Abstract
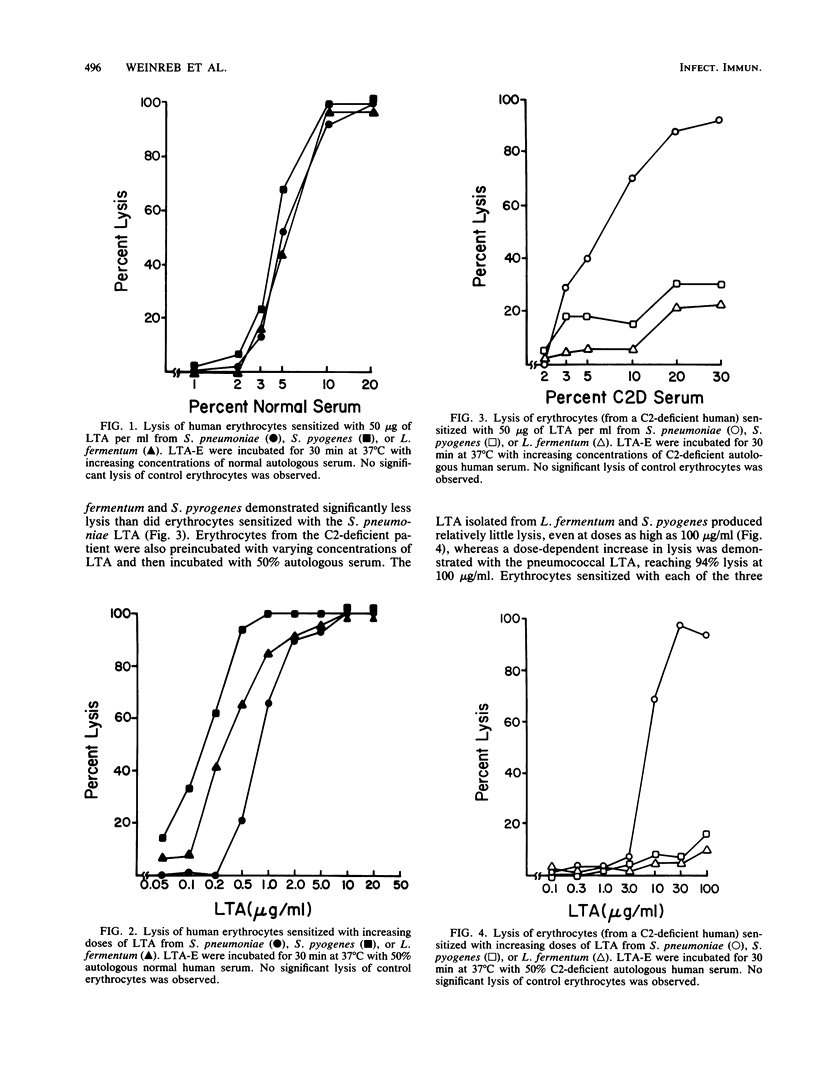
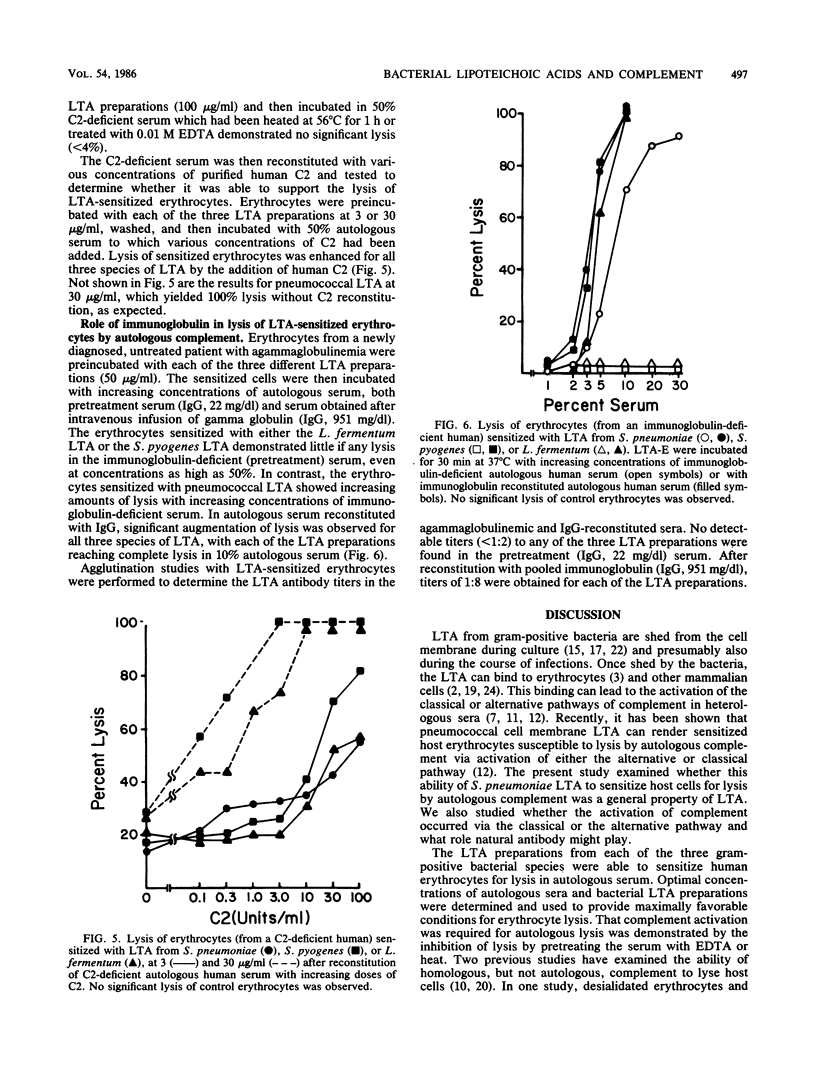
Previous studies have demonstrated that lipoteichoic acid (LTA) from Streptococcus pneumoniae binds to erythrocytes and renders them susceptible to lysis by autologous complement. The present study was performed to determine whether LTA from two other gram-positive bacterial species had the ability to render mammalian cells susceptible to lysis by autologous complement. Human erythrocytes were sensitized with LTA from S. pneumoniae, Streptococcus pyogenes, or Lactobacillus fermentum. Under incubation in normal autologous serum, lysis was observed with each of the LTA-sensitized erythrocyte preparations. When erythrocytes from a C2-deficient patient were sensitized with the LTA preparations and then incubated in autologous, C2-deficient serum, the erythrocytes sensitized with S. pyogenes or L. fermentum LTA demonstrated relatively little lysis, whereas the erythrocytes sensitized with S. pneumoniae LTA yielded near-total lysis. After reconstitution of the C2-deficient serum with purified human C2, lysis was observed with all three LTA preparations. When erythrocytes from an agammaglobulinemic patient were sensitized with either the S. pyogenes or the L. fermentum LTA, they were not lysed in the presence of autologous agammaglobulinemic serum, whereas the erythrocytes sensitized with S. pneumoniae LTA were completely lysed. Serum obtained from the agammaglobulinemic patient after reconstitution with intravenous pooled gamma globulin was able to lyse autologous erythrocytes sensitized with each of the three LTA preparations. These results demonstrate that the ability to render host cells susceptible to lysis by autologous complement is a general property of LTA. Whether activation of the autologous complement occurs by the classical or alternative pathways and whether it is antibody dependent depends on the nature of the bacterial LTA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M. L., Beachey E. H. Excretion of lipoteichoic acid by group A streptococci. Influence of penicillin on excretion and loss of ability to adhere to human oral mucosal cells. J Clin Invest. 1978 Mar;61(3):671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Dale J. B., Simpson W. A., Evans J. D., Knox K. W., Ofek I., Wicken A. J. Erythrocyte binding properties of streptococcal lipoteichoic acids. Infect Immun. 1979 Mar;23(3):618–625. doi: 10.1128/iai.23.3.618-625.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Brissette J. L., Shockman G. D., Pieringer R. A. Effects of penicillin on synthesis and excretion of lipid and lipoteichoic acid from Streptococcus mutans BHT. J Bacteriol. 1982 Aug;151(2):838–844. doi: 10.1128/jb.151.2.838-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Activation of the alternative complement pathway by a streptococcal lipoteichoic acid. Infect Immun. 1978 Oct;22(1):286–287. doi: 10.1128/iai.22.1.286-287.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS T. N., HARRIS S. Agglutination by human sera of erythrocytes incubated with streptococcal culture concentrates. J Bacteriol. 1953 Aug;66(2):159–165. doi: 10.1128/jb.66.2.159-165.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle J. J., Hoffmann E. M. Evidence for restriction of the ability of complement to lyse homologous erythrocytes. J Immunol. 1984 Sep;133(3):1444–1452. [PubMed] [Google Scholar]
- Hummell D. S., Swift A. J., Tomasz A., Winkelstein J. A. Activation of the alternative complement pathway by pneumococcal lipoteichoic acid. Infect Immun. 1985 Feb;47(2):384–387. doi: 10.1128/iai.47.2.384-387.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummell D. S., Winkelstein J. A. Bacterial lipoteichoic acid sensitizes host cells for destruction by autologous complement. J Clin Invest. 1986 May;77(5):1533–1538. doi: 10.1172/JCI112468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt A. C., Altenburger K. M., Johnston R. B., Jr, Winkelstein J. A. Increased susceptibility to severe pyogenic infections in patients with an inherited deficiency of the second component of complement. J Pediatr. 1981 Mar;98(3):417–419. doi: 10.1016/s0022-3476(81)80708-1. [DOI] [PubMed] [Google Scholar]
- Jackson D. E., Wong W., Largen M. T., Shockman G. D. Monoclonal antibodies to immunodeterminants of lipoteichoic acids. Infect Immun. 1984 Mar;43(3):800–803. doi: 10.1128/iai.43.3.800-803.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Tanaka H., Okada N. Prevention of complement activation on the homologous cell membrane of nucleated cells as well as erythrocytes. Eur J Immunol. 1983 Apr;13(4):340–344. doi: 10.1002/eji.1830130413. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E., ZUCKERMAN A. Hemolysis and hemagglutination by normal and immune serums of erythrocytes treated with a nonspecies specific bacterial substance. J Infect Dis. 1956 Mar-Apr;98(2):211–222. doi: 10.1093/infdis/98.2.211. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Ofek I., Beachey E. H. Binding of streptococcal lipoteichoic acid to the fatty acid binding sites on serum albumin. J Biol Chem. 1980 Jul 10;255(13):6092–6097. [PubMed] [Google Scholar]
- Simpson W. A., Ofek I., Sarasohn C., Morrison J. C., Beachey E. H. Characteristics of the binding of streptococcal lipoteichoic acid to human oral epithelial cells. J Infect Dis. 1980 Apr;141(4):457–462. doi: 10.1093/infdis/141.4.457. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Bacterial cell surface amphiphiles. Biochim Biophys Acta. 1980 May 27;604(1):1–26. doi: 10.1016/0005-2736(80)90583-0. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]


