Abstract
Intratracheal instillation of L-selectin-deficient (L-Sel−/−) mice with an adenovirus 2 (Ad2) vector resulted in the lack of respiratory Ad2- or β-galactosidase (βgal)-specific CTLs with concomitant long-lived βgal transgene expression in the lungs. The absence of Ag-specific CTLs was attributed to a deficiency in lymphoid CD11c+ CD8+ dendritic cells (DCs) in the lower respiratory lymph nodes (LRLNs). To enable L-Sel−/− CTL activity, cell-sorted L-Sel−/− CD8+ T cells were co-cultured with cell-sorted L-Sel+/+ CD8+ or CD8− DCs or L-Sel−/− CD8− DCs. Only the CD8+ DCs restored CTL activity; L-Sel−/− CD8− DCs failed to support L-Sel+/+ CTLs because these remained immature, lacking the ability to express co-stimulatory molecules CD40, CD80, or CD86. While no lung CD8+ DCs were detected, the DC environment remained suppressive in L-Sel−/− mice evident by the lack of CTL responses following adenoviral challenge with ovalbumin in recipient L-Sel−/− adoptively transferred with OT-1 CD8+ T cells. To assess whether the L-Sel−/− CD8− DCs could be induced into maturity, microbial stimulation studies were performed showing the failure of L-Sel−/− LRLN to make matured DCs. When L-Sel−/− mice were subjected in vivo to microbial activation prior to Ad2 vector dosing, CTL activity was restored stimulating the renewed presence of LRLN CD8+ DCs in L-Sel−/− mice. These studies show that impairment of L-Sel−/− DCs maturaion results in insufficient mature DCs that require microbial activation to restore increases in respiratory CD8+ DCs to support CTL responses.
Keywords: mucosal immunity, dendritic cells, tolerance
Introduction
Few studies have assessed the role of L-selectin in mucosal responses despite the importance of L-selectin in Peyer’s patches (1–4), nasal-associated lymphoid tissue (NALT3; 5, 6), head and neck lymph nodes (HNLNs; 5, 7, 8), and human tonsils (9). The homing receptor, L-selectin, mediates naive lymphocyte binding through its interaction with peripheral node addressin (PNAd), which is the predominant addressin expressed in peripheral LNs (PLNs; 10, 11). It is also responsible for some binding in mucosal tissues, including the mesenteric LNs (12) and the various HNLNs (5). Because PNAd is a composite of carbohydrate moieties expressed on different glycoprotein backbones (13), PNAd-L-selectin interactions have also been shown to mediate some binding of naive lymphocytes to the Peyer’s patches and NALT through the expression of PNAd carbohydrate on the mucosal addressin cellular adhesion molecule-1 glycoprotein backbone (6, 14, 15).
The development of L-selectin-deficient (L-Sel−/−) mice has enabled testing of L-selectin’s importance to Ag-specific memory responses. These mice show diminished DTH responses (16) and primary Ag-specific T cell proliferative responses (16), but not mitogen-stimulated T cell proliferative responses (17). This failure in Ag-specific T cell proliferative responses is not attributed to defective Ag presentation since this aspect remains functionally intact and capable of presenting Ag to wild-type T cells (18). Interestingly, defects in secretory IgA responses were observed in L-Sel−/− mice that were mucosally immunized. When L-Sel−/− mice were immunized intranasally with the potent mucosal adjuvant, cholera toxin, systemic IgG and gut IgA responses were not affected, but upper respiratory and vaginal IgA responses were delayed (7, 8). L-Sel−/− mice orally immunized with an attenuated Salmonella vaccine vector expressing the fimbriae from enterotoxigenic E. coli showed no fimbriae-specific mucosal IgA Abs despite elevations in systemic IgG Ab titers (19).
While most studies have focused on L-selectin expression on B and T lymphocytes, few studies have evaluated the role of L-selectin expression on dendritic cells (DCs; 20 – 22). In particular, L-Sel−/− mice were found to show a reduced expression of CD11c+ CD11b− DCs in their LN when compared to L-Sel+/+ BALB/c mice (22), but the functional consequence of this observation was not pursued. In this current study, in addition to the L-selectin deficiency, these mice showed an overall deficiency in DCs numbers, with the CD8+ DCs subset being particularly affected in the lower respiratory lymph nodes (LRLNs), as evidenced by an overall reduction in their numbers, resulting in a concomitant loss of CTL function. The absence of this CD11c+ CD8+ DCs resulted in the loss of Ad2- and βgal-specific CTL responses despite the presence of other lymphoid DCs.
Materials and Methods
Virus
The non-replicating Ad2/βGal-2 vector (23) was kindly provided by the Virus Production Unit of Genzyme Corporation (Framingham, MA) and was subsequently expanded in 293 cells (CRL-1573; American Type Culture Collection, Manassas, VA) and purified by cesium chloride-gradient centrifugation (23, 24). Wild-type adenovirus 2 (Ad2) stock was purchased from Quantum Biotechnologies (Montreal, Canada) and grown in HeLa cells (CCL-2.2; ATCC) for 48 hrs following infection. The virus was harvested by repeated freeze thawing and titered by serial dilutions. A stock of Ad5 CMV Trf-OVA (25) was kindly provided by the University of Iowa Gene Transfer Vector Core, referred to here as Ad5-OVA.
Mice
C57BL/6N mice (Frederick Cancer Research Facility, National Cancer Institute, Frederick, MD) and C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were used throughout this study. L-Sel−/− and OT-I breeder pairs were obtained from The Jackson Laboratory (Bar Harbor, ME) to establish our colony. All mice were maintained at Montana State University Animal Resources Center under pathogen-free conditions in individually ventilated cages under HEPA-filtered barrier conditions and were fed sterile food and water ad libitum. Mice (5–10/group) at 6 to 8 wks of age were anesthetized with isofluorane (Abbott Laboratories, North Chicago, IL) inhalation to effect and received a single intratracheal (i.t.) dose of 1x109 pfu of Ad2/β Gal-2 vector in 50 μ.l (24, 26). In some instances, mice were dosed i.t. with 0.1 μg, 10 μg, or 100 μg E. coli O55:B5 LPS (Sigma-Aldrich Chemical Co., St. Louis, MO) 3 days before or 2 days after i.t. instillation with Ad2 vector. Most evaluations were conducted after 12 days, except for the kinetic studies.
Lymphocyte and DC isolation
The lungs, LRLNs, HNLNs, and spleens were used throughout these studies for cytolytic assays or FACS analysis. Lymphocytes were isolated from the LN and spleens by mechanical disruption followed by Ficoll-Hypaque (Lymphocyte M, Accurate Chemical, Westbury, NY) density gradient centrifugation (24,26). Lung mononuclear cells were isolated and subjected to collagenase (Worthington, Freehold, NJ) digestion (24,26) with > 95% cell viability recovered.
DCs were isolated from LRLN by collagenase (50 U/ml Type IV; Sigma-Aldrich) digestion + DNAse (0.8 U/ml; Promega, Madison, WI) in Teflon flasks under gentle stir for 30 min at 37°C. The digested LRLN were passed through Nitex (Fairview Fabrics, Hercules, CA) and incubated at 37°C for 30 minutes. Cell suspensions were washed in complete media (CM) consisting of RPMI 1640 medium supplemented with 0.2 mM L-glutamine, 10 mM HEPES, 0.1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FBS (Gibco BRL-Life Technologies, Grand Island, NY). Total lymphocytes were resuspended in 2.0 ml of Hank’s Balanced Salt Solution (HBSS; BRL-Life Technol.) and then added to 1.0 ml OptiprepTM (Axis-Shield PoC AS, Oslo, Norway) and mixed gently. This was then layered with a 1:3.2 (14.3%) solution of diluent, which consisted of 0.88% NaCl, 1.0 mM EDTA, and 0.5% (w/v) bovine serum albumin (BSA), and 10 mM Hepes-NaOH, pH 7.4) and OptiprepTM. The gradient was then topped with 3.0 ml of HBSS. Lymphocytes were subjected to density gradient centrifugation for 15 minutes at 20°C. DCs were removed from the top of the 12% Optiprep gradient and washed in CM. Typically, DCs were enriched to >80% purity, as evaluated by immunofluorescent staining with anti-CD11c (B–D Pharmingen, San Diego, CA) and DEC205 (Serotec, Inc., Raleigh, NC) mAbs.
To assess conversion of CD8− to CD8+ DCs, DCs were isolated using a magnetic labeling kit to isolate CD8+ DC (Miltenyi Biotec, Gladbach, Germany). Total LRLN and lung lymphocytes were subfractionated to remove non-DCs. Following enrichment, CD8+ DCs were isolated by positive selection, and the CD8− DC fraction was passed through the column. Both the CD8+ and CD8− DCs were evaluated by FACS and showed >80% purity, and the CD8− DCs contained ≤2.2% CD8+ DCs. The purified CD8+ DCs were stained with 1.25 μM carboxy-fluorescein diactetate succinimidyl ester CFDA (Molecular Probes-Invitrogen, Carlsbad, CA) in RPMI for 5 minutes at RT in the dark and then washed three times. The purified CD8− DCs were stained with 2 μl of CM-Dil (Molecular Probes-Invitrogen) in 1 ml of RPMI for 20 minutes at 37°C and then washed three times. The CFDA-labeled CD8+ and CM-DiI-labeled CD8− DCs were co-cultured without or with 10 μg/ml LPS for 24 hrs. Some cultures were then infected with Ad2/βgal-2 at 500:1 ratio for additional 24 hrs. DCs were harvested, washed, and stained for CD11c, CD8α, MHC class II, and TCRβ.
Cell lines
EL4 thymoma (TIB39) and E.G7-OVA (CRL2113) (H-2b) cells were obtained from ATCC. E22 cells are EL4 cells transfected with the lacZ gene and were kindly provided by Dr. Yvonne Paterson (27).
Cytotoxicity assay
To assess CTL activity in mononuclear cell suspensions from lungs, LRLN, and spleens following a single i.t. dose of Ad2/βGal-2, a standard 51Cr release assay was adapted (24). For Ad2-infected targets, E22, and EL-4 cells were prepared and loaded with 100 mCi of Na251CrO4 (NEN Life Science Products, Inc./Dupont, Boston, MA), and cytotoxic assays were performed identically to those previously described (26).
To determine the supportive DCs for Ad2- and βgal-specific CTLs, L-Sel+/+ and L-Sel−/− LRLN and HNLN CD8+ T cells, L-Sel+/+ LRLN CD11c+ CD8+, and L-Sel+/+ and L-Sel−/− LRLN CD11c+ CD8− were sorted by flow cytometry to > 98% purity. DCs were co-cultured with CD8+ T cells at 1:5 ratio and restimulated with mitomycin C-treated Ad2-infected splenocytes for 5 days.
To assess role of lung DCs support, 5x106 purified splenic and respiratory LN transgenic OT-1 anti-OVA CD8+ T cells (obtained to >95% purity) were adoptively transferred into L-Sel+/+ or L-Sel−/− mice, and one day later, mice were dosed i.t. with 1x109 pfu of Ad5-OVA. L-Sel+/+ and L-Sel−/− lungs were evaluated two and three days after Ad5-OVA challenge for OVA257–264 H-2Kb pentamer+ (PE-labeled Pro5™ MHC pentamer; Proimmune Limited, Oxford Science Park, United Kingdom) CD8+ T cells by flow cytometry and CTL responses to OVA using the E.G7-OVA as target cells in a chrominum release assay, as described above.
βgal transgene expression in lungs
Mice dosed i.t. with Ad2/βGal-2 were evaluated for the expression of βgal according to the modified method by Parsons et al. (28) at varying intervals between 3 and 28 days. Following exsanguination, lungs were perfused with 2 mls of cold 2% paraformaldehyde in Dulbeccos PBS (DPBS) containing 2 mM MgCl2 and 1.25 mM EGTA. The fixed, inflated lungs were dissected from chest cavity and immersed in paraformaldehyde at 4°C overnight. Lungs were rinsed in rinse buffer (DPBS containing 2 mM MgCl2 and 0.1% Triton X 100) for three changes, 30 minutes each on a rotator. XGAL (40 mg/ml; Fisher Scientific, Hampton, NH) dissolved in dimethylformamide was diluted to 1.0 mg/ml in XGAL staining buffer (5 mM potassium ferrocyanide and 5 mM potassium ferricyanide in Rinse Buffer). Rinsed lungs were immersed into XGAL staining solution up to 3 days at 30°C to 32°C. After the blue color developed, stained lungs were immersed into 30% sucrose in DPBS overnight at 4°C, and then embedded in OCT (Sakura Finetek, Torrance, CA), snap frozen, and cryosectioned at −26°C. Five μm frozen sections were mounted on Plus Charge® (Erie Scientific, Portsmouth, NH) microscope slides, air dried at room temp, counterstained with 10 dips in nuclear fast red, rinsed with water, air dried, and cover slipped using permanent mounting media.
Flow cytometry
To determine the type of CD11c+ DCs recruited to the LRLN following one i.t. instillation with Ad2 vector, lymphocytes from lungs and LRLN were assessed. Fluorochrome-conjugated mAbs (B-D PharMingen) for mouse CD11c (N418), TCRβ (H57–597), Mac-3, CD4 (RM4–5), CD8α (53-6.7), B220 (RA3-6B2), CD11b (M1/70), L-Sel (Mel-14), Gr1 (RB6-8C5), CD40 (3/23), CD80 (16-10A1), and CD86 (GL-1). The mAbs for plasmacytoid DC included cJF05-1C2.4.1 (mPDCA-1 clone, Miltenyi Biotec) and anti-Siglec H mAb (29; clone 440c; Cell Sciences, Inc., Canton, MA). Immunofluorescent staining was then measured by flow cytometry using a forward scatter gate set for lymphocytes in the LRLN and a forward scatter gate set for lymphocytes and myeloid cells in the lungs.
For cell sorting of CD8+ T (CD8+ TCRβ+) cells, LRLN lymphocytes were labeled with fluorochrome-conjugated anti-CD4, anti-CD8, and anti-TCRβ mAbs for 30 min on ice. For cell sorting of DCs (CD11clow CD8+ vs CD8− TCRβ −), LRLN lymphocytes were labeled with anti-CD11c, anti-CD8α, and anti-TCRβ mAbs. The excess label was washed, and cells were subjected to cell sorting using a FACSVantage (B-D Biosciences). Greater than 98% purity of single-positive populations was isolated for in vitro cell culture.
Statistical analysis
The Student’s t test was used to evaluate the differences between experimental parameters in each experiment.
Results
Absence of Ad2- and βgal-specific CTLs in L-Sel−/− mice
I.t. instillation with the Ad2/βGal-2 vector elevates perforin- and FasL-dependent cytolytic Ad2- and βgal-specific CD8+, not CD4+ T cells (24). To learn the DCs that support these CD8+ T cell responses, L-Sel+/+ and L-Sel−/− mice were given a single i.t. dose of 1 x 109 i.u. of Ad2/βGal-2. L-Sel+/+ mice gave the expected dose-dependent (varying effector to target cell ratios) CTL response against Ad2-infected targets using freshly isolated lung, mediastinal and hilar lymph nodes, referred to as lower respiratory lymph nodes (LRLN), HNLN, and splenic lymphocytes as effector cells (Fig. 1A). Ag-specific reactivity was determined since no lysis of mocked-infected cells occur (Figure 1A) nor lysis of YAC-1 cells (data not shown). Analysis of lymphocytes freshly isolated from Ad2/βGal-2-dosed L-Sel−/− mice showed no cytolytic activity against Ad2- or mock-infected targets (Fig. 1B) nor after in vitro Ag-restimulation for 5 days in any of the tissues tested (Fig. 1D), which contrasted with Ag-restimulated L-Sel+/+ lymphocytes (Fig. 1C). This observed inactivity was not time-dependent since weekly sampling to six wks post-instillation of L-Sel−/− mice failed to reveal CTL responsive in any of the tested tissues (Fig. 1E–J) nor by Ag restimulation assays (data not shown). In contrast, L-Sel+/+ lung and splenic CTLs showed increasing cytolytic activity against Ad2-infected targets and βgal-expressing (E22 cells) targets for at least to six wks post-instillation (Fig. 1E–J).
Figure 1.
L-Sel−/− mice fail to develop CTL responses to Ad2 after i.t. instillation with Ad2 vector even after 6 wks post-i.t. instillation, resulting in sustained lung βgal transgene expression. L-Sel+/+ and L-Sel−/− mice were i.t. instilled with Ad2/βGal-2 vector, and (A and B) freshly isolated mononuclear cells from lungs, LRLNs, HNLNs, and spleens were assessed 12 days later for CTL activity against wild-type Ad2- or mock-infected targets. (C and D) Following in vitro restimulation for 5 days with Ad2-infected targets, LRLN, HNLN, and splenic CTL activity was assessed. Varying effector to target (E:T) cell ratios were tested and showed that despite antigen-restimulation, lymphocytes from L-Sel−/− mice failed to lyse virus-infected cells. The percent cytotoxicity was expressed as the level of cytotoxicity obtained at each E:T cell ratio corrected for spontaneous release divided by total release of 51Cr corrected for spontaneous release. Data are representative of eight experiments and depicted as mean ± SD. To determine time dependence, L-Sel+/+ and L-Sel−/− mice were given a single i.t. dose Ad2/βGal-2 vector and freshly isolated lymphocytes (ex vivo) from the lungs, LRLNs, and spleens were evaluated 3, 4, or 6 wks later against (E, G, and I) Ad2-infected targets or (F, H, and J) βgal-expressing EL-4 (E22) cells at varying effector to target (E:T) cell ratios. L-Sel−/− mice remained unresponsive to Ad2 or βgal at any of the time points examined. Lungs from (K and L) 3 and (N and O) 28 day post-i.t. instilled L-Sel−/− and L-Sel+/+ mice were evaluated for βgal expression. The βgal activity diminished after 3 days in L-Sel+/+ mice, but endured for (N) at least 28 days in L-Sel−/− mice. The kinetic CTL data (mean ± SD) are representative of two experiments and the depicted histology are representative of 5 mice/group.
Because of the observed immune unresponsiveness, we questioned whether in vivo transgene expression by the lacZ gene delivered by this Ad2 vector would be sustained. I.t. instillation of L-Sel−/− and L-Sel+/+ mice was conducted using the Ad2/βGal-2 vector, and at varying intervals, mice lungs were sampled for βgal expression. When compared to L-Sel+/+ mice, L-Sel−/− lungs showed sustained βgal expression for at least 28 days (Fig. 1N) contrasting with transgene expression in L-Sel+/+ mice, which lost its βgal expression (Fig. 1O). Such evidence suggests that the βgal protein was not stimulating a CTL response in L-Sel−/− mice. Thus, these studies show that the Ad2/βGal-2 vector was unable to stimulate a CD8 T cell-dependent response in L-Sel−/− mice.
Absence of LRLN CD8+ DCs contributes to CD8+ T cell unresponsiveness to Ad2/βGal-2 vector
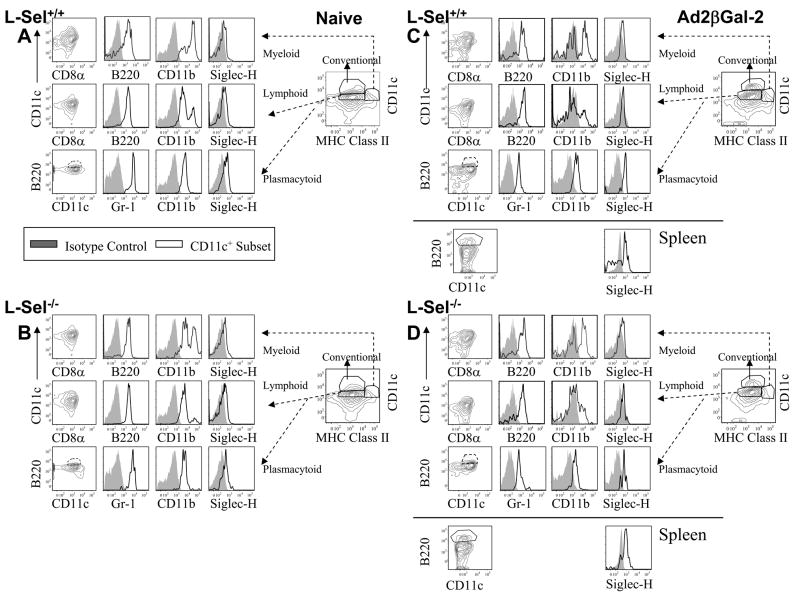
FACS analysis was performed to determine whether the observed unresponsiveness by L-Sel−/− mice might be attributed to a defect or an absence of a particular DC subset. Determination of DC subsets was based upon descriptions for respiratory DCs (29–33) in which forward and side-scatter profiles were evaluated, and to exclude lung macrophages, MHC classlow, CD11clow, and Mac-3+ cells were removed from the analysis. For the lymphocyte gate, CD11c+ TCRβ+ cells were also excluded from the analysis. Four DC subsets determined relevant to respiratory tissues (29–33) were evaluated: conventional DCs (cDCs), CD11chigh MHC class II+; lymphoid DCs, CD11c+ MHC class II+, CD8+ or −; myeloid DCs (mDCs), CD11c+ MHC class IIhigh, CD11bhigh, SiglecH−; and plasmacytoid DCs (pDCs), CD11c+ MHC class II+, B220high, SiglecH+ (Figs. 2 and 3).
FIGURE 2.
In naive (B) or in (D) Ad2 vector-dosed L-Sel−/− LRLNs, the CD11clow CD8+ DCs are reduced when compared to (A and C) L-Sel+/+ mice. FACS analysis was performed on (A and C) L-Sel+/+ and (B and D) L-Sel−/− LRLN CD11clow MHC class II+ (TCRβ −) DCs to discern CD8− (dashed lines) and CD8+ (solid lines) DC subsets. The majority of the (B and D) L-Sel−/− LRLN DCs were CD11clow CD8− when compared to (B and D) L-Sel+/+ LRLN DCs regardless of instillation with (C and D) Ad2 vector. Cell surface analyses were performed on L-Sel+/+ and L-Sel−/− LRLN to discern the DC phenotypes: mDCs, CD11c+, MHC class II+, CD11bhigh, Siglec-H−, B220+/−; lymphoid DCs, CD11c+, MHC class II+, CD8+/−, Gr-1+, B220+; and pDCs, CD11c+, MHC class II+, B220high, Gr-1+, Siglec-H+, CD11b+/−. The depicted data are representative from at least 10 mice/group.
FIGURE 3.
Differences are found in L-Sel−/− lung DC subsets in naive mice following i.t. Ad2 vector instillation. FACS analysis of naive lung show in both (A) L-Sel+/+and (B) L-Sel−/− mice showed the presence of mDCs (CD11c+ MHC class IIhigh, CD11bhigh, B220+ Siglec-H−), cDCs (CD11chigh MHC class II+), lymphoid DCs (CD11c+ MHC class II+ CD11b+ B220+), and pDCs (CD11c+ MHC class II+ CD11b+ B220high Siglec-H+). (C and D) By 12 days post-i.t. instillation with the Ad2 vector, less cDCs were present in (D) L-Sel−/− lungs (Table III). Positive (CD11c+ B220high Siglec-H+) pDC histograms for splenic pDCs obtained from Ad2-dosed (C) L-Sel−/− and (D) L-Sel+/+ mice are depicted. The depicted data are representative of at least 10 mice/group.
Evaluation of naive L-Sel−/− and L-Sel+/+ LRLN DC subsets revealed that the CD11chigh cDCs were nearly nonexistent, and in fact, for both mice, nearly all the DCs were CD11clow (Fig. 2A and B). Subsequent evaluations of these DC subsets revealed that the overall percentages of lymphoid, mDCs, and pDCs did not vary between naive L-Sel−/− and L-Sel+/+ LRLNs (Table I); however, of the lymphoid DCs, the CD8+ DC subset was diminished in L-Sel−/− LRLN (Fig. 2B; Table II). The percentage of the CD11clow CD8+ DCs of the total CD11clow DCs in naive L-Sel−/− LRLN was 14.4%, which was reduced by nearly half when compared to L-Sel+/+ mice (Fig. 2B; Table II).
Table I.
DC subsets in the LRLNs
| Treatmentb |
L-Selectinc |
P1d | P2e Naive vs. Treatment
|
||||
|---|---|---|---|---|---|---|---|
| DC Subset a | Ad2/β Gal-2 | LPS | −/− | +/+ | −/− vs. +/+ | −/− | +/+ |
| Lymphoid | – | – | 72.4 ± 3.1 | 77.8 ± 2.6 | NS | - | - |
| “ | + | – | 76.8 ± 1.9 | 81.2 ± 2.1 | NS | NS | NS |
| “ | + | – | 75.8 ± 2.7 | 78.0 ± 1.1 | NS | NS | NS |
| Plasmacytoid | – | – | 10.8 ± 2.3 | 11.8 ± 1.8 | NS | - | - |
| “ | + | – | 17.1 ± 1.2 | 14.6 ± 1.5 | NS | 0.038 | NS |
| “ | + | + | 15.3 ± 1.7 | 17.6± 2.3 | NS | NS | NS |
| Myeloid | – | – | 26.8 ± 3.0 | 21.5 ± 2.5 | NS | - | - |
| “ | + | – | 21.9 ± 2.0 | 17.2 ± 2.0 | NS | NS | NS |
| “ | + | + | 23.6 ± 1.0 | 21.1 ± 1.1 | NS | NS | NS |
Pulmonary DCs were gated on lymphocytes that were CD11clow TCRα β−; no CD11chigh cDCs were detected in the LNs.
DCs were derived from naive, Ad2/βGal-2-dosed, or Ad2/βGal-2 + LPS-dosed mice.
−/− = L-Selectin-deficient mice; +/+ = B6 mice. Data presented as mean ± SEM.
p-value between L-Sel−/− and L-Sel+/+ DC subsets (n = 10 mice/group); NS = not significant.
p-value between treatments within L-Sel−/− or L-Sel+/+ groups.
Table II.
Lymphoid DC subsets in the LRLN
| Treatmentb |
L-Selectinc |
P1d | P2e Naive vs Treatment
|
||||
|---|---|---|---|---|---|---|---|
| DC Subset a | Ad2/βGal-2 | LPS | −/− | +/+ | −/− vs. +/+ | −/− | +/ + |
| LRLN CD8+ | – | – | 14.4 ± 1.6 | 28.4 ± 3.6 | 0.004 | - | - |
| “ | + | – | 16.7 ± 2.9 | 29.4 ± 3.1 | 0.006 | NS | NS |
| “ | + | + | 31.7 ± 4.7 | 36.9 ± 4.3 | NS | 0.003 | NS |
| LRLN CD8− | – | – | 84.5 ± 1.6 | 71.0 ± 3.8 | 0.007 | - | - |
| “ | + | – | 81.6 ± 2.8 | 69.6 ± 3.1 | 0.009 | NS | NS |
| “ | + | + | 67.1 ± 5.0 | 61.8 ± 4.5 | NS | 0.004 | NS |
Pulmonary DCs were gated on lymphocytes that were CD11c low TCRαβ−.
DCs were derived from naive, Ad2/βGal-2-dosed, or Ad2/βGal-2 + LPS-dosed mice.
−/− = L-Selectin-deficient mice; +/+ = B6 mice. Data presented as mean ± SEM.
p-value between L-Sel−/− and L-Sel +/+ DC subsets (n = 10 mice/group); NS = not significant.
p-value between treatments within L-Sel−/− or L-Sel +/+ groups.
Twelve days after dosing with the Ad2/βGal-2 vector revealed that only slight significant increase in pDCs (CD8− Gr-1+ Siglec-H+ B220+) were observed in L-Sel−/− LRLNs (Table I), and the percentage of CD11c+ CD8+ DCs (Gr-1+ Siglec-H− B220−) in the L-Sel−/− or L-Sel+/+ LRLN remained unchanged from their respective naive percentages, leaving the L-Sel−/− CD11c+ CD8+ DCs reduced (Fig. 2D; Table II). Although instillation of L-Sel−/− mice with Ad2/βGal-2 vector did not result in the absence of a specific DC subset, the percentage of CD11c+ CD8+ DCs remained less (p = 0.006) than in L-Sel+/+ LRLNs. Thus, the observed failure to induce Ad2- and βgal-specific CTLs may be due to the inability to mobilize L-Sel−/− CD11c+ CD8+ allowing the accumulation of CD11c+ CD8− DCs (p = 0.009; Table II). No changes in LRLN mDCs (CD11bhigh Gr-1+ Siglec-H− B220+) for either L-Sel−/− or L-Sel+/+ mice were observed following instillation with the Ad2 vector (Fig. 2 C, D and Table I).
Examination of the naive lung DCs revealed minimal differences in the percentages of the various subsets, with the pDCs and cDCs being slightly less in L-Sel−/− mice than L-Sel+/+ mice (Fig. 3A, B; Table III). By 12 days after i.t. delivery with the Ad2 vector, the lymphoid DCs did decrease in the L-Sel−/− lungs with a concomitant increase in cDCs and no significant change in mDCs (Fig. 3C, D; Table III). A similar pattern was also observed in the L-Sel+/+ lungs, although more cDCs and less lymphoid DCs were obtained when compared to the L-Sel−/− mice. The pDCs in the L-Sel−/− lungs did increase by ~70% when compared to naive lungs (p < 0.001), but the percentage of pDCs in these lungs were not significantly different from the percentage of pDCs from the Ad2 vector-dosed L-Sel+/+ lungs (Table III). There were minimal to no CD8+ DCs in and, and these remained unchanged (data not shown).
Table III.
DC subsets in the lungs
| Treatmentb |
L-Selectinc |
P1d | P2e Naive vs. Treatment
|
||||
|---|---|---|---|---|---|---|---|
| DC Subset a | Ad2/β Gal-2 | LPS | −/− | +/+ | −/− vs. +/+ | −/− | +/+ |
| Lymphoid | – | – | 57.6 ± 2.0 | 54.4 ± 4.4 | NS | - | - |
| “ | + | – | 44.5 ± 3.7 | 33.9 ± 2.7 | 0.035 | 0.007 | <0.001 |
| “ | + | – | 53.5 ± 2.9 | 38.6 ± 2.3 | <0.001 | NS | 0.004 |
| Plasmacytoid | – | – | 10.2 ± 0.7 | 14.2 ± 0.8 | 0.001 | - | - |
| “ | + | – | 17.3 ± 1.5 | 17.1 ± 1.6 | NS | <0.001 | NS |
| “ | + | + | 11.9 ± 1.4 | 14.5 ± 1.5 | NS | NS | NS |
| Myeloid | – | – | 11.4 ± 0.9 | 14.3 ± 2.0 | NS | - | - |
| “ | + | – | 17.5 ± 2.9 | 17.6 ± 3.8 | NS | NS | NS |
| “ | + | + | 17.8 ± 1.3 | 23.4 ± 1.3 | 0.006 | <0.001 | <0.001 |
| Conventional | – | – | 17.8 ± 1.0 | 24.0 ± 2.4 | 0.025 | - | - |
| “ | + | – | 33.8 ± 3.6 | 46.3 ± 3.9 | 0.029 | <0.001 | <0.001 |
| “ | + | + | 19.5 ± 2.5 | 31.8 ± 3.0 | 0.004 | NS | NS |
Pulmonary DCs were gated on lymphocytes that were CD11c TCRα β.
DCs were derived from naive, Ad2/βGal-2-dosed, or Ad2/βGal-2 + LPS-dosed mice.
−/− = L-Selectin-deficient mice; +/+ = B6 mice. Data presented as mean ± SEM.
p-value between L-Sel−/− and L-Sel+/+ DC subsets (n = 10 mice/group); NS = not significant.
p-value between treatments within L-Sel−/− or L-Sel+/+ groups.
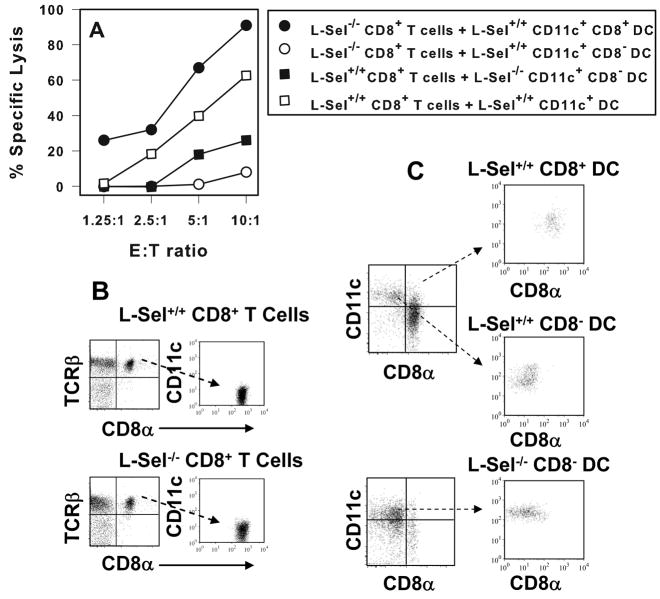
Addition of L-Sel+/+ CD11c+ CD8+ DCs, not CD11c+ CD8− DCs, drives L-Sel−/− CD8+ T cells to be responsive to Ad2 and βgal
Since CD11c+ CD8+ DCs were greatly reduced in L-Sel−/− LRLN, their addition should restore the cytolytic activity by L-Sel−/− CD8+ T cells. To test for this possibility, naive CD11c+ CD8+ or CD11c+ CD8− DCs were cell-sorted from L-Sel+/+ LRLN and co-cultured for five days in the presence of Ag and cell-sorted CD8+ T cells obtained from L-Sel−/− mice given a single i.t. dose of Ad2/βGal-2 vector, after which CTL activity was assessed. The cultures containing the CD8+ DCs restored cytolytic activity, whereas those co-cultured with CD8− DCs did not (Fig. 4A). Such evidence suggested that Ad2-specific CTLs may be induced in L-Sel−/− mice, but the frequency of supportive CD11c+ CD8+ DCs was insufficient in these mice, leaving L-Sel−/− mice in an unresponsive state. To test this possibility, L-Sel+/+ CD8+ T cells were co-cultured without or with L-Sel−/− LRLN CD8− DCs, and after 5 days, CTL responses were measured. The L-Sel+/+ CTL responses were normal; however, the cultures containing the L-Sel−/− LRLN DCs were compromised (Fig. 4A). Thus, this collective evidence shows that in the absence of L-selectin, LRLN CD11c+ CD8− DCs are not supportive, whereas CD11c+ CD8+ DCs support CTL responses.
FIGURE 4.
L-Sel−/− CTL activity is restored upon co-culture with L-Sel+/+ CD11c+ CD8+ DCs. Cell-sorted (B) L-Sel−/− or L-Sel+/+ LRLN and HNLN CD8+ T cells from 12 day post-instillation with Ad2 vector were co-cultured with (C) cell-sorted L-Sel+/+ CD11c+ CD8+ or L-Sel+/+ or L-Sel−/− CD11c+ CD8− DCs for 5 days and then evaluated for CTL activity against Ad2-infected targets. L-Sel+/+ CD11c+ CD8+ DCs restored CTL activity to L-Sel−/− CD8+ T cells contrasting the inability of L-Sel+/+ CD11c+ CD8− DCs or L-Sel−/− CD11c+ CD8− DCs to support L-Sel−/− CTLs. L-Sel+/+ CD8+ T cells co-cultured with total L-Sel+/+ CD11c+ DCs showed CTL activity. Data are representative of four experiments.
Are the L-Sel−/− CD8− DCs immature?
The observed inactivity by L-Sel−/− CD8+ T cells could be attributed to a lack of DC maturation. A kinetics analysis was performed to assess LRLN CD11clow DCs for co-stimulatory molecule expression of B7.1, B7.2, and CD40 (Fig. 5). Both L-Sel+/+ and L-Sel−/− CD8+ DCs showed enhanced expressions of B7.1 (p < 0.001) on day 3 post-i.t. instillation and no changes in CD40 levels at 3 or 12 days post-i.t. instillation (Fig. 5C). There was a significant increase in the amount of B7.2 expression on L-Sel+/+ (p < 0.001), but not on L-Sel−/− CD8+ DCs three days post-i.t. instillation with the Ad2 vector (Fig. 5B). Both L-Sel+/+ and L-Sel−/− CD8− DCs showed no changes in B7.1 or B7.2 levels, although the L-Sel+/+ LRLN showed more constitutive B7.2+ DCs (Fig. 5E). A reduction in CD40 was also observed in L-Sel−/− LRLN CD8− DCs at 3 days post-i.t. instillation (Fig. 5F). In the lung CD11clow DCs, even fewer changes were observed (data not shown). No differences in B7.1 or B7.2 were observed for either L-Sel+/+ and L-Sel−/− CD8+ or CD8− DCs. These findings collectively show the CD8− DCs are immature, even following i.t. dosing with the Ad2 vector, and the increased presence of CD8− DCs in L-Sel−/− LRLN may pre-empt a responsive phenotype.
FIGURE 5.

Reduced expression of co-stimulatory molecules by L-Sel−/− LRLN CD11c+ DCs. A kinetic analysis was performed to discern expression levels of co-stimulatory molecules (A and D) B7.1, (B and E) B7.2, or (C and F) CD40 following i.t. dosing with Ad2/βGal-2 vector. Evaluations were done by cell surface staining of LRLN CD11c+ (A–C) CD8+ and (D–F) CD8− DCs and analyzed by flow cytometry. Both L-Sel−/− and L-Sel+/+ CD11c+ CD8+ DCs showed increased B7.1 at 3 days post-i.t. instillation with the Ad2 vector. Only L-Sel+/+ CD11c+ CD8+ DCs showed increased expression of B7.2, and neither mice showed changes in CD40. For both L-Sel−/− and L-Sel+/+ CD11c+ CD8− DCs, no changes in B7.1 or B7.2 were observed as a consequence of Ad2 vector instillation; however, L-Sel−/− CD11c+ CD8− DCs were significantly lower in B7.2 expression and on three days post-i.t. instillation were significantly reduced in B7.1 expression when compared to L-Sel+/+ mice. Data depict results from 8 individual mice. Differences between L-Sel−/− and L-Sel+/+ CD11c+ DC: *p ≤ 0.005; **p = 0.018; ***p = 0.044. Differences in co-stimulatory molecule expression within the L-Sel−/− or L-Sel+/+ CD11c+ DC subset: ¶ p ≤ 0.001;¶¶¶ p = 0.03.
Lack of CTL activity by L-Sel−/− mice is a function of lung DC environment
Since CD8+ DCs were absent from the lungs at the time points examined (Fig. 3 C, D), the lack of lung CTL activity in L-Sel−/− mice is suggestive that environment or DC content of L-Sel−/− lungs contributes to the observed unresponsiveness. To test this possibility, transgenic OT-I CD8+ T cells specific for OVA257–264 were isolated and adoptively transferred into L-Sel+/+ or L-Sel−/− mice, and one day later, challenged i.t. with 1x109 pfu of Ad5-OVA. Total lung lymphocytes were harvested from their lungs and evaluated by flow cytometry for CD8+ T cells capable of binding soluble MHC class I loaded with OVA257–264 peptide (pentamer+) T cells. In the first experiment, no differences in the percentage of pentamer+ CD8+ T cells were obtained in L-Sel−/− or in L-Sel+/+ recipient lungs two or three days post-challenge with Ad5-OVA vector (Table IV). In contrast, the L-Sel−/− lungs remained unresponsive despite receiving transgenic OT-1 T cells, as evidence by the lack of lytic activity against E.G7-OVA target cells for either two or three days post-challenge (Table IV). OT-I T cells adoptively transferred into L-Sel+/+ mice remained responsive and capable of lysing E.G7-OVA target cells (Table IV). When this experiment was repeated, similar results were obtained in that two days after adoptive transfer of OT-1 into L-Sel−/− or in L-Sel+/+ recipients. No difference in pentamer+ CD8+ T cells were observed, but after three days, less pentamer+ CD8+ T cells were observed in the L-Sel+/+ lungs, yet CTL activity only associated with L-Sel+/+, not L-Sel−/− lungs (Table IV). Thus, the lack of activity in L-Sel−/− mice appears to be attributed to differences between the lung DCs.
Table IV.
Transgenic OT-1 CD8+ T cells remain unresponsive in Ad5-OVA-dosed L-Sel−/− lungs
| Experiment 1
| |||||||
|---|---|---|---|---|---|---|---|
| OT-1 → Recipient Tissuea | Day Testedb | % pentamer+ CD8+ T cellsb | P-Valuec | Effector: Targetd | % Lysisd | P-Valuee | |
| L-Sel+/+ lungs | 2 | 24.5 ± 10.1 | NS | 25:1 | 15.7 ± 4.3 | 0.005 | |
| 50:1 | 36.9 ± 2.3 | < 0.05 | |||||
| L-Sel−/− lungs | 2 | 21.1 ± 2.7 | 25.1 | −1.1 ± 2.6 | |||
| 50:1 | 8.8 ± 2.9 | ||||||
| L-Sel +/+ lungs | 3 | 15.2 ± 4.1 | NS | 25:1 | 38.0 ± 8.3 | < 0.05 | |
| 50:1 | 66.1 ± 10.2 | 0.005 | |||||
| L-Sel−/− lungs | 3 | 13.0 ± 0.3 | 25:1 | 6.5 ± 1.8 | |||
| 50:1 | 8.5 ± 1.6 | ||||||
|
| |||||||
| Experiment 2 | |||||||
| L-Sel+/+ lungs | 2 | 8.5 ±0. 9 | NS | 25:1 | 11.8 ±3.7 | NS | |
| 50:1 | 20.0 ± 4.3 | NS | |||||
| L-Sel−/− lungs | 2 | 9.0 ±2.1 | 25:1 | 5.5 ± 3.2 | |||
| 50:1 | 11.8 ±4.0 | ||||||
| L-Sel +/+ lungs | 3 | 4.6 ± 0.4 | 0.040 | 25:1 | 17.0 ±3.8 | 0.007 | |
| 50:1 | 24.0 ± 1.4 | <0.001 | |||||
| L-Sel−/− lungs | 3 | 8.4 ±1.2 | 25:1 | 2.1 ±3.4 | |||
| 50:1 | 5.9 ±3.3 | ||||||
Transgenic OT-1 T cells were purified to > 95% CD8+ T cells, and 5 x 106 cells were injected i.v. into L-Sel−/− or L-Sel+/+ mice and challenged 24 h later with 1 x 109 Ad5-OVA. Recipient lungs were harvested 2 or 3 days after challenge.
Total lymphocytes were harvested from lungs and stained with OVA257–264 peptide in soluble MHC class I pentamer and with anti-CD8 mAb. Percent pentamer+ CD8+ T cells ±SEM were determined by flow cytometry.
P-value between the percentage of pentamer+ CD8+ T cells in L-Sel+/+ and L-Sel−/− lungs.
CTL experiments were performed using freshly isolated L-Sel+/+ or L-Sel−/− lymphocytes as effector cells measuring their lytic activity against E.G7-OVA target cells (percent lysis) at the indicated effector to target cell ratios.
P-value for OVA-specific cytolysis between L-Sel+/+ and L-Sel−/− lung lymphocytes.
Microbial activation restores the percentage of CD11c+ CD8+ DCs in L-Sel−/− LRLN and restoration of CTL function
To test if microbial activation by LPS would enable stimulation of functional CTLs, L-Sel−/− mice were i.t. dosed with 0.1, 10, or 100 μg of LPS either three days prior or 2 days after i.t. instillation with Ad2/βGal-2 vector, and CTL responses were measured 12 days later (Fig. 6A–F). The lower dose of LPS, 0.1 μg, had no effect upon stimulating Ad2-specific CTLs (Fig. 6A). The 10 μg dose showed an impact (Fig. 6B), but clearly the 100 μg dose was effective in priming respiratory APC to become responsive (Fig. 6C). This priming event had to occur prior to exposure to the Ad2 vector because when the LPS was administered two days after i.t. Ad2 vector instillation, the absence of a CTL response remained (Fig. 6D–F). Subsequent experiments showed that LPS priming enhanced CTL responsiveness to βgal targets, as well (Fig. 6H, J, L, and N). While not necessary for L-Sel+/+ mice, the cytolytic activity was unchanged by the LPS activation (Fig. 6G–N). Furthermore, in vitro Ag restimulation with either Ad2 or βgal markedly improved cytolytic activity by L-Sel−/− respiratory effector cells (Fig. 6G and H). Such studies suggest that activation with the microbial product, LPS, overcomes the unresponsive state by respiratory APC to Ad2/βGal-2.
FIGURE 6.
In vivo microbial stimulation prior to i.t. instillation with Ad2 vector promotes Ad2-specific CTLs in L-Sel−/− mice because of restored CD8+ DCs in the LRLN. L-Sel−/− mice were given a single i.t. dose of (A and D) 0.1, (B and E) 10, or (C and F) 100 μg LPS (A–C) 3 days before or (D–F) 2 days after i.t. instillation with Ad2/βGal-2 vector. Lung and splenic CTL ex vivo analysis was conducted on day 12. LPS exposure prior to Ad2 vector delivery restored Ad2-specific CTL activity in L-Sel−/− mice, as opposed to exposure subsequent to Ad2 vector administration, which had minimal impact even at the 100 μg dose. Reactivity against mock-infected cells was not detected. Data are representative of 3 experiments. (G–N) Microbial activation restores CTL activity in L-Sel−/− mice similarly with those obtained in L-Sel+/+ mice. CTL responses were measured against (G, I, K, and M) Ad2-infected targets or (H, J, L, and N) βgal-expressing E22 cells. Mice were i.t. dosed 3 days with 100 μg LPS prior to i.t. instillation with Ad2/βGal-2 vector, and (G–J) ex vivo lung and splenic and (K–N) in vitro Ag-restimulated LRLN and splenic CTL responses were measured. LPS had a minimal impact upon L-Sel+/+ CTLs in contrast to L-Sel−/− CTLs, which were restored to or near to L-Sel+/+ cytolytic activities. Results depict one representative experiment of three.
To determine the impact of LPS upon the L-Sel−/− DC subsets, L-Sel−/− and L-Sel+/+ mice were dosed with 100 μg of LPS three days prior to Ad2/βGal-2 instillation. Evaluation of LRLN DCs 12 days post-i.t. instillation revealed an increase in the percentage of lymphoid CD8+ DCs in L-Sel−/− LRLNs to 31.7 ± 4.7, which was equivalent to that found in L-Sel+/+ LRLNs, 36.9 ± 4.3 (Table II). Stimulation with LPS and Ad2/βGal-2 more than doubled the percentage of lymphoid CD8+ DCs in the L-Sel−/− LRLNs (p = 0.003) with no changes in cell surface expression (data not shown) from mice dosed with only Ad2 vector (Fig. 2). In contrast, this same LPS stimulation did not impact the percentage of CD8+ DCs in the L-Sel+/+ LRLN (Table II). These increases in the CD11c+ CD8+ DCs suggest that the inability to induce or expand this DC subset in L-Sel−/− mice may account for the observed immune unresponsiveness after a single i.t. dose of Ad2 vector, whereas in L-Sel competent mice, increases in CD11c+ CD8+ DCs were achieved. No significant differences in the percentages of pDCs or mDCs were observed as a consequence of in vivo LPS treatment nor were there differences between L-Sel−/− and L-Sel+/+ LRLNs following LPS treatment (Table I).
Examination of L-Sel−/− and L-Sel+/+ lung DCs revealed that the LPS treatment restored lymphoid, pDCs, and cDCs to nearly the same levels as with naive lungs, each of which were less than mice treated with the Ad2 vector only (Table III). The mDCs increased in both L-Sel−/− and L-Sel+/+ lungs (p < 0.001).
Microbial activation of L-Sel−/− lung DCs results in the conversion CD8+ DCs
To assess whether LPS could induce conversion of L-Sel−/− CD8− DCs to CD8+ DCs, naive L-Sel+/+ and L-Sel−/− lung and LRLN CD8+ and CD8− DCs were isolated. CD8+ DCs were green labeled with CFDA; CD8− DCs were red labeled with CM-DiI. DC subsets were co-cultured and treated with LPS for 24 hrs prior to infection with Ad2/βGal-2 vector. Treatment of L-Sel−/− lung CD8− DCs with Ad2/βGal-2 or LPS only or both resulted in the conversion into CD8+ DCs with nearly a four-fold increase in CM-DiI CD8+ DCs (Fig. 7A), whereas only a 1.7-fold increase was obtained for L-Sel+/+ lung DCs (Fig. 7B). Concomitant reductions in lung CM-DiI-labeled CD8− DCs were observed for both L-Sel−/− and L-Sel+/+ lung CD8− DCs. This increase in lung CD8+ DCs was not attributed to increased proliferation of endogenous CD8+ DCs because the percentage of CFDA-labeled CD8+ DCs did not significantly change as a result of treatments, although a slight conversion into CD8− DCs was observed more so for the L-Sel−/− lung DCs (Fig. 7A) than the L-Sel+/+ lung DCs (Fig. 7B). Neither the L-Sel−/− nor L-Sel+/+ LRLN CD8− DCs showed conversion into CD8+ DCs (Fig. 7C and D). Thus, these data suggest that treatment with LPS can cause increased numbers of lung CD8+ DCs, which then can migrate to the draining LN.
Figure 7.
In vitro microbial activation of lung DCs results in the expansion of CD8+ DCs derived from CD8- DCs. (A and B) Naive lung and (C and D) LRLN CD8+ and CD8− DCs were isolated from (A and C) L-Sel−/− and (B and D) L-Sel+/+ mice. CD8+ DCs were labeled with CFDA (green open circles; residual CD8− DCs are depicted as solid green circles), and CD8− DCs were labeled with CM-DiI (red open circles; residual CD8+ DCs are depicted as solid red circles). Media treatments represent baseline for each lung and LRLN population. DCs were stimulated in vitro without or with LPS for 24 hrs followed by an additional stimulation with Ad2/βGal-2 for 24 hrs. DCs were harvested and immunostained for CD8 expression by CD11c+ MHC class II+ TCRβ− DCs. Increases in (A) new L-Sel−/− lung CD8+ DCs were observed by the conversion of CD8− (open red) DCs into CD8+ (solid red) DCs upon microbial stimulation, and not by expansion of CD8+ (open green) DCs. (C and D) LRLN DCs showed no conversion of CD8− (open red) DCs into CD8+ (solid red) DCs. Depicted are the combined results from two experiments.
Discussion
In this study, we describe the importance of L-selectin in the development of lymphoid CD8+ DCs to support CTL responses. Expression of CD8 has been shown to be associated with lymphoid DCs (28). L-Sel−/− mice i.t. dosed with a nonreplicating Ad2 vector failed to stimulate CTL responses, either to the Ad2 vector or its βgal transgene. Failure to display such cytolytic activities was not linked to a delay in responding since L-Sel−/− mice remained unresponsive even after six wks from initial i.t. dosing. By contrast, the observed CTL responses by the L-Sel+/+ mice were consistent with previous studies (24,26). To probe the reason for such attenuation of the Ad2- and βgal-specific CTLs, we hypothesized that the supportive DCs in L-Sel−/− mice may be compromised. In an attempt to obtain a correlative phenotype to explain this lack of CTL reactivity, FACS analysis was performed on both L-Sel−/− and L-Sel+/+ respiratory DCs. In the LRLNs, L-Sel−/− mice shared similar phenotypes and percentages of the different DC subsets with L-Sel+/+ mice. The majority of the DCs in both mice were the lymphoid DCs with some pDCs and mDCs, but no cDCs; however, a notable reduction in the percentage of lymphoid CD8+ DCs was apparent. Ad2-immunized L-Sel−/− mice failed to elicit CD8+ DC subset in the LRLN, unlike that observed with the L-Sel+/+ mice in which LRLN CTL responses to both Ad2 and βgal were induced with significantly more CD8+ DCs, suggesting that these supported the observed CTL responses. In the lungs, the most notable changes were observed in the percentages of DC subsets following i.t. delivery of the Ad2 vector. While both L-Sel−/− and L-Sel+/+ mice showed a decrease in the percentage of lymphoid DCs with concomitant increases in cDCs, ~70% increase in pDCs, and no changes in mDCs in the L-Sel−/− lungs. The cDCs in the L-Sel+/+ lungs nearly doubled. The lymphoid CD8+ DCs were minimally detectable in the L-Sel−/− lungs, and these mice remained unable to support CTL responses using either Ad2 or Ad5 vectors. It may be that this combination of differences in DC subsets, e.g., more pDCs, and possibly differences in magnitude of changes in cDCs and lymphoid DCs could account for the L-Sel−/− mice unresponsiveness in the lungs. This was further evidenced when even the adoptive transfer of immunocompetent transgenic (L-Sel+/+) OT-1 CD8 T cells into L-Sel−/− mice was still unable to support OVA-specific CTL responses following i.t. challenge with the Ad5-OVA. Thus, using three different Ags, it is clear that L-Sel−/− mice are unresponsive in their respiratory CD8 T cell responses.
The absence of CD8+ DCs in the L-Sel−/− LRLN contributed to the observed impairment in CD8+ T cell responses. Consequently, there was an increased presence of CD8− or immature DCs since these failed to show increased co-stimulatory molecule expression following i.t. instillation with Ad2/βGal-2 vector, unlike that observed with C57BL/6 LRLN CD8+ or CD8− DCs. The cell-sorting experiments showed that the lymphoid CD8+ DCs were responsible for Ag presentation to CD8+ T cells. Neither the L-Sel+/+ nor the L-Sel−/− CD8− DCs could support cell-sorted CD8+ CTL responses; only the L-Sel+/+ CD8+ DCs were able to support such responses. The L-Sel−/− mice could produce CTL responses, but only when virus was presented by L-Sel+/+ CD8+ DCs. Since the L-Sel−/− mice lacked mature DCs in their LRLNs, the absence of CTL responses manifests as unresponsiveness to Ad2 vectors when in fact the L-Sel−/− mice are responsive to Ad2, but require the presence of CD8+ DCs. This defect in lymphoid DCs was not compensated by mDCs since these remained unchanged in either L-Sel+/+ or L-Sel−/− LRLNs. The CD11chigh (cDC) subset was absent from both L-Sel+/+ and L-Sel−/− LRLNs. Thus, these collective findings suggest that the lack of mature lymphoid CD8+ DCs is the major defect in the L-Sel−/− LRLNs.
It is not surprising that fewer DCs were detected in the L-Sel−/− LRLN since naive LN in these mice tend to be smaller (17, 35), but their ability to induce immunity still remains intact. L-Sel−/− mice have been shown to be responsive to immunization, although dependent upon the type of Ag and its route of administration. No significant differences in their Ab responses to KLH were observed in L-Sel−/− mice when compared to similarly immunized C57BL/6 (L-Sel+/+) mice (16). Serum Ab responses to T cell-independent type 2 Ag were greatly enhanced when administered i.p. rather than s.c. (35). Oral immunization of L-Sel−/− mice showed that systemic Ab responses to passenger fimbriae were retained, albeit, with a more proinflammatory bias than in L-Sel+/+ mice (19). Because of this redirection of Th cell responses, the S-IgA responses were severely attenuated. Similarly, S-IgA responses were compromised in mucosal secretions when L-Sel−/− mice were nasally immunized with cholera toxin (7, 8).
Recent studies suggest that DCs are responsible for priming viral (33, 36) or bacterial CTL responses (37) by CD8+ DCs (38, 39). The rapid migration of respiratory DCs to the pulmonary draining LN after an influenza infection suggests that lung DCs may act a source of viral Ags for the regional LN (33). Thus, an inability to migrate from the site of infection to LN could possibly explain the relevance of functional L-selectin expression by DCs (36). The evidence shows that L-Sel−/− LRLNs have a reduced percentage of CD8+ DCs by 43% following Ad2/βGal-2 delivery when compared to L-Sel+/+ LRLNs; thus, the possibility that lung DC migration to the LRLNs is compromised cannot be excluded. Accumulation of DCs in regional LNs has been observed following upper respiratory infection with influenza virus (33) or footpad infection with mouse mammary tumor virus (40). However, the presence of CD8+ DCs was difficult to identify in the lungs from either L-Sel−/− or L-Sel+/+ mice. Adoptive transfer of L-Sel−/− DCs in L-Sel−/− or L-Sel+/+ mice showed migration to the recipients’ lungs and LRLNs can occur (data not shown), suggesting that the adoptive transfer may bypass the in vivo block in migration. Instead, the defect in L-Sel−/− mice appeared to be contributed by the lack of DC maturation. The L-Sel−/− LRLN DCs showed reduced expression of co-stimulatory molecules, and only upon microbial activation was there an increase in CD8+ DCs. The in vitro labeling studies revealed that the lung CD8− DCs served as the source of mature DCs, and these DCs matured upon LPS stimulation to become CD8+ DCs. The Ad2 vector only had a modest impact upon this conversion and required microbial activation to enhance increases in L-Sel−/− CD8+ DCs. This conversion did not appear to take place in the LRLN since no changes were detected with the LRLN DC cultures. Consequently, being unable to mature upon infection with the Ad2 vector, the L-Sel−/− CD8− DCs are unable to support CTL responses. Functionally, the CD8+ DCs are important since subsequent cell sorting experiments showed that these were necessary for supporting Ag-specific CTL responses, and only the CD8+ DCs supported both L-Sel+/+ and L-Sel−/− CD8+ anti-Ad2 CTL responses. Even the sorted L-Sel+/+ CD8− DCs failed to support L-Sel−/− CD8+ T cell cytolytic activity. Fully functional L-Sel+/+ CD8+ T cells were also not supported by L-Sel−/− CD8− DCs, suggesting that these CTLs were unresponsive, which is consistent with CD8− DCs being tolerogenic (41, 42).
Immature DCs have been shown to be important for tolerance induction, resulting in the induction of regulatory T cell subset; whereas mature DCs support Ag-reactive responses (43). This maturation appears to be IFN-α-dependent, as previously shown, when chimpanzee and human Ad vectors were used to infect DCs directly, albeit, at very high doses (44). However, we were unable to detect IFN-α secretion by any of these DC subsets.
In summary, in the absence of L-selectin, a defect resulted in the failure to stimulate productive CTL responses attributed to a lack of mature DCs in the LRLN. Microbial activation could reverse this effect, enhancing the numbers of CD8+ DCs in the LRLN. The cell isolation studies revealed that CTLs could be induced in L-Sel−/− mice, but these required the presence of lymphoid CD8+ DCs. Addition of lymphoid CD8+ DCs subset restored cytolytic activity, consistent with the notion that L-Sel−/− DCs have Ag presenting capabilities (18). Microbial activation increased lung CD8+ DC conversion from CD8− DCs, or possibly due to increased recruitment in vivo, but this additional stimulus was required to obtain sufficient mature CD8+ DCs.
Acknowledgments
We thank Dr. Johanne M. Kaplan and Dr. Kathleen Heir of Genzyme for providing us with the Ad2/βGal-2 vector stocks used in this study, the University of Iowa Gene Transfer Vector Core for providing the Ad5 CMV Trf-OVA vector, Ms. Larrisa Jackiw for the cell-sorting experiments, and Ms. Nancy Kommers for her assistance in preparing this manuscript.
Footnotes
This work was supported by Public Health Service grant AI-55563 and in part by Montana Agricultural Station and U.S. Department of Agriculture Formula Funds. The VMB flow cytometry facility was in part supported by NIH/National Center for Research Resources, Centers of Biomedical Excellence P20 RR-020185, and in part from a grant from M.J. Murdock Charitable Trust.
Disclosures
The authors have no financial conflict of interest.
Abbreviations used in this paper: NALT, nasal-associated lymphoid tissue; HNLNs, head and neck lymph nodes; PNAd, peripheral node addressin; LN, lymph nodes; PLNs, peripheral lymph nodes; L-Sel−/−, L-selectin-deficient; DC, dendritic cell; LRLNs, lower respiratory lymph nodes; Ad2, adenovirus 2; CM, complete media; βgal, β-galactosidase; DPBS, Dulbeccos PBS; i.t., intratracheal; mDC, myeloid DC; cDC, conventional DC; pDC, plasmacytoid DC.
References
- 1.Hamann A, Jablonski-Westrich D, Jonas P, Thiele HG. Homing receptors reexamined: mouse LECAM-1 (MEL-14 antigen) is involved in lymphocyte migration into gut-associated lymphoid tissue. Eur J Immunol. 1991;21:2925–2929. doi: 10.1002/eji.1830211205. [DOI] [PubMed] [Google Scholar]
- 2.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten GD, Capon J, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 3.Steeber DA, Tang ML, Zhang XQ, Müller W, Wagner N, Tedder TF. Efficient lymphocyte migration across high endothelial venules of mouse Peyer's patches requires overlapping expression of L-selectin and β7 integrin. J Immunol. 1998;161:6638–6647. [PubMed] [Google Scholar]
- 4.Wagner N, Löhler J, Tedder TF, Rajewsky K, Müller W, Steeber DA. L-selectin and β7 integrin synergistically mediate lymphocyte migration to mesenteric lymph nodes. Eur J Immunol. 1998;28:3832–3839. doi: 10.1002/(SICI)1521-4141(199811)28:11<3832::AID-IMMU3832>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Csencsits KL, Jutila MA, Pascual DW. Mucosal addressin expression and binding interactions with native lymphocytes vary among the cranial, oral, and nasal associated lymphoid tissues. Eur J Immunol. 2002;32:3029–3039. doi: 10.1002/1521-4141(200211)32:11<3029::AID-IMMU3029>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Csencsits KL, Jutila MA, Pascual DW. Nasal-associated lymphoid tissue (NALT): phenotypic and functional evidence for the primary role of peripheral node addressin in naive lymphocyte adhesion to HEV in a mucosal site. J Immunol. 1999;163:1382–1389. [PubMed] [Google Scholar]
- 7.Csencsits KL, Walters N, Pascual DW. Cutting Edge: Dichotomy of homing receptor dependence by mucosal effector B cells: αE versus L-selectin. J Immunol. 2001;167:2441–2445. doi: 10.4049/jimmunol.167.5.2441. [DOI] [PubMed] [Google Scholar]
- 8.Csencsits KL, Pascual DW. Absence of L-selectin delays mucosal B cell responses in non-intestinal effector tissues. J Immunol. 2002;169:5649–5659. doi: 10.4049/jimmunol.169.10.5649. [DOI] [PubMed] [Google Scholar]
- 9.Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ. The human peripheral lymph node vascular addressin: an inducible endothelial antigen involved in lymphocyte homing. Am J Path. 1993;143:1688–1698. [PMC free article] [PubMed] [Google Scholar]
- 10.Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallatin WM, Weissman IL, Butcher EC. A cell surface molecule involved in organ-specific homing of lymphocytes. Nature (Lond) 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 12.Streeter PR, Berg EL, Rouse BTN, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature (Lond) 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 13.Streeter PR, Rouse BTN, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 15.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin mediated lymphocyte rolling on MAdCAM-1. Nature (Lond) 1993;366:695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Grewal IS, Geba GP, Flavell RA. Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med. 1996;183:589–598. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steeber DA, Green NE, Sato S, Tedder TF. Lymphocyte migration in L-selectin-deficient mice. Altered subset migration and aging of the immune system. J Immunol. 1996;157:1096–1106. [PubMed] [Google Scholar]
- 18.Catalina MD, Carroll MC, Arizpe H, Takashima A, Estess P, Siegelman MH. The route of antigen entry determines the requirement for L-selectin during immune responses. J Exp Med. 1996;184:2341–2351. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascual DW, White MD, Larson T, Walters N. Impaired mucosal immunity in L-selectin-deficient mice orally immunized with a Salmonella vaccine vector. J Immunol. 2001;167:407–415. doi: 10.4049/jimmunol.167.1.407. [DOI] [PubMed] [Google Scholar]
- 20.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 21.Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Hayem R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J Immunol. 2000;165:4062–4068. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- 22.Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan JM, Armentano D, Sparer TE, Wynn SG, Peterson PA, Wadsworth SC, Couture KK, Pennington PE, St George JA, Gooding LR, Smith AE. Characterization of factors involved in modulating persistence of transgene expression from recombinant adenovirus in the mouse lung. Hum Gene Ther. 1997;8:45–56. doi: 10.1089/hum.1997.8.1-45. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Hillemeyer P, Pascual DW. Segregation of mechanisms for CTL killing between lungs and regional lymph nodes subsequent to intratracheal delivery of an adenovirus 2 vector. Viral Immunol. 2003;16:525–539. doi: 10.1089/088282403771926346. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000;7:1034–1038. doi: 10.1038/sj.gt.3301197. [DOI] [PubMed] [Google Scholar]
- 26.Pascual DW, Walters N, Hillemeyer P. Repeated intratracheal instillations of nonreplicating adenovirus 2 vector attenuate CTL responses and IFN-γ production. J Immunol. 1998;160:4465–4472. [PubMed] [Google Scholar]
- 27.Schafer R, Portnoy DA, Brassell SA, Paterson Y. Induction of cellular immune response to a foreign antigen by a recombinant Listeria monocytogenes. J Immunol. 1992;4:53–59. [PubMed] [Google Scholar]
- 28.Parsons DW, Grubb BR, Johnson LG, Boucher RC. Enhanced in vivo airway gene transfer via transient modification of host barrier properties with a surface-active agent. Hum Gene Ther. 1998;9:2661–2672. doi: 10.1089/hum.1998.9.18-2661. [DOI] [PubMed] [Google Scholar]
- 29.Blasius A, Vermi W, Krug A, Facchetti F, Cella M, Colonna M. A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion of interferon-alpha. Blood. 2004;103:4201–4206. doi: 10.1182/blood-2003-09-3108. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, Cerundolo V, Crocker PR. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 31.Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, Agapov E, Holtzman MJ. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–1448. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- 32.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 αEβ7 integrin+ epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 33.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 34.de Haan BJMM, Lehar SM, Bevan MJ. CD8+ not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1695. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steeber DA, Green NE, Sato S, Tedder TF. Humoral immune responses in L-selectin-deficient mice. J Immunol. 1996;157:4899–4907. [PubMed] [Google Scholar]
- 36.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 37.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith CM, Belz GT, Wilson NS, Villadangos JA, Shortman K, Carbone FR, Heath WR. Cutting Edge: Conventional CD8α+ dendritic cells are preferentially involved in CTL priming after footpad infections with herpes simplex virus-1. J Immunol. 2003;170:4437–4440. doi: 10.4049/jimmunol.170.9.4437. [DOI] [PubMed] [Google Scholar]
- 39.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting Edge: Conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 40.Martin P, Ruiz SR, del Hoyo GM, Anjuère F, Vargas HH, López-Bravo M, Ardavin C. Dramatic increase in lymph node dendritic cell number during infection by the mouse mammary tumor virus occurs by a CD62L-dependent blood-borne DC recruitment. Blood. 2002;99:1282–1288. doi: 10.1182/blood.v99.4.1282. [DOI] [PubMed] [Google Scholar]
- 41.Legge KL, Gregg RK, Maldonado-Lopez R, Li L, Caprio JC, Moser M, Zaghouani H. On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity. J Exp Med. 2002;196:217–227. doi: 10.1084/jem.20011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiele AT, Sumpter TL, Walker IA, Xu Q, Chang CH, Bacallae RL, Kher R, Wilkes DS. Pulmonary immunity to viral infection: adenovirus infection of lung dendritic cells renders T cells nonresponsive to interleukin-2. J Virol. 2006;80:1826–1836. doi: 10.1128/JVI.80.4.1826-1836.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charbonnier LM, van Duivenvoorde L, Apparailly F, Cantos C, Han WG, Noël D, Duperray C, Huizinga TW, Toes RE, Jorgensen C, Louis-Plence P. Immature dendritic cells suppress collagen-induced arthritis by in vivo expansion of CD49b+ regulatory T cells. J Immunol. 2006;177:3806–3813. doi: 10.4049/jimmunol.177.6.3806. [DOI] [PubMed] [Google Scholar]
- 44.Hensley SE, Giles-Davis W, McCoy KC, Weninger W, Ertl HC. Dentritic cell maturation but no CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J Immunol. 2005;175:6032–6041. doi: 10.4049/jimmunol.175.9.6032. [DOI] [PubMed] [Google Scholar]