Abstract
Three groups of cattle, each group comprising six animals, were inoculated intravenously with populations of bloodstream forms of Trypanosoma vivax. The first group received T. vivax clone ILDat 1.3 derived from an isolate from Nigeria, while the other two received T. vivax stocks IL 1875 or IL 2133 isolated from Coast Province, Kenya. One animal from the group that was infected with IL 1875 died 8 weeks postinfection. The remaining 17 animals became aparasitemic in 8 to 12 weeks without intervention by drug therapy. The recovered animals developed serodeme-specific immunity against Glossina morsitans subsp. centralis-transmitted challenge. There was complete cross-protection between the two East African T. vivax stocks, although they were isolated from areas 80 to 90 km apart, indicating that they belong to the same serodeme. Antibodies to the homologous metacyclic variable antigen types (VATs) were not detected in sera from recovered animals, suggesting that the immunity displayed by the recovered animals was directed at the bloodstream and not the metacyclic VATs. It is thus suggested that recovery in these animals is due to exhaustion of the repertoire of bloodstream VATs expressed in the animals by the infecting T. vivax clone or stocks.
Full text
PDF
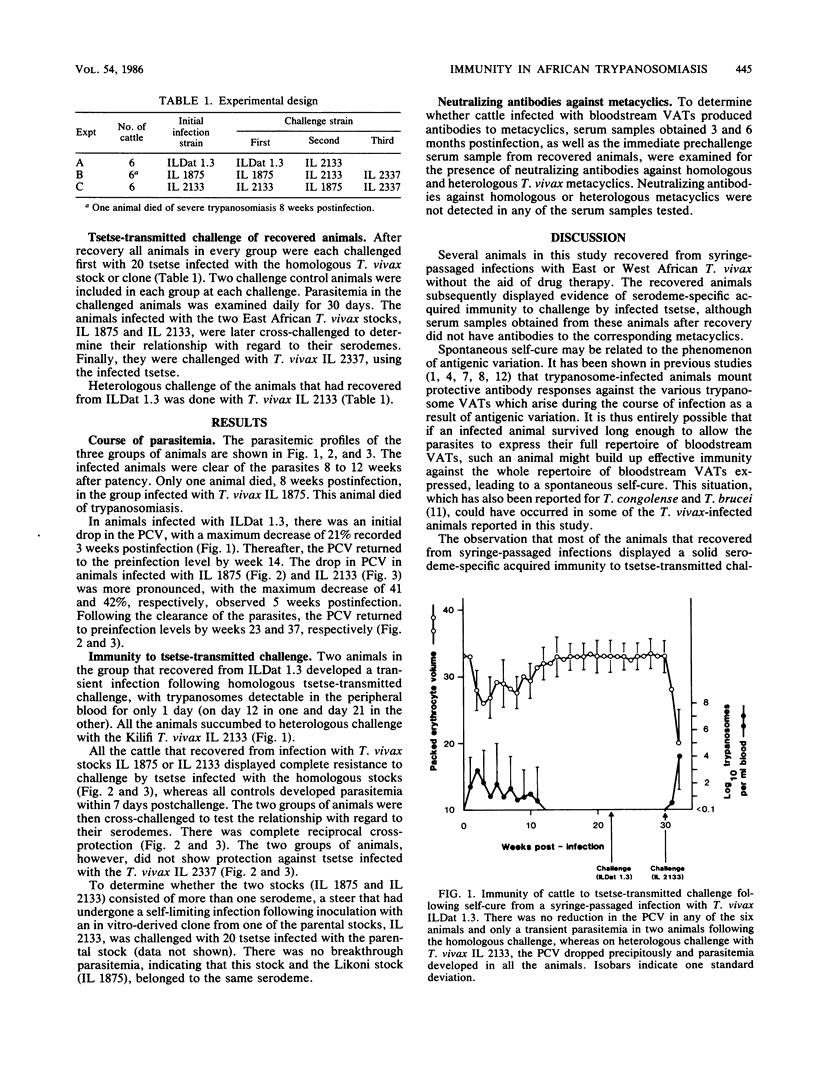
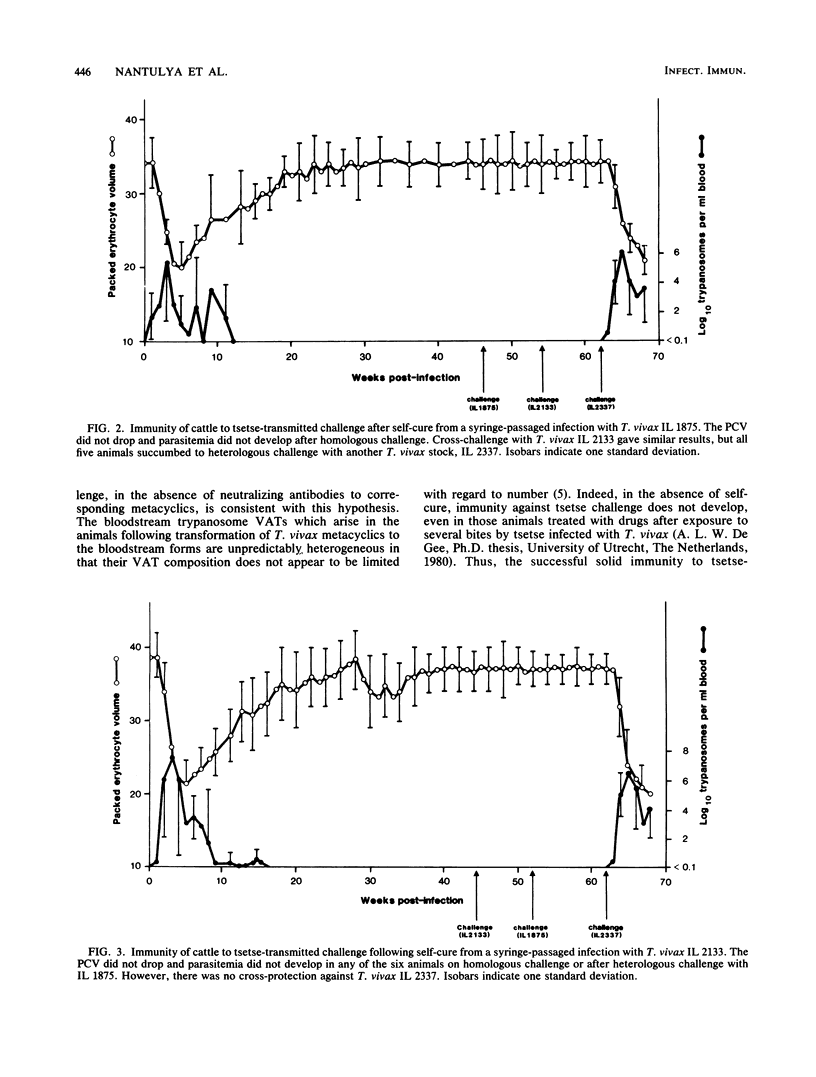

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry J. D. Antigenic variation during Trypanosoma vivax infections of different host species. Parasitology. 1986 Feb;92(Pt 1):51–65. doi: 10.1017/s0031182000063447. [DOI] [PubMed] [Google Scholar]
- Barry J. D., Gathuo H. Antigenic variation in Trypanosoma vivax: isolation of a serodeme. Parasitology. 1984 Aug;89(Pt 1):49–58. doi: 10.1017/s0031182000001128. [DOI] [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. Trypanosoma equiperdum: etude des variations antigéniques au cours de la trypanosomose experimentale du lapin. Exp Parasitol. 1977 Jun;42(1):6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Doyle J. J. Antigenic variation in the salivarian trypanosomes. Adv Exp Med Biol. 1977;93:31–63. doi: 10.1007/978-1-4615-8855-9_4. [DOI] [PubMed] [Google Scholar]
- Gardiner P. R., Thatthi R., Gathuo H., Nelson R., Moloo S. K. Further studies of cyclical transmission and antigenic variation of the ILDar 1 serodeme of Trypanosoma vivax. Parasitology. 1986 Jun;92(Pt 3):581–593. doi: 10.1017/s003118200006546x. [DOI] [PubMed] [Google Scholar]
- Leeflang P., Buys J., Blotkamp C. Studies on Trypanosoma vivax: infectivity and serial maintenance of natural bovine isolates in mice. Int J Parasitol. 1976 Oct;6(5):413–417. doi: 10.1016/0020-7519(76)90027-8. [DOI] [PubMed] [Google Scholar]
- Masake R. A., Musoke A. J., Nantulya V. M. Specific antibody responses to the variable surface glycoproteins of Trypanosoma congolense in infected cattle. Parasite Immunol. 1983 Jul;5(4):345–355. doi: 10.1111/j.1365-3024.1983.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Murray M., Murray P. K., McIntyre W. I. An improved parasitological technique for the diagnosis of African trypanosomiasis. Trans R Soc Trop Med Hyg. 1977;71(4):325–326. doi: 10.1016/0035-9203(77)90110-9. [DOI] [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Barbet A. F., Kironde F., McGuire T. C. Bovine immune response to African ;trypanosomes: specific antibodies to variable surface glycoproteins of Trypanosoma brucei. Parasite Immunol. 1981 Summer;3(2):97–106. doi: 10.1111/j.1365-3024.1981.tb00388.x. [DOI] [PubMed] [Google Scholar]
- Nantulya V. M., Musoke A. J., Moloo S. K., Ngaira J. M. Analysis of the variable antigen composition of Trypanosoma brucei brucei metacyclic trypanosomes using monoclonal antibodies. Acta Trop. 1983 Mar;40(1):19–24. [PubMed] [Google Scholar]
- Nantulya V. M., Musoke A. J., Rurangirwa F. R., Moloo S. K. Resistance of cattle to tsetse-transmitted challenge with Trypanosoma brucei or Trypanosoma congolense after spontaneous recovery from syringe-passaged infections. Infect Immun. 1984 Feb;43(2):735–738. doi: 10.1128/iai.43.2.735-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngaira J. M., Nantulya V. M., Musoke A. J., Hirumi K. Phagocytosis of antibody-sensitized Trypanosoma brucei in vitro by bovine peripheral blood monocytes. Immunology. 1983 Jun;49(2):393–400. [PMC free article] [PubMed] [Google Scholar]
- Wellde B. T., Chumo D. A., Adoyo M., Kovatch R. M., Mwongela G. N., Opiyo E. A. Haemorrhagic syndrome in cattle associated with Trypanosoma vivax infection. Trop Anim Health Prod. 1983 May;15(2):95–102. doi: 10.1007/BF02239803. [DOI] [PubMed] [Google Scholar]


