SUMMARY
Previous tendon and ligament studies demonstrated a role for mechanical loading in tissue homeostasis and healing. In uninjured musculoskeletal tissues, increased loading leads to an increase in mechanical properties, while decreased loading leads to a decrease in properties. The role of loading on healing tissues is less clear. We studied tendon-to-bone healing in a canine flexor tendon-to-bone injury and repair model. To examine the effect of muscle loading on healing, repaired tendons were either cut proximally to remove all load from the distal phalanx repair site (unloaded group) or left intact proximally (loaded group). All paws were cast post-operatively and subjected to daily passive motion rehabilitation. Specimens were tested to determine functional properties, biomechanical properties, repair-site gapping, and bone mineral density. Loading across the repair site led to improved functional and biomechanical properties (e.g., stiffness for the loaded group was 8.2 ± 3.9 vs. 5.1 ± 2.5 N/mm for the unloaded group). Loading did not affect bone mineral density or gapping. The formation of a gap between the healing tendon and bone correlated with failure properties. Using a clinically relevant model of flexor tendon injury and repair, we found that muscle loading was beneficial to healing. Complete removal of load by proximal transection resulted in tendon-to-bone repairs with less range of motion and lower biomechanical properties compared to repairs in which the muscle-tendon-bone unit was left intact.
Keywords: flexor tendon, insertion site, enthesis, animal model, hand
INTRODUCTION
Soft tissue injuries to the upper extremity affect workers, athletes, and the elderly.1,2 Hand injuries lead to over 1.5 million days lost from work per year,1 and many require surgery, including tendon-to-bone reconstruction. Little scientific basis exists for current clinical management of flexor tendon-to-bone injuries. Our previous studies demonstrated that the tendon-to-bone insertion site has poor healing capacity, with bone mineral density and biomechanical properties declining over the first 21 days of healing.3–6 These findings motivate our search for new repair treatments to improve the properties of the healing insertion during the critical early time interval where the repair is at risk for gap formation and failure.
Previous tendon and ligament studies demonstrated a role for mechanical loading in tissue homeostasis and healing. Uninjured tendons, ligaments, and their bony insertions are highly sensitive to their mechanical environment.7–11 Increased loading typically leads to an increase in mechanical properties, while decreased loading leads to a decrease in properties,7–9 mostly at the bony end of the insertion.7 In healing tissues, the loading effect is less clear. Some studies showed a beneficial effect of increased loading8,9, while others showed a detrimental effect12,13. While low load levels appear beneficial8,9 (e.g., by preventing adhesion formation during healing), high levels may lead to microinjuries and decreased mechanical properties12,13. Loading also influences bone mineral density (BMD) and mechanical properties. Unloading leads to a decrease in BMD while increased loading increases BMD.14,15 In our canine flexor tendon model, we observed significant bone loss and a drop in structural properties after tendon-to-bone injury and repair.5,16 Therefore, our goal in this study was to examine the effect of muscle loading on flexor tendon-to-bone healing. We hypothesized that loading would prevent bone loss and improve range of motion)and mechanical properties in the early healing period.
MATERIALS AND METHODS
Animal model
20 flexor digitorum profundus (FDP) tendons were injured and repaired into bone tunnels in the distal phalanx in 10 canines. Animals were euthanized at 21 days. 14 additional flexor digitorum profundus (FDP) tendons from canine cadavers were injured and repaired using identical methods and served as time zero controls (Control group). All animal procedures were approved by the Animal Studies Committee of Washington University.
Each dog had the 2nd and 5th FDP tendons injured and repaired (Fig. 1). The tendon was approached via a v-shaped lateral incision and transected sharply at its insertion. The cut end was grasped using a 4-strand modified Becker stitch (the core suture). A 5mm deep × 3mm diameter tunnel was drilled at the volar aspect of the distal phalanx. Two needle holes for the core suture were drilled through the distal phalanx out through the nail. Two further parallel needle holes were drilled through the side of the distal phalanx (medial side for the 2nd digit and lateral side for the 5th digit) that communicates with the tunnel for a supplemental suture.17 Using a double-armed caprolactam suture, each needle was placed retrograde through the hole and delivered proximally out of the tunnel. This supplemental suture was used to augment the time zero structural properties to prevent early rupture of the repair.17 While not yet customary clinically for zone one flexor tendon repairs, the supplemental suture is comparable to the commonly used epitenon suture for mid-substance repairs. A 1mm diameter stainless steel bead was sutured to the tendon end to mark its position in the tunnel. Beads were prefabricated with sutures passed through a small central hole. The core sutures were passed through the needle holes and tied over the dorsal surface of the toenail, pulling the tendon stump into the tunnel. A supplemental suture was added through the medial or lateral needle holes, grasping the tendon at the tunnel entrance with a simple stitch and completing the repair.
Figure 1.
The FDP tendon was transected at its insertion and grasped using a Becker stitch (the ‘core’ suture). A tunnel was drilled at the base of the distal phalanx. Two parallel needle holes were drilled through the medial side of the phalanx for the ‘supplemental’ suture. The core sutures were tied over the dorsal surface of the toenail, and a supplemental suture added to complete the repair. Gap formation was determined from positions of stainless steel beads (indicated with the arrow in the bottom panel) implanted at surgery. Gap was calculated as the difference between the bead position at surgery and at euthanasia.
To examine the effect of muscle loading, repaired tendons were cut proximally at the metacarpophalangeal level to remove all load from the distal phalanx repair site (Unloaded group) or left intact proximally (Loaded group). Post-operatively, forelimbs were placed in fiberglass casts at 70° wrist flexion and subjected to passive motion as described previously.4,5 Briefly, the volar portion of the cast was removed daily to allow for passive motion of the wrist and digits. Digits were flexed and extended with the wrist at 70° for 5 min/day. Our previous studies indicated that structural properties declined over the first 21 days of healing, leaving the repair at risk for failure.4,5 We therefore chose to study the effect of loading at 21 days.
Histology
Specimens for histology (2 per group) were fixed post mortem in 10% neutral buffered formalin for 48 h, decalcified for 4 weeks in 14% EDTA, and embedded in paraffin. Longitudinal sections (5–8μm) were cut in the mid-sagittal plane and stained with H&E, Toluidine blue, or Masson’s Trichrome. Sections were blinded and evaluated under bright-field illumination for the appearance of a fibrocartilaginous tendon-to-bone interface, the level of inflammation and numbers of inflammatory cells (mononuclear cells and polymorphonuclear leukocytes), and the level of vascular proliferation.
Gap formation
Gap formation (10 specimens per group) was quantified at euthanasia by determining the positions of the stainless steel beads. To determine the initial position of the bead at surgery, calipers were used to measure tunnel depth, the length of the Becker suture, and the length of the Becker suture that remained outside the tunnel after repair. Based on cadaver trials, the precisions of the tunnel and suture length measurements were 5.1% and 1.4%, respectively. At euthanasia, a radiograph was taken of the specimen (including a calibration bar to determine magnification), and the bead position determined using image analysis software (Scion Image, Frederick, MD) (Fig. 1). “Gap” was the difference between the bead position at surgery and at euthanasia relative to the tunnel entrance. These specimens were then evaluated biomechanically. All markers remained attached to the tendon ends based on visual inspection.
Biomechanics
The 2nd and 5th digits were disarticulated at the metacarpophalangeal joints. The FDP tendons in the Loaded group were transected proximally, resulting in tendon lengths equivalent to the Unloaded group. Specimens were blinded to group and timepoint before biomechanical assessment. Functional properties were assessed using a motion analysis system (PC Reflex, Qualisys), and a passive motion protocol described below (8 per group).4,5 A reflective marker (2 mm diam) was glued to the proximal end of the tendon, a pair of reflective markers were pinned to the middle phalanx, and a second pair was pinned to the toe nail of each digit (attached to the distal phalanx). The proximal middle phalanx was held in a vertical orientation, and the marker coordinates were sampled first with the digit in a flexed and then in an extended position. Flexion was produced by suspending a 1.5 N weight from the proximal stump of the flexor tendon and a 0.15 N counterweight from the extensor tendon. For extension, the 1.5 N weight was suspended from the extensor tendon and the 0.15 N weight from the flexor tendon. Based on differences between the flexed and extended positions, range of motion of the distal interphalangeal (DIP) joint and tendon excursion were determined. Precisions were 7% for range of motion and 13% for excursion.18
After range of motion testing, tendon-bone specimens were pulled in uniaxial tension until failure (8 for Unloaded and Loaded groups, 14 for Control group). Specimens were isolated from the digits and tested with a material testing machine (8500R; Instron Corp); the distal phalanx was held by a rigid clamp and the proximal tendon was held by a soft-tissue clamp.3–5 The tendon was displaced at 0.375 mm/s until failure, consistent with our previous studies.3–5 Force-elongation data were recorded using a data acquisition system (Labview 7.0; National Instruments); marker displacement data were recorded using a motion analysis system (PC-Reflex). The two markers, spaced ~ 10 mm apart and spanning the repair site, were tracked to determine repair-site elongation. From force-elongation curves, we determined the structural ultimate properties (maximum load) and repair-site stiffness (the slope of the linear portion of the curve). Elongation was then normalized to compute average repair-site strain, based on the initial marker distance. From the force-strain curves, we determined repair-site rigidity (the slope of the linear portion of the force-strain curve), ultimate strain, and repair-site strain at 20 N force (a physiologically relevant level of load).
Bone densitometry
BMD of the distal phalanges (8 per group) was assessed after mechanical testing using peripheral quantitative CT (pQCT; XCT Research M, Stratec).3–5 Two transverse slices were obtained 1 and 2 mm distal to the FDP insertion site (0.5 mm thickness; 0.07 mm voxel size). Using the manufacturer’s software (CALCBD routine) and a threshold of 280 mg/cm3 to segment bone from soft tissues, we determined average BMD for the two slices. BMD determined by this method represents an “apparent” value, as the volume of interest is the entire cross-section of the distal phalanx, including the cortical and trabecular bone and the bone tunnel. Specimens were blinded to group before bone densitometry analysis.
Statistics
Data were tested for normality with the Shapiro-Wilk test. To test whether gap size or BMD correlated with biomechanical properties, a Pearson correlation matrix was created. Probabilities were then tested using a Bonferroni correction for multiple comparisons. Probabilities were considered significant for p<0.05, with trends noted at p < 0.10. Correlated variables were plotted with linear regression fits. The ‘Loaded’ group was compared to the ‘Unloaded’ group using a paired t-test. To compare these groups to Control (unpaired) data), we performed an ANOVA followed by a Fisher’s least squares differences post-hoc test.
RESULTS
The tendon-to-bone insertion site in the Control specimens consisted of the classically described four zone transition (tendon, fibrocartilage, mineralized fibrocartilage, and bone) (Fig. 2A). The insertions of the Loaded and Unloaded specimens, on the other hand, consisted of fibrous tissue, with no fibrocartilage (Figs. 2B, 2C). No apparent differences were found between the Loaded and Unloaded groups. Compared to Control, there was an increase in cellularity, inflammatory cells, and vascularity in both Loaded and Unloaded specimens (Fig. 2).
Figure 2.

The Control insertion demonstrated the classic four-zone insertion site (A). The Loaded (B) and Unloaded (C) groups had a fibrous insertion, without a transition zone, between the healing tendon (‘t’) and bone (‘b’). [Masson’s trichrome stain, 10× objective].
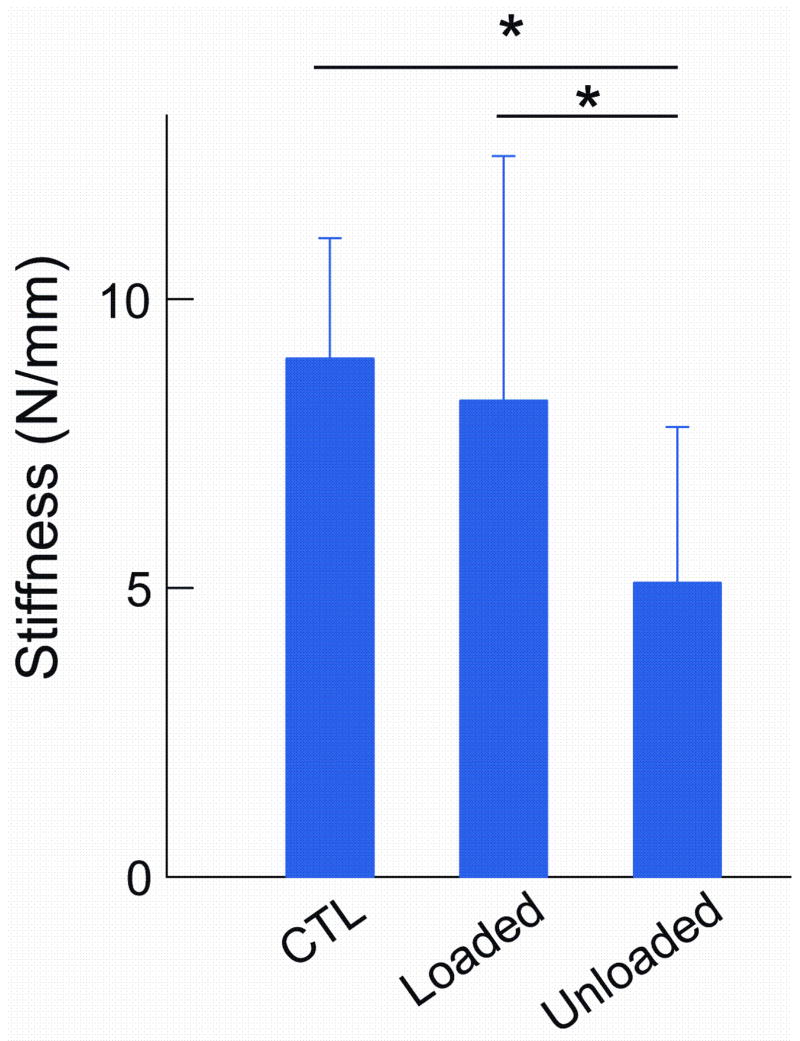
Loading across the repair site improved biomechanical properties (Table 1). Range of motion was significantly higher in the Loaded compared to the Unloaded group (Fig. 3). Both groups, however, had significantly lower range of motion than the Control group. Similar results were seen for tendon excursion. Repair-site stiffness and rigidity in the Loaded group were not different from Controls (Fig. 4). Stiffness and rigidity, on the other hand, were significantly lower in the Unloaded group than in the Loaded and Control groups. Ultimate load was not significantly different between Loaded and Unloaded groups (Fig. 5); both had significantly lower load values than the Control group. There were no differences in repair-site elongation at 20 N between groups (Table 1)
TABLE I.
Functional and biomechanical properties of the tendon-to-bone repair.
| Group | Excursion (mm)# | Strain at 20N | Rigidity (N/mm/mm)* | Ultimate Strain# |
|---|---|---|---|---|
| Control | 6.9 ± 0.8 | 0.12 ± 0.04 | 298.4 ± 68.7 | 0.36 ± 0.09 |
| Loaded | 5.2 ± 1.5§ | 0.097 ± 0.05 | 301.5 ± 167.7 | 0.29 ± 0.09 |
| Unloaded | 4.0 ± 1.0§ | 0.15 ± 0.11 | 163.2 ± 71.9§ | 0.42 ± 0.13 |
Loaded vs. Unloaded -
: p<0.10;
: p<0.05
Control vs. Loaded/Unloaded -
: p<0.05
Figure 3.
Range of motion at the DIPjoint (nearest to the repair site) was significantly higher in the Loaded compared to the Unloaded group. Both groups had significantly lower range of motion that the uninjured Control group. [* p < 0.05 ].
Figure 4.
The Loaded group had significantly higher stiffness than the Unloaded group. Stiffness in the Unloaded group was significantly lower than the Control group. [* p < 0.05 ].
Figure 5.
Ultimate load was not different between Loaded and Unloaded groups. Both groups had significantly lower load compared to Controls. Gap length was not different between the Loaded and Unloaded groups. A significant correlation existed between gap length and ultimate load. [* p < 0.05 ].
Gap length was not significantly different between the Loaded and Unloaded groups (Fig.5). A significant correlation existed between gap length and ultimate load.
BMD was not different between Loaded and Unloaded groups (Fig. 6). However, both groups had significantly lower BMD compared to Controls (Fig. 6). There was a significant correlation between BMD and ultimate load.
Figure 6.
pQCT analysis showed that BMD was not different between Loaded and Unloaded groups. Both groups had lower BMD than Controls. A significant correlation existed between BMD and ultimate load. [* p < 0.05 ].
DISCUSSSION
The response to loading of tendons, ligaments, and their bony insertions has been evaluated extensively in vivo7–9 and in vitro10,11. In vivo, increased loading (e.g., through exercise) leads to an increase in mechanical properties, while decreased loading (e.g., through immobilization) leads to a decrease in properties7–9. In vitro, Hannafin et al showed that ligament explants responded to cyclic loading.10 Cyclic loading was necessary to maintain mechanical properties. Bone is also sensitive to loading environment.14,15 Unloading bone (e.g., through casting or weightlessness) leads to rapid resorption and a decrease in BMD. Increased loading, on the other hand, promotes new bone formation and increases BMD.
The response to tendon loading in healing tissues is less clear. Increased static stress is beneficial to the properties of the healing medial collateral ligament.9 Increased cyclic stress, on the other hand, is detrimental to healing in a rabbit MCL mid-substance model.13 Tendon mechanical loading was effective in stimulating matrix production in a rat rotator cuff model, but ineffective in improving material properties.12 In contrast, passive tendon motion applied to flexor tendon mid-substance injuries in a canine model was beneficial to healing.8,12 An optimal loading level likely exists for tendon-to-bone healing. High loads may lead to microdamage at the tendon-bone interface and a decrease in biomechanical properties. Low loads may reduce cell stimulation and extracellular matrix synthesis and remodeling. In our study, casting alone led to better healing than casting plus removal of muscle force. Completely unloading the tendon-to-bone repair may have insufficiently stimulated cells, decreasing in biomechanical properties.
In support of our hypothesis, we saw improvement in functional properties with loading. Both range of motion and tendon excursion were improved when the tendon remained intact. The tendons in our Unloaded group, however, may not have experienced the same motion as tendons in the Loaded group. The paw was moved through the same range, but tendons in the Unloaded group may have remained stationary due to the proximal cut. It is unclear therefore whether improvements in functional properties were due to increased loading (stress in the tendon) or increased motion (gliding in the sheath). Our previous work in flexor tendon midsubstance healing showed that increased motion benefitted functional properties8,19 but increased loading had no effect20. If similar physiologic mechanisms apply, improved functional properties seen in the current study were likely due to increases in motion and not in load.
Muscle loading resulted in significantly improved biomechanical properties, especially in the physiologic sub-failure range. Previous studies showed that loading stimulates matrix production e.g.,21,22, collagen alignment e.g.,23,24, and bone formation15. Loading in our study, however, did not affect BMD. Therefore, the improvements in stiffness, rigidity, and strain at 20 N may have been due to increased matrix production and/or better collagen organization. Alternatively, the unloaded state may have led to a catabolic environment and a loss of tissue properties. The loaded state, on the other hand, may have prevented this catabolic environment, maintaining tissue properties. Future studies will examine the biologic effect of increased loading to determine the mechanisms leading to biomechanical changes.
Gap formation between the healing tendon and bone correlated with failure properties. No difference in gap formation was found comparing the Loaded and Unloaded groups, but a strong negative correlation existed between ultimate load and gap formation, similar to our previous study of flexor tendon mid-substance healing.25 In that study, gaps >3mm led to dramatically weaker structural properties 3 and 6 weeks after repair. In the current study, gaps >2.5mm led to dramatically weaker failure properties. These studies imply that regardless of treatment (Loaded vs. Unloaded repair), tissue will be prone to failure if a gap forms.
In our study, loading did not significantly affect BMD, suggesting that other factors may be more important for maintaining bone density during tendon-to-bone healing. Surprisingly, a strong negative correlation existed between ultimate load and BMD, contrary to our initial hypothesis that there would be a positive correlation between biomechanical properties and BMD. Our previous study showed that preventing bone loss after tendon-to-bone repair using bisphosphonates led to improved biomechanical properties.3 However, that study also suggested that bone density above a certain threshold may be detrimental to biomechanical properties. Dense bone at the healing interface may inhibit integration of unmineralized collagen fibers. The bony interface in this case may serve as a barrier to healing rather than a scaffold for integration.
Vascular supply may play a role in flexor tendon healing. At the time of surgical injury and repair, there was a long avascular zone in the Unloaded group due to the proximal cut. However, this scenario is similar to the Loaded group and to what is seen clinically in a zone I injury. Prior injection and clearing studies demonstrated a consistent 3 cm avascular zone proximal to the vinvculum breve in uninjured human FDP tendons.26 This zone does not revascularize during repair. The canine hand anatomy lacks a vinculum breve. Simulation of a zone I injury in the canine model extends the avascular zone from 3cm to 5cm, whether or not a proximal cut is made. Our prior study showed that a tendon stump (left unrepaired) remained viable and had reparative capability even when lacking a blood supply.27 Unlike the tendon midsubstance, the healing tendon-to-bone insertion re-vascularizes by 21 days after repair.28 Therefore, the tendon stump is nourished primarily by synovial fluid convection in the early period and by a vascular supply from the healing insertion in the late period after repair. Our two groups (Loaded and Unloaded) began with the same vascular supply proximal to the repair (i.e., a 3 cm avascular zone) and a disrupted vascular supply directly at the tendon-to-bone insertion. The only difference between the two groups, therefore, was the loading environment.
There are several limitations to this study. We only examined one early time point of healing. Previous studies demonstrated that structural and normalized mechanical properties did not improve in the first 3 weeks of healing, and often decreased relative to time zero.4,5 Therefore, we chose to study the 3 week time point in an attempt to influence the phase of tendon-to-bone healing where the construct is at greatest risk for failure. Our study included a number of assessments, but we did not include extensive biologic analysis. Future studies will examine matrix synthesis at the genetic and protein levels and structural analysis at the collagen fiber orientation and ultrastructural levels to determine the biologic mechanisms for improved tendon-to-bone healing. Finally, we have no quantitative measure of loading across the repair site in either of our groups. Presumably, tendon transection led to zero loading across the site in the Unloaded group. However, adhesions may have formed between the tendon and surrounding structures, leading to some loading at the repair site. The level of load in the Loaded group, on the other hand, is likely close to that measured in our previous flexor tendon study.29
In conclusion, we found that muscle loading was beneficial to healing. Complete removal of load through casting and proximal tendon transection resulted in less range of motion and lower biomechanical properties than casting alone. A balance must likely be reached during tendon-to-bone healing between too much loading (leading to damage at the repair) and too little loading (leading to insufficient cell stimulation). Gap formation between the healing tendon and bone, which correlated with ultimate load, should be prevented during the post-operative period.
Acknowledgments
The authors thank Tim Morris for providing animal care and rehabilitation. This study was supported by the NIH grants (EB004347 and AR033097).
References
- 1.Kelsey JL. Upper extremity disorders: frequency, impact and cost. Churchill Livingstone; New York, NY: 1997. [Google Scholar]
- 2.Praemer A, Furner S, Rice D. Musculoskeletal Conditions in the US. American Academy of Orthopaedic Surgeons; Park Ridge IL: 1992. [Google Scholar]
- 3.Thomopoulos S, Matsuzaki H, Zaegel M, et al. Alendronate prevents bone loss and improves tendon-to-bone repair strength in a canine model. J Orthop Res. 2007;25:473–479. doi: 10.1002/jor.20293. [DOI] [PubMed] [Google Scholar]
- 4.Silva MJ, Thomopoulos S, Kusano N, et al. Early healing of flexor tendon insertion site injuries: Tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24:990–1000. doi: 10.1002/jor.20084. [DOI] [PubMed] [Google Scholar]
- 5.Silva MJ, Boyer MI, Ditsios K, et al. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury and suture repair. J Orthop Res. 2002;20:447–453. doi: 10.1016/S0736-0266(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 6.Boyer MI, Harwood F, Ditsios K, et al. Two-portal repair of canine flexor tendon insertion site injuries: histologic and immunohistochemical characterization of healing during the early postoperative period. J Hand Surg [Am] 2003;28:469–474. doi: 10.1053/jhsu.2003.50091. [DOI] [PubMed] [Google Scholar]
- 7.Woo SL, Gomez MA, Sites TJ, et al. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg [Am] 1987;69:1200–1211. [PubMed] [Google Scholar]
- 8.Gelberman RH, Woo SL, Lothringer K, et al. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg [Am] 1982;7:170–175. doi: 10.1016/s0363-5023(82)80083-x. [DOI] [PubMed] [Google Scholar]
- 9.Gomez MA, Woo SL, Amiel D, et al. The effects of increased tension on healing medical collateral ligaments. Am J Sports Med. 1991;19:347–354. doi: 10.1177/036354659101900405. [DOI] [PubMed] [Google Scholar]
- 10.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907–914. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H, Manske PR, Pruitt DL, Larson BJ. Effect of cyclic tension on lacerated flexor tendons in vitro. J Hand Surg [Am] 1995;20:467–473. doi: 10.1016/S0363-5023(05)80109-1. [DOI] [PubMed] [Google Scholar]
- 12.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 13.Kamps BS, Linder LH, DeCamp CE, Haut RC. The influence of immobilization versus exercise on scar formation in the rabbit patellar tendon after excision of the central third. Am J Sports Med. 1994;22:803–811. doi: 10.1177/036354659402200612. [DOI] [PubMed] [Google Scholar]
- 14.Wolff J. Translated as: The Law of Bone Remodeling. Springer-Verlag; Berlin: 1892. Das Gesetz der Transformation der Knochen (Berlin A. Hirchwild) [Google Scholar]
- 15.Bostrom MPG, Boskey A, Kauffman JK, Einhorn TA. Form and function of bone. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science. 2. AAOS; 2000. pp. 319–370. [Google Scholar]
- 16.Ditsios K, Boyer MI, Kusano N, et al. Bone loss following tendon laceration, repair and passive mobilization. J Orthop Res. 2003;21:990–996. doi: 10.1016/S0736-0266(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 17.Dovan TT, Gelberman RH, Kusano N, et al. Zone I flexor digitorum profundus repair: an ex vivo biomechanical analysis of tendon to bone repair in cadavera. J Hand Surg [Am] 2005;30:258–266. doi: 10.1016/j.jhsa.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Silva MJ, Brodt MD, Boyer MI, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 19.Woo SL, Gelberman RH, Cobb NG, et al. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981;52:615–622. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 20.Boyer MI, Gelberman RH, Burns ME, et al. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg [Am] 2001;83-A:891–899. [PubMed] [Google Scholar]
- 21.Mauck RL, Soltz MA, Wang CC, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 22.Chiquet M, Renedo AS, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman SD, Karlon WJ, Holmes JW, et al. Structural and mechanical factors influencing infarct scar collagen organization. Am J Physiol Heart Circ Physiol. 2000;278:H194–200. doi: 10.1152/ajpheart.2000.278.1.H194. [DOI] [PubMed] [Google Scholar]
- 24.Thomopoulos S, Fomovsky GM, Holmes JW. The development of structural and mechanical anisotropy in fibroblast populated collagen gels. J Biomech Eng. 2005;127:742–750. doi: 10.1115/1.1992525. [DOI] [PubMed] [Google Scholar]
- 25.Gelberman RH, Boyer MI, Brodt MD, et al. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975–982. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Leversedge FJ, Ditsios K, Goldfarb CA, et al. Vascular anatomy of the human flexor digitorum profundus tendon insertion. J Hand Surg [Am] 2002;27:806–812. doi: 10.1053/jhsu.2002.35080. [DOI] [PubMed] [Google Scholar]
- 27.Silva MJ, Ritty TM, Ditsios K, et al. Tendon injury response: assessment of biomechanical properties, tissue morphology and viability following flexor digitorum profundus tendon transection. J Orthop Res. 2004;22:990–997. doi: 10.1016/j.orthres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Ditsios K, Leversedge FJ, Gelberman RH, et al. Neovascularization of the flexor digitorum profundus tendon after avulsion injury: an in vivo canine study. J Hand Surg [Am] 2003;28:231–236. doi: 10.1053/jhsu.2003.50025. [DOI] [PubMed] [Google Scholar]
- 29.Lieber RL, Silva MJ, Amiel D, Gelberman RH. Wrist and digital joint motion produce unique flexor tendon force and excursion in the canine forelimb. J Biomech. 1999;32:175–181. doi: 10.1016/s0021-9290(98)00154-7. [DOI] [PubMed] [Google Scholar]