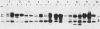Abstract
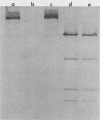
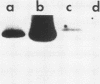
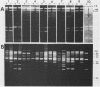
The ability to bind Congo red (Crb+) is associated with virulence of Shigella flexneri and is encoded by a large, 220-kilobase plasmid. We cloned fragments of this plasmid to isolate the sequences encoding Congo red binding, to determine the degree of conservation of these sequences among S. flexneri strains, and to study the molecular basis for loss of the Crb+ phenotype. At least two separate BamHI fragments cloned into plasmid vectors encode Congo red binding in E. coli or S. flexneri. One Crb+ clone, pTKS2, contains a copy of IS1 adjacent to the crb sequences. IS1 appears to be responsible for deletions leading to loss of Congo red binding in this clone. In addition, this clone was found to integrate into the chromosome at relatively high frequency. Integration resulted in loss of the Crb+ phenotype. A second clone, pTKS15, which has only limited homology to pTKS2, also encodes Congo red binding. The Crb+ phenotype of transformants carrying pTKS15 was detected at 37 degrees C but not at 30 degrees C, and thus it resembles Congo red binding in wild-type S. flexneri. HindIII digests of plasmid DNA from 10 different S. flexneri strains were hybridized to both of these Crb+ clones and to an IS1 probe. More than one fragment hybridized to pTKS2 or pTKS15. In general, the sizes of these fragments were the same in S. flexneri strains of different serotypes, indicating conservation of these sequences. Three of five copies of IS1 were also found on the large S. flexneri plasmids. Two of the copies were on fragments of the same size in each strain. Analysis of Crb- derivatives of the 10 strains indicated that, although IS1 may be closely linked to crb sequences on the 220-kilobase plasmid, it is not responsible for the majority of deletions of this plasmid associated with loss of Congo red binding.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskaleros P. A., Payne S. M. Cloning the gene for Congo red binding in Shigella flexneri. Infect Immun. 1985 Apr;48(1):165–168. doi: 10.1128/iai.48.1.165-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B., Willetts N. S. Plasmid co-integrates of prophage lambda and R factor R100. J Bacteriol. 1976 Apr;126(1):166–176. doi: 10.1128/jb.126.1.166-176.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. A Chromosomal Locus Which Controls the Ability of Shigella flexneri to Evoke Keratoconjunctivitis. Infect Immun. 1971 Jan;3(1):73–79. doi: 10.1128/iai.3.1.73-79.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. Genetic Transfer of Shigella flexneri Antigens to Escherichia coli K-12. Infect Immun. 1970 Mar;1(3):279–287. doi: 10.1128/iai.1.3.279-287.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E., Stevenson P., Hale T. L., Formal S. B. Synthesis of aerobactin and a 76,000-dalton iron-regulated outer membrane protein by Escherichia coli K-12-Shigella flexneri hybrids and by enteroinvasive strains of Escherichia coli. Infect Immun. 1985 Jul;49(1):67–71. doi: 10.1128/iai.49.1.67-71.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Oaks E. V., Formal S. B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985 Dec;50(3):620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S., Meyer J., Arber W. Genesis and natural history of IS-mediated transposons. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):27–43. doi: 10.1101/sqb.1981.045.01.006. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor K. M., Payne S. M. Aerobactin genes in Shigella spp. J Bacteriol. 1984 Oct;160(1):266–272. doi: 10.1128/jb.160.1.266-272.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Blackmon B., Curtiss R., 3rd Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect Immun. 1984 Jan;43(1):397–401. doi: 10.1128/iai.43.1.397-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Blackmon B., Curtiss R., 3rd Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984 Jan;43(1):195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977 Oct;18(1):94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif H. J., Saedler H. IS1 is involved in deletion formation in the gal region of E. coli K12. Mol Gen Genet. 1975;137(1):17–28. doi: 10.1007/BF00332538. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., d'Hauteville H., Ecobichon C., Pourcel C. Molecular comparison of virulence plasmids in Shigella and enteroinvasive Escherichia coli. Ann Microbiol (Paris) 1983 May-Jun;134A(3):295–318. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]