with positive culture or cytologic/histologic results
Statistical methods
The primary endpoints in this followup study were chronic GVHD at 2 years, the probabilities of overall survival (OS) at 2 years and the incidence of serious viral and fungal opportunistic infections.
Diagnosis of chronic GVHD was based on standard clinical criteria (10) with histopathologic confirmation where possible. The cumulative incidence of GVHD was calculated by treating deaths from other causes as competing risks. Probabilities and 95% confidence intervals (CI) of infections and chronic GVHD, were calculated using the cumulative incidence function (11). The statistical endpoint of overall survival (OS) was estimated by the Kaplan-Meier method (12).
Statistical comparison of time-to-event curves was completed by the Log-Rank test. Comparison of the demographic factors was performed by the Chi-square test or Fischer’s exact test if patient numbers were small. Continuous factors were compared by the non-parametric General-Wilcoxon test.
Results
Graft-versus-host disease
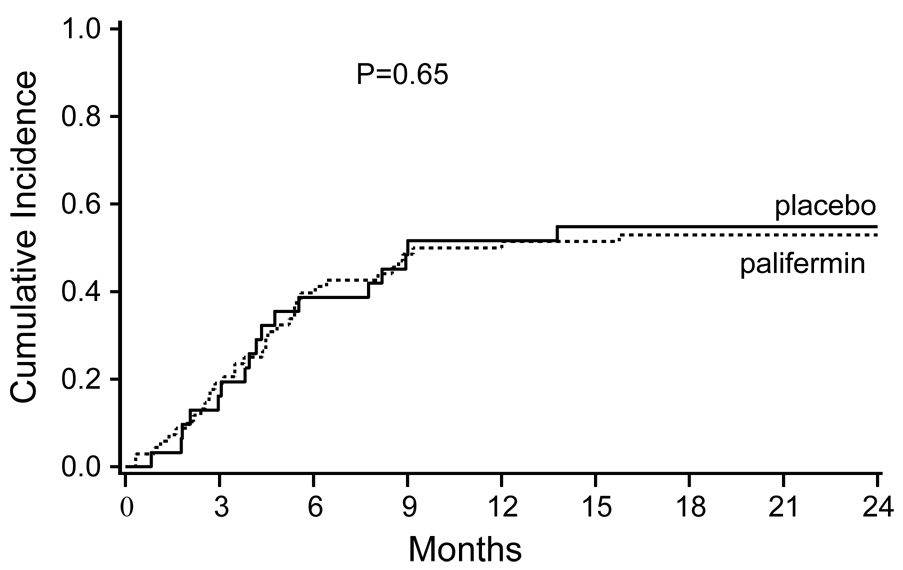
As originally reported, there was no significant difference in the incidence of grades II-IV or grades III-IV acute GVHD in patients receiving palifermin compared to placebo nor was there a difference in involvement or severity of individual organs. As shown in Table 2 and Figure 1, the cumulative incidence of chronic GVHD was nearly identical for both the palifermin and placebo cohorts (55% vs 53%, p=0.65). There were no statistically significant differences in time to onset of chronic GVHD or organ involvement.
Table 2.
Cumulative Incidence of Chronic GVHD
| Chronic GVHD | ||||||
|---|---|---|---|---|---|---|
| N | # with cGVHD | 2 year cum inc (95% CI) | P | 2 year competing risk (95% CI) | P | |
| Total | 98 | 53 | 54% (43–65%) | 27% (18–36%) | ||
| Placebo | 31 | 17 | 55% (35–75%) | 0.65 | 32% (16–48%) | 0.26 |
| 67 | 36 | 53% (39–67%) | 25% (15–35%) | |||
| Palifermin | ||||||
The competing risks (death without chronic GVHD) are shown.
Figure 1.
Cumulative incidence of chronic GVHD in the palifermin treated and placebo cohorts.
Infections
As shown in Table 3, the one-year cumulative incidence of CMV infection was 16% for both the palifermin and placebo cohorts (p=0.94). There was no evidence of a protective effect during the first 100 days after transplant (when CMV infections are most likely to occur) nor was there a trend for decreasing CMV infection rates with increasing palifermin dose.
Table 3.
Cumulative Incidence of Infections
| Infections | |||||||
|---|---|---|---|---|---|---|---|
| N | # with infections | 100 days (95% CI) | 1 year (95% CI) | P | 1 year competing risk (95% CI) | P | |
| CMV infections | |||||||
| Total | 98 | 16 | 15% (8–22%) | 16% (9–23%) | 30% (21–39%) | ||
| Palifermin | 67 | 11 | 16% (7–25%) | 16% (7–25%) | 0.94 | 29% (18–30%) | 0.65 |
| Placebo | 31 | 5 | 13% (2–24%) | 16% (3–29%) | 32% (15–49%) | ||
| Fungal infections | |||||||
| Total | 98 | 37 | 28% (19–37%) | 37% (27–47%) | 20% (12–28%) | ||
| Palifermin | 67 | 28 | 29% (18–40%) | 41% (29–53%) | 0.38 | 19% (10–28%) | 0.65 |
| Placebo | 31 | 9 | 26% (11–41%) | 29% (13–45%) | 23% (8–38%) | ||
The competing risks (death without infection) are shown.
Palifermin administration was not associated with protection against invasive fungal infections. Yeasts were the predominant fungal infection encompassing 26/28 cases in palifermin treated patients and all nine invasive fungal infections in the placebo cohort. In addition to yeast infections, there was one aspergillus and one penicillium infection in the palifermin treated patients. As shown in Table 3, the one year cumulative incidence of invasive fungal infections was 41% for the palifermin cohort and 29% for the placebo cohort (p=0.38).
Survival
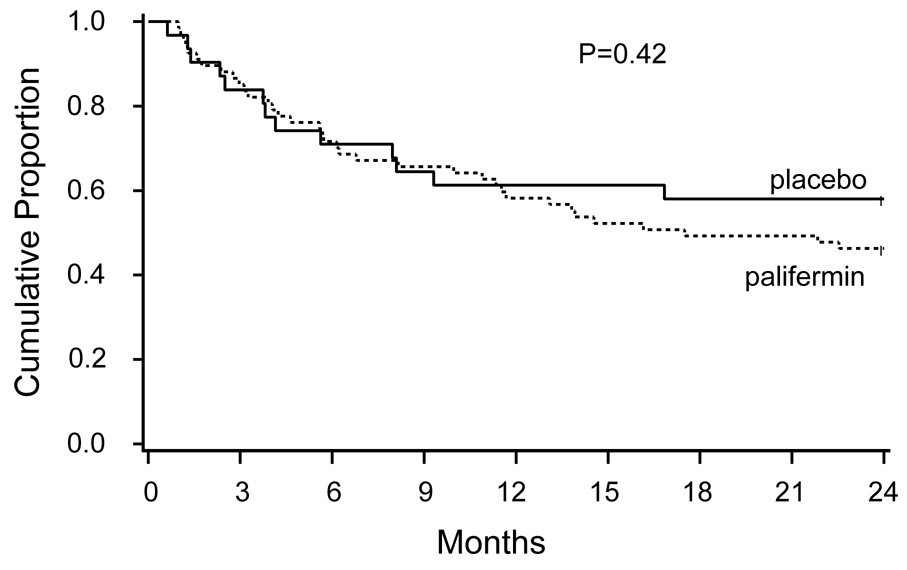
We previously reported that palifermin did not provide a survival benefit for survival during the first 100 days. This lack of benefit did not change over time as the two-year survival for the palifermin cohort vs the placebo cohort was 46% vs 58%, respectively, p=0.42 (Table 4, Figure 2).
Table 4.
Survival at 100 days, 1 year, and 2 years from HCT
| Survival | |||||||
|---|---|---|---|---|---|---|---|
| N | # dead | 100 day survival (95% CI) | 1 year (95% CI) | P | 2 year (95% CI) | P | |
| Total | 98 | 49 | 83% (76–90%) | 59% (49–69%) | 50% (40–60%) | ||
| Palifermin | 67 | 36 | 82% (73–91%) | 58% (46–70%) | .85 | 46% (34–58%) | .42 |
| Placebo | 31 | 13 | 84% (71–97%) | 61% (44–78%) | 58% (41–75%) | ||
Figure 2.
Kaplan-Meier survival curves for the palifermin treated and placebo cohorts.
Discussion
Our previous study demonstrated that palifermin could be safely administered to patients undergoing allogeneic HCT. Although not monitored on a daily basis, we also showed that palifermin reduced mucositis severity in patients undergoing a cyclophosphamide/total body irradiation conditioning regimen, but not in patients receiving the less mucotoxic regimen of busulfan and cyclophosphamide. At these doses and schedules, palifermin did not alter the incidence, organ involvement, or severity of acute GVHD, nor did it protect against death. Unanswered at the time of the original publication however, was whether palifermin may have conferred protection against infections, chronic GVHD or late mortality. We now conclude that chronic GVHD was unaffected in this study.
Animal studies in rodents indicated that palifermin can reduce acute GVHD associated lethality when given with total body irradiation (5, 6). However, the potency of this protective effect varied between models and severity of the acute GVHD lethality in controls. When total body irradiation was combined with cyclophosphamide, acute GVHD was more aggressive and palifermin rescued ~13% of mice from lethality as assessed at the conclusion of the study (5.5 weeks post-BMT) (5). In these latter studies, a transient epithelial protective effect by palifermin was observed on day 7 but not at later times post-BMT. Therefore, the clinical data on acute GVHD lethality in cyclophosphamide and total body irradiation conditioned rodents is similar in many respects to the acute GVHD results in this human clinical trial. Both rodent (13, 14), and non-human primates (15) studies have demonstrated that palifermin protects thymic epithelium from radiation induced damage resulting in improved thymopoiesis and peripheral immune recovery. We were not able to perform detailed immune recovery assays, therefore, we can not conclude that palifermin does not alter immune recovery in a favorable way. However, in this study we observed no evidence of clinical benefit in terms of reduced fungal or CMV infections in the palifermin cohort.
The mechanisms that lead to chronic GVHD are complex and poorly understood, but a major risk factor for chronic GVHD is preceding acute GVHD (16). Therefore, it is perhaps not surprising that we observed no reduction in the incidence of chronic GVHD in the palifermin cohort. It therefore follows that one year survival was not improved in the palifermin cohort, given the major role GVHD plays in transplant related mortality, particularly following the first few months. A lack of survival benefit with palifermin was previously reported in the autologous HCT setting (3).
Palifermin is routinely administered to reduce the severity, incidence, and duration of mucositis following autologous HCT and we have previously reported a similar benefit in the allogeneic setting when using highly mucotoxic regimens. However, palifermin, when administered in the doses and schedule used in this study, resulted in no long term benefit. Results of ongoing studies in other allogeneic HCT trials will determine whether the acute and chronic GVHD results seen in palifermin treated patients enrolled on this trial can be extrapolated to other clinical venues.
Acknowledgments
The authors thank Pam James and Julie Formosa for excellent data collection and management. This work was supported by the following grants: National Institutes of Health grants R01 HL073794, NIH P01 CA03952, and 2P30CA046592; Food and Drug Administration grant FRD 020201; and Amgen.
Contribution: D.J.W. and J.E.L. designed the study. J.E.L. wrote the paper. T.D. performed the statistical analysis. All authors critically reviewed the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare no competing financial interests.
REFERENCES
- 1.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. [PubMed] [Google Scholar]
- 2.Ferrara JL, Cooke KR, Teshima T. The pathophysiology of acute graft-versus-host disease. Int J Hematol. 2003;78:181–187. doi: 10.1007/BF02983793. [DOI] [PubMed] [Google Scholar]
- 3.Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 4.Krijanovski OI, Hill GR, Cooke KR, et al. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood. 1999;94:825–831. [PubMed] [Google Scholar]
- 5.Panoskaltsis-Mortari A, Lacey DL, Vallera DA, Blazar BR. Keratinocyte growth factor administered before conditioning ameliorates graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood. 1998;92:3960–3967. [PubMed] [Google Scholar]
- 6.Panoskaltsis-Mortari A, Taylor PA, Rubin JS, et al. Keratinocyte growth factor facilitates alloengraftment and ameliorates graft-versus-host disease in mice by a mechanism independent of repair of conditioning-induced tissue injury. Blood. 2000;96:4350–4356. [PubMed] [Google Scholar]
- 7.Rossi S, Blazar BR, Farrell CL, et al. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 8.Min D, Taylor PA, Panoskaltsis-Mortari A, et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- 9.Blazar BR, Weisdorf DJ, Defor T, et al. A phase I/II randomized, placebo-control trial of Palifermin to prevent graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) Blood. 2006 doi: 10.1182/blood-2006-04-017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socie G. Chronic graft-versus-host disease: clinical features and grading systems. Int J Hematol. 2004;79:216–220. doi: 10.1532/ijh97.03174. [DOI] [PubMed] [Google Scholar]
- 11.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier O. Nonparametric estimation from incomplete observations. J American Stat Association. 1958;53:457–481. [Google Scholar]
- 13.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, et al. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution following murine bone marrow transplantation. Blood. 2008 doi: 10.1182/blood-2008-01-136531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alpdogan O, Hubbard VM, Smith OM, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seggewiss R, Lore K, Guenaga FJ, et al. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood. 2007;110:441–449. doi: 10.1182/blood-2006-12-065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Remberger M, Kumlien G, Aschan J, et al. Risk factors for moderate-to-severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:674–682. doi: 10.1053/bbmt.2002.v8.abbmt080674. [DOI] [PubMed] [Google Scholar]