Abstract
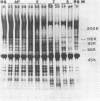
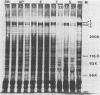
Pseudomonad proteases disrupted the function and structure of demembranated cilia (axonemes) extracted from porcine tracheae. Proteolytic degradation by the two pseudomonad proteases elastase and alkaline protease and by trypsin and subtilisin impaired motility of ATP-activated axonemes. In addition, electron microscopic observation of negatively stained axonemes indicated that exposure to proteases caused dissociation into individual doublet or singlet microtubules. Inhibition of motility and axonemal fraying occurred when axonemes were treated with less than 5 U of proteolytic activity of any of the four proteases tested. When the effects of 2 U of each protease were compared, trypsin and subtilisin were able to produce immotility in less time than pseudomonad elastase and alkaline protease, while alkaline protease and subtilisin caused the most axonemal fraying in 10 min. Proteolytic digestion of axonemal proteins was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. All four proteases cleaved dynein proteins (proteins necessary for motility), though treatment with trypsin resulted in the most extensive solubilization of axonemal proteins. Trypsin and subtilisin both produced more changes in the protein profiles of treated axonemes, using fewer units of proteolytic activity, than the pseudomonad proteases. However, the limited alteration of only a few axonemal proteins by pseudomonad proteases indicates that cleavage need not be extensive to produce dysfunction. Thus, ciliary axonemes are susceptible to proteolytic attack. Degradation of axonemal proteins by pseudomonad proteases, which are released during active infection, may contribute to the impaired ciliary function associated with pseudomonad colonization of the respiratory tract.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Isolation of ciliated or unciliated basal bodies from the rabbit oviduct. J Cell Biol. 1974 Feb;60(2):393–404. doi: 10.1083/jcb.60.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. R. Role of exotoxin A and proteases of Pseudomonas aeruginosa in respiratory tract infections. Can J Microbiol. 1982 Feb;28(2):248–255. doi: 10.1139/m82-033. [DOI] [PubMed] [Google Scholar]
- Bell C. W., Gibbons I. R. Structure of the dynein-1 outer arm in sea urchin sperm flagella. II. Analysis by proteolytic cleavage. J Biol Chem. 1982 Jan 10;257(1):516–522. [PubMed] [Google Scholar]
- Bowman B. H., Lockhart L. H., McCombs M. L. Oyster ciliary inhibition by cystic fibrosis factor. Science. 1969 Apr 18;164(3877):325–326. doi: 10.1126/science.164.3877.325. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Elastase digestion of demembranated sperm flagella. Science. 1980 Mar 21;207(4437):1365–1367. doi: 10.1126/science.6898364. [DOI] [PubMed] [Google Scholar]
- Bruce M. C., Poncz L., Klinger J. D., Stern R. C., Tomashefski J. F., Jr, Dearborn D. G. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis. 1985 Sep;132(3):529–535. doi: 10.1164/arrd.1985.132.3.529. [DOI] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Peterson L. P., Baseman J. B. Pathogenesis of infection with Bordetella pertussis in hamster tracheal organ culture. J Infect Dis. 1977 Aug;136 (Suppl):S196–S203. doi: 10.1093/infdis/136.supplement.s196. [DOI] [PubMed] [Google Scholar]
- Döring G., Goldstein W., Röll A., Schiøtz P. O., Høiby N., Botzenhart K. Role of Pseudomonas aeruginosa exoenzymes in lung infections of patients with cystic fibrosis. Infect Immun. 1985 Sep;49(3):557–562. doi: 10.1128/iai.49.3.557-562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K., Flehmig B., Høiby N., Hofmann A. Proteases of Pseudomonas aeruginosa in patients with cystic fibrosis. J Infect Dis. 1983 Apr;147(4):744–750. doi: 10.1093/infdis/147.4.744. [DOI] [PubMed] [Google Scholar]
- FISHER E., Jr, ALLEN J. H. Corneal ulcers produced by cell-free extracts of Pseudomonas aeruginosa. Am J Ophthalmol. 1958 Jul;46(1 Pt 2):21–27. doi: 10.1016/0002-9394(58)90030-8. [DOI] [PubMed] [Google Scholar]
- Gray L., Kreger A. Microscopic characterization of rabbit lung damage produced by Pseudomonas aeruginosa proteases. Infect Immun. 1979 Jan;23(1):150–159. doi: 10.1128/iai.23.1.150-159.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie A. T., Dicker D. T., Hingley S. T., Kueppers F., Higgins M. L., Weinbaum G. Isolation of cilia from porcine tracheal epithelium and extraction of dynein arms. Cell Motil Cytoskeleton. 1986;6(1):25–34. doi: 10.1002/cm.970060105. [DOI] [PubMed] [Google Scholar]
- Hingley S. T., Hastie A. T., Kueppers F., Higgins M. L., Weinbaum G., Shryock T. Effect of ciliostatic factors from Pseudomonas aeruginosa on rabbit respiratory cilia. Infect Immun. 1986 Jan;51(1):254–262. doi: 10.1128/iai.51.1.254-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder I. A., Haidaris C. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: extracellular protease and elastase as in vivo virulence factors. Can J Microbiol. 1979 May;25(5):593–599. doi: 10.1139/m79-085. [DOI] [PubMed] [Google Scholar]
- Jagger K. S., Bahner D. R., Warren R. L. Protease phenotypes of Pseudomonas aeruginosa isolated from patients with cystic fibrosis. J Clin Microbiol. 1983 Jan;17(1):55–59. doi: 10.1128/jcm.17.1.55-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger K. S., Robinson D. L., Franz M. N., Warren R. L. Detection by enzyme-linked immunosorbent assays of antibody specific for Pseudomonas proteases and exotoxin A in sera from cystic fibrosis patients. J Clin Microbiol. 1982 Jun;15(6):1054–1058. doi: 10.1128/jcm.15.6.1054-1058.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M., Bottone E. J. Pseudomonas aeruginosa enzyme profiling: predictor of potential invasiveness and use as an epidemiological tool. J Clin Microbiol. 1981 Jul;14(1):55–60. doi: 10.1128/jcm.14.1.55-60.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Carter-Hamm B., Dralle W. M. Inactivation of human bronchial mucosal proteinase inhibitor by Pseudomonas aeruginosa elastase. Am Rev Respir Dis. 1982 Dec;126(6):1070–1073. doi: 10.1164/arrd.1982.126.6.1070. [DOI] [PubMed] [Google Scholar]
- Kawaharajo K., Homma J. Y., Aoyama Y., Morihara K. In vivo studies on protease and elastase from Pseudomonas aeruginosa. Jpn J Exp Med. 1975 Apr;45(2):89–100. [PubMed] [Google Scholar]
- Klinger J. D., Straus D. C., Hilton C. B., Bass J. A. Antibodies to proteases and exotoxin A of Pseudomonas aeruginosa in patients with cystic fibrosis: Demonstration by radioimmunoassay. J Infect Dis. 1978 Jul;138(1):49–48. doi: 10.1093/infdis/138.1.49. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- Melby K., Toews G. B., Pierce A. K. Pulmonary elastase activity in response to Streptococcus pneumoniae and Pseudomonas aeruginosa. Am Rev Respir Dis. 1985 Apr;131(4):559–563. doi: 10.1164/arrd.1985.131.4.559. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oda K. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect Immun. 1979 Apr;24(1):188–193. doi: 10.1128/iai.24.1.188-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchelle E., Tournier J. M., Petit A., Zahm J. M., Lauque D., Vidailhet M., Sadoul P. The frog palate for studying mucus transport velocity and mucociliary frequency. Eur J Respir Dis Suppl. 1983;128(Pt 1):293–303. [PubMed] [Google Scholar]
- Sale W. S., Satir P. Direction of active sliding of microtubules in Tetrahymena cilia. Proc Natl Acad Sci U S A. 1977 May;74(5):2045–2049. doi: 10.1073/pnas.74.5.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallman L. A., Hill S. L., Stockley R. A. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: a serine proteinase effect. Thorax. 1984 Sep;39(9):663–667. doi: 10.1136/thx.39.9.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley R. A., Hill S. L., Morrison H. M., Starkie C. M. Elastolytic activity of sputum and its relation to purulence and to lung function in patients with bronchiectasis. Thorax. 1984 Jun;39(6):408–413. doi: 10.1136/thx.39.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3092–3096. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. Effects of trypsin digestion on flagellar structures and their relationship to motility. J Cell Biol. 1973 Sep;58(3):618–629. doi: 10.1083/jcb.58.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Tegner H., Ohlsson K., Toremalm N. G., von Mecklenburg C. Effect of human leukocyte enzymes on tracheal mucosa and its mucociliary activity. Rhinology. 1979 Sep;17(3):199–206. [PubMed] [Google Scholar]
- Warner F. D., Mitchell D. R. Dynein: the mechanochemical coupling adenosine triphosphatase of microtubule-based sliding filament mechanisms. Int Rev Cytol. 1980;66:1–43. doi: 10.1016/s0074-7696(08)61970-1. [DOI] [PubMed] [Google Scholar]
- Warner F. D., Mitchell D. R. Structural conformation of ciliary dynein arms and the generation of sliding forces in Tetrahymena cilia. J Cell Biol. 1978 Feb;76(2):261–277. doi: 10.1083/jcb.76.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985 Feb;40(2):125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B., Plummer J., Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol. 1978 Mar;76(3):729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Cryz S. J., Friedman R. L., Iglewski B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]