Abstract
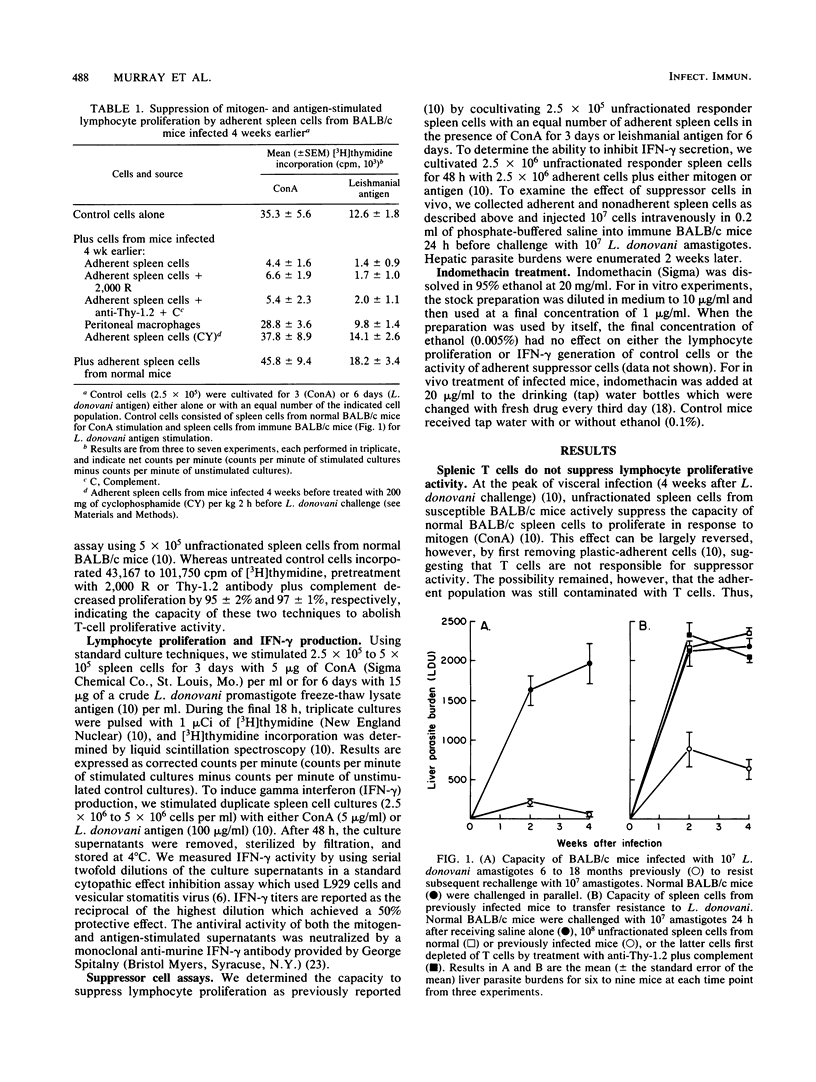
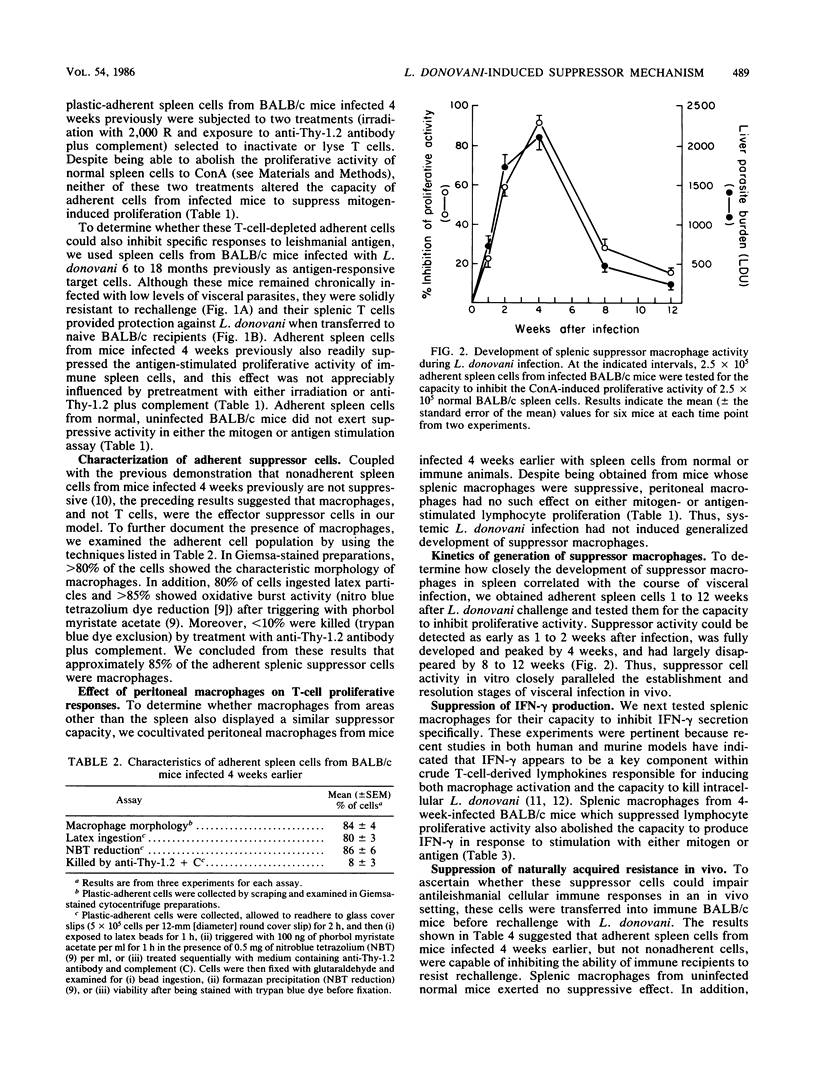
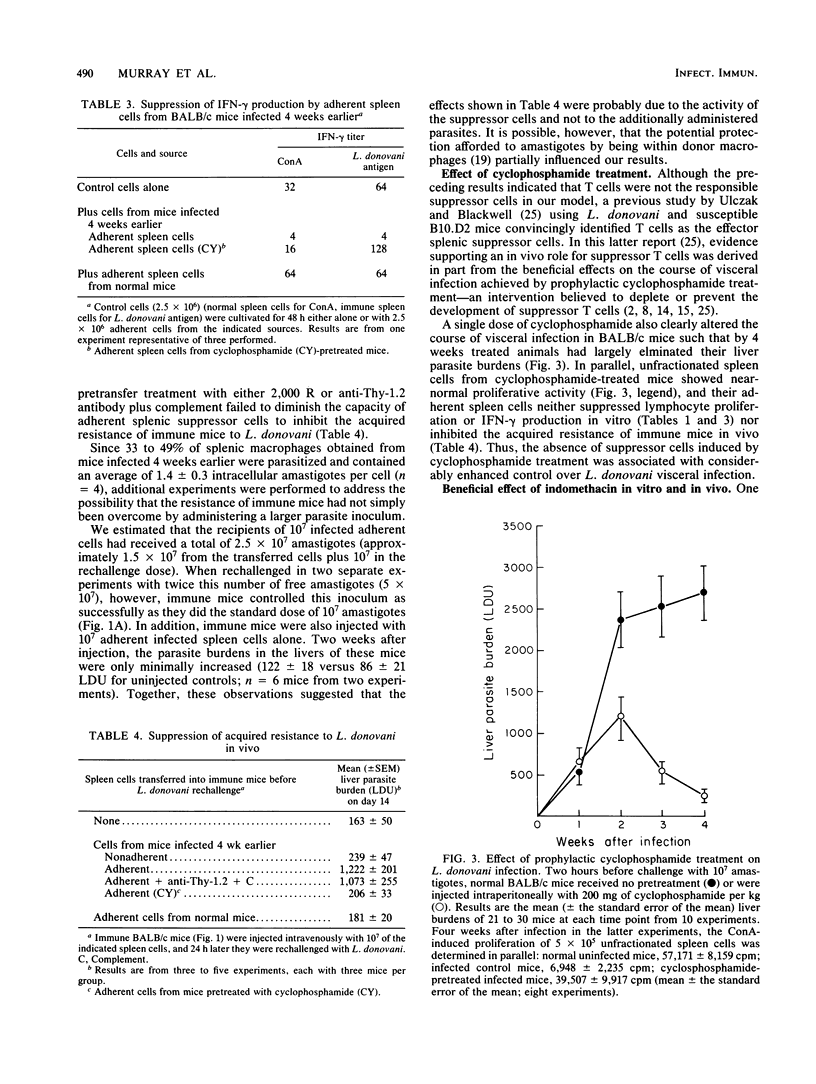
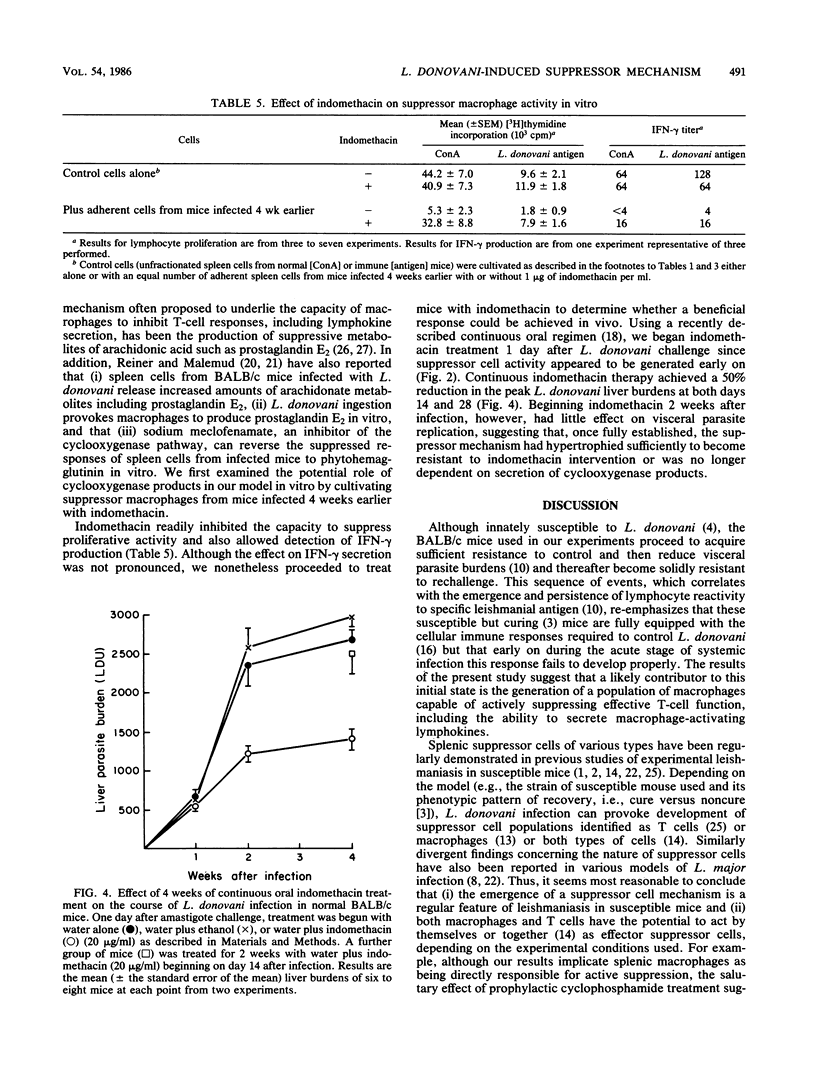
In susceptible BALB/c mice, systemic intracellular infection with Leishmania donovani provokes generation of adherent spleen cells which can suppress both mitogen- and specific-antigen-stimulated T-cell responses. To characterize the responsible suppressor cell, we irradiated (2,000 R) adherent spleen cells from L. donovani-infected mice or treated them with anti-Thy-1.2 antibody plus complement. Neither anti-T-cell treatment diminished the capacity to inhibit lymphocyte proliferative activity. In addition, as judged by morphologic and functional criteria, 80 to 90% of the adherent cells appeared to be macrophages. Four observations suggested an immunopathogenic role for these suppressor macrophages. (i) Their appearance and disappearance paralleled the establishment and resolution of L. donovani visceral infection in vivo. (ii) Suppressive effects included inhibition of production of the macrophage-activating lymphokine gamma interferon (IFN-gamma). (iii) On transfer into immune mice, suppressor macrophages impaired naturally acquired resistance to L. donovani. (iv) Inhibition of macrophage prostaglandin metabolism by indomethacin reduced suppressor activity in vitro and resulted in a 50% decrease in parasite visceral replication in vivo. In addition, prophylactic cyclophosphamide treatment inhibited the development of suppressor macrophages, and under these conditions visceral infection was rapidly controlled. These results suggested that disseminated L. donovani infection provokes a macrophage-mediated suppressor mechanism which appears to contribute to establishment of visceral leishmaniasis in a susceptible host.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arredondo B., Pérez H. Alterations of the immune response associated with chronic experimental leishmaniasis. Infect Immun. 1979 Jul;25(1):16–22. doi: 10.1128/iai.25.1.16-22.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenase P. W., Hayden B. J., Gershon R. K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975 Mar 1;141(3):697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J., Freeman J., Bradley D. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature. 1980 Jan 3;283(5742):72–74. doi: 10.1038/283072a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol. 1977 Oct;30(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Teixeira R. S., Johnson W. D., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981 Aug;33(2):498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L., Patel P. J. Enhanced production of murine interferon gamma by T cells generated in response to bacterial infection. J Exp Med. 1982 Jul 1;156(1):112–127. doi: 10.1084/jem.156.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Koech D. K., Iha D. W., Bryceson A. D. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983 Feb;51(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J Exp Med. 1981 Mar 1;153(3):557–568. doi: 10.1084/jem.153.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Interaction of Leishmania with a macrophage cell line. Correlation between intracellular killing and the generation of oxygen intermediates. J Exp Med. 1981 Jun 1;153(6):1690–1695. doi: 10.1084/jem.153.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Visceral leishmaniasis in congenic mice of susceptible and resistant phenotypes: T-lymphocyte-mediated immunosuppression. Infect Immun. 1985 Oct;50(1):169–174. doi: 10.1128/iai.50.1.169-174.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Visceral leishmaniasis in congenic mice of susceptible and resistant phenotypes: immunosuppression by adherent spleen cells. Infect Immun. 1985 Oct;50(1):160–168. doi: 10.1128/iai.50.1.160-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982 Apr 1;155(4):1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Wheeler D. A., Harrison L. H., Kay H. D. The immunobiology of leishmaniasis. Rev Infect Dis. 1983 Sep-Oct;5(5):907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- Petersen E. A., Neva F. A., Oster C. N., Bogaert Diaz H. Specific inhibition of lymphocyte-proliferation responses by adherent suppressor cells in diffuse cutaneous leishmaniasis. N Engl J Med. 1982 Feb 18;306(7):387–392. doi: 10.1056/NEJM198202183060702. [DOI] [PubMed] [Google Scholar]
- Potter M., Wax J. S., Anderson A. O., Nordan R. P. Inhibition of plasmacytoma development in BALB/c mice by indomethacin. J Exp Med. 1985 May 1;161(5):996–1012. doi: 10.1084/jem.161.5.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W. Mechanisms of immunity to leishmaniasis. II. Significance of the intramacrophage localization of the parasite. Clin Exp Immunol. 1980 Apr;40(1):25–35. [PMC free article] [PubMed] [Google Scholar]
- Reiner N. E., Malemud C. J. Arachidonic acid metabolism by murine peritoneal macrophages infected with Leishmania donovani: in vitro evidence for parasite-induced alterations in cyclooxygenase and lipoxygenase pathways. J Immunol. 1985 Jan;134(1):556–563. [PubMed] [Google Scholar]
- Reiner N. E., Malemud C. J. Arachidonic acid metabolism in murine leishmaniasis (Donovani): ex-vivo evidence for increased cyclooxygenase and 5-lipoxygenase activity in spleen cells. Cell Immunol. 1984 Oct 15;88(2):501–510. doi: 10.1016/0008-8749(84)90181-3. [DOI] [PubMed] [Google Scholar]
- Scott P. A., Farrell J. P. Experimental cutaneous leishmaniasis. I. Nonspecific immunodepression in BALB/c mice infected with Leishmania tropica. J Immunol. 1981 Dec;127(6):2395–2400. [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber L. A. The origin and significance of the distribution of parasites in visceral leishmaniasis. Trans N Y Acad Sci. 1966 Mar;28(5):635–643. doi: 10.1111/j.2164-0947.1966.tb02382.x. [DOI] [PubMed] [Google Scholar]
- Ulczak O. M., Blackwell J. M. Immunoregulation of genetically controlled acquired responses to Leishmania donovani infection in mice: the effects of parasite dose, cyclophosphamide and sublethal irradiation. Parasite Immunol. 1983 Sep;5(5):449–463. doi: 10.1111/j.1365-3024.1983.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L., Arenzana-Seisdedos F., Rothhut B., Huerta J. M., Russo-Marie F., Fiers W. Defective IFN-gamma production in the human neonate. II. Role of increased sensitivity to the suppressive effects of prostaglandin E. J Immunol. 1985 Jan;134(1):172–176. [PubMed] [Google Scholar]
- Walker C., Kristensen F., Bettens F., deWeck A. L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983 Apr;130(4):1770–1773. [PubMed] [Google Scholar]



