Abstract
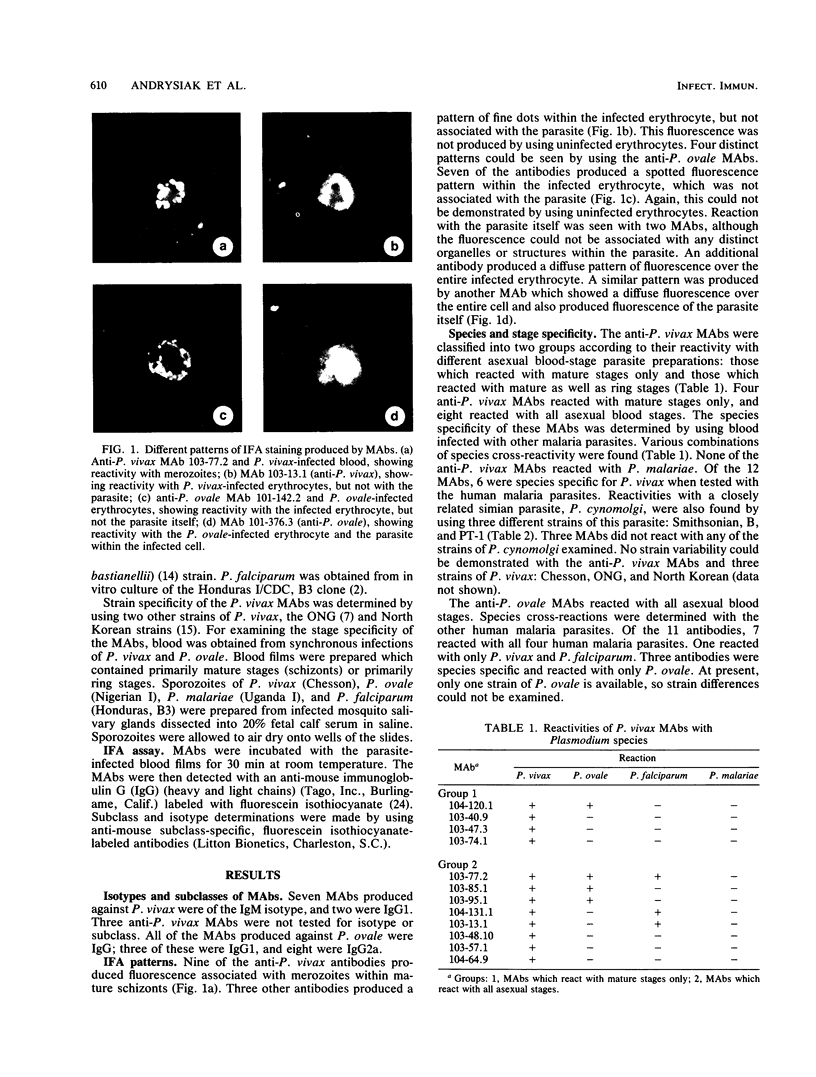
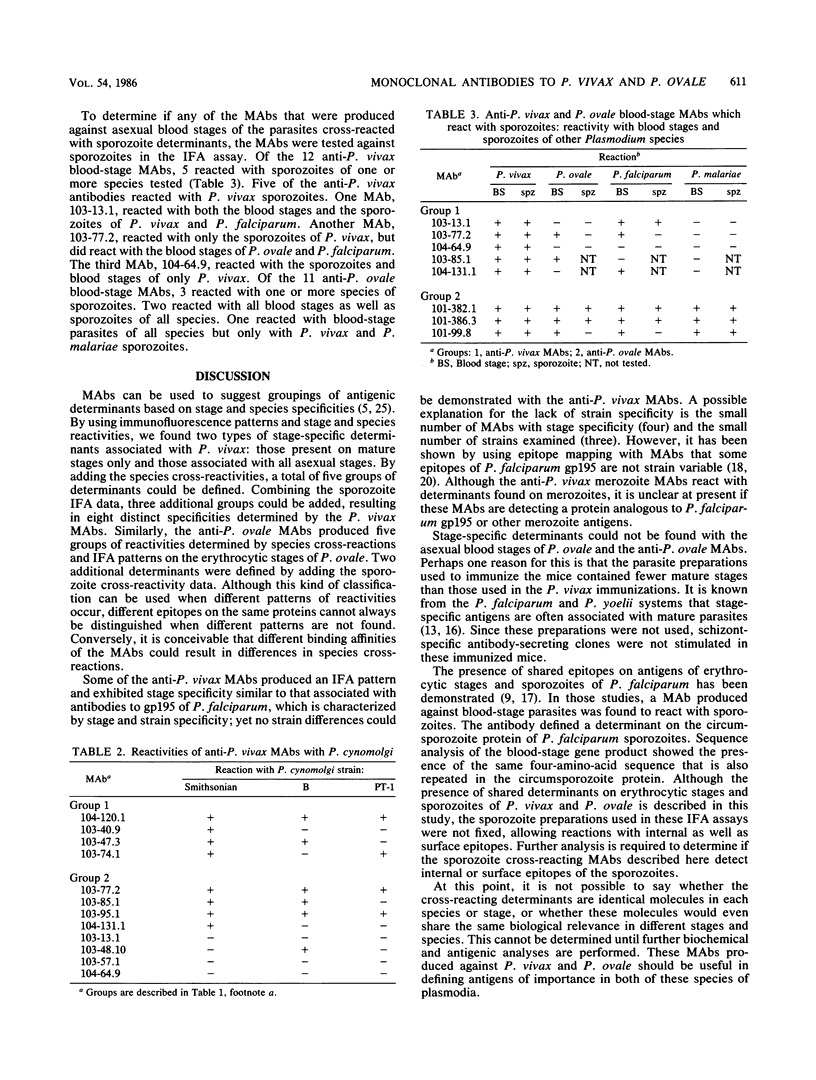
Monoclonal antibodies (MAbs) were produced against the asexual blood stages of Plasmodium vivax and Plasmodium ovale and used to define antigens of plasmodial parasites in an indirect fluorescent antibody assay. The anti-P. vivax MAbs produced two distinct patterns in the indirect fluorescent antibody assay. Four patterns were found with the anti-P. ovale MAbs. Species-specific epitopes were defined for P. vivax and P. ovale; epitopes shared among all four species of human malaria parasites were also defined. Some of the anti-P. vivax MAbs reacted only with mature stages, and others reacted with all asexual stages. No asexual blood-stage specificity could be found with the anti-P. ovale antibodies. Five of the anti-P. vivax MAbs and three of the anti-P. ovale MAbs also reacted with sporozoites.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrysiak P. M., Collins W. E., Campbell G. H. Concentration of Plasmodium ovale- and Plasmodium vivax-infected erythrocytes from nonhuman primate blood using Percoll gradients. Am J Trop Med Hyg. 1986 Mar;35(2):251–254. doi: 10.4269/ajtmh.1986.35.251. [DOI] [PubMed] [Google Scholar]
- Bhasin V. K., Trager W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am J Trop Med Hyg. 1984 Jul;33(4):534–537. doi: 10.4269/ajtmh.1984.33.534. [DOI] [PubMed] [Google Scholar]
- Chin W., Contacos P. G. A recently isolated West African strain of plasmodium ovale. Am J Trop Med Hyg. 1966 Jan;15(1):1–2. doi: 10.4269/ajtmh.1966.15.1. [DOI] [PubMed] [Google Scholar]
- Cochrane A. H., Gwadz R. W., Barnwell J. W., Kamboj K. K., Nussenzweig R. S. Further studies on the antigenic diversity of the circumsporozoite proteins of the Plasmodium cynomolgi complex. Am J Trop Med Hyg. 1986 May;35(3):479–487. doi: 10.4269/ajtmh.1986.35.479. [DOI] [PubMed] [Google Scholar]
- Collins W. E., Schwartz I. K., Skinner J. C., Broderson J. R. Studies on the Uganda I/CDC strain of Plasmodium malariae in bolivian Aotus monkeys and various anophelines. J Parasitol. 1984 Oct;70(5):677–681. [PubMed] [Google Scholar]
- Collins W. E., Warren M., Skinner J. C., Huong A. Y., Nguyen-Dinh P. Observations on the infectivity of two strains of Plasmodium vivax from Vietnamese refugees to Aotus monkeys and anopheline mosquitoes. J Parasitol. 1983 Aug;69(4):689–695. [PubMed] [Google Scholar]
- Collins W. E., Warren M., Skinner J. C., Richardson B. B., Kearse T. S. Effect of sequential infection with Plasmodium vivax and P. falciparum in the Aotus trivirgatus monkey. J Parasitol. 1979 Aug;65(4):605–608. [PubMed] [Google Scholar]
- Coppel R. L., Favaloro J. M., Crewther P. E., Burkot T. R., Bianco A. E., Stahl H. D., Kemp D. J., Anders R. F., Brown G. V. A blood stage antigen of Plasmodium falciparum shares determinants with the sporozoite coat protein. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5121–5125. doi: 10.1073/pnas.82.15.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman F. C., Ellis J. M., Young M. D. PLASMODIUM VIVAX CHESSON STRAIN. Science. 1945 Apr 13;101(2624):377–377. doi: 10.1126/science.101.2624.377. [DOI] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Trejdosiewicz A. J., Cross G. A. Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature. 1980 Mar 27;284(5754):366–368. doi: 10.1038/284366a0. [DOI] [PubMed] [Google Scholar]
- Garnham P. C., Bray R. S., Bruce-Chwatt L. J., Draper C. C., Killick-Kendrick R., Sergiev P. G., Tiburskaja N. A., Shute P. G., Maryon M. A strain of Plasmodium vivax characterized by prolonged incubation: morphological and biological characteristics. Bull World Health Organ. 1975;52(1):21–32. [PMC free article] [PubMed] [Google Scholar]
- Hall R., McBride J., Morgan G., Tait A., Zolg J. W., Walliker D., Scaife J. Antigens of the erythrocytes stages of the human malaria parasite Plasmodium falciparum detected by monoclonal antibodies. Mol Biochem Parasitol. 1983 Mar;7(3):247–265. doi: 10.1016/0166-6851(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Hall R., Simmons D. L., Hyde J. E., Scaife J. G. Evidence for immunological cross-reaction between sporozoites and blood stages of a human malaria parasite. Nature. 1984 Mar 8;308(5955):191–194. doi: 10.1038/308191a0. [DOI] [PubMed] [Google Scholar]
- Howard R. F., Stanley H. A., Campbell G. H., Langreth S. G., Reese R. T. Two Plasmodium falciparum merozoite surface polypeptides share epitopes with a single Mr 185 000 parasite glycoprotein. Mol Biochem Parasitol. 1985 Oct;17(1):61–77. doi: 10.1016/0166-6851(85)90128-8. [DOI] [PubMed] [Google Scholar]
- Jeffery G. M. Epidemiological significance of repeated infections with homologous and heterologous strains and species of Plasmodium. Bull World Health Organ. 1966;35(6):873–882. [PMC free article] [PubMed] [Google Scholar]
- Lyon J. A., Geller R. H., Haynes J. D., Chulay J. D., Weber J. L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2989–2993. doi: 10.1073/pnas.83.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig V., Nussenzweig R. S., Collins W. E., Harinasuta K. T., Tapchaisri P., Chomcharn Y. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982 Jul 1;156(1):20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Ramirez E., Er-Hsiang L., Lambert P. H. Plasmodium falciparum: characterization of defined antigens by monoclonal antibodies. Clin Exp Immunol. 1980 Jul;41(1):91–96. [PMC free article] [PubMed] [Google Scholar]
- Sulzer A. J., Wilson M., Hall E. C. Indirect fluorescent-antibody tests for parasitic diseases. V. An evaluation of a thick-smear antigen in the IFA test for malaria antibodies. Am J Trop Med Hyg. 1969 Mar;18(2):199–205. [PubMed] [Google Scholar]
- Sulzer A. J., Wilson M. The indirect fluorescent antibody test for the detection of occult malaria in blood donors. Bull World Health Organ. 1971;45(3):375–379. [PMC free article] [PubMed] [Google Scholar]
- Taylor D. W., Kim K. J., Munoz P. A., Evans C. B., Asofsky R. Monoclonal antibodies to stage-specific, species-specific, and cross-reactive antigens of the rodent malarial parasite, Plasmodium yoelii. Infect Immun. 1981 May;32(2):563–570. doi: 10.1128/iai.32.2.563-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



