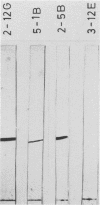
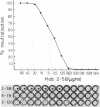
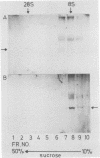
Abstract
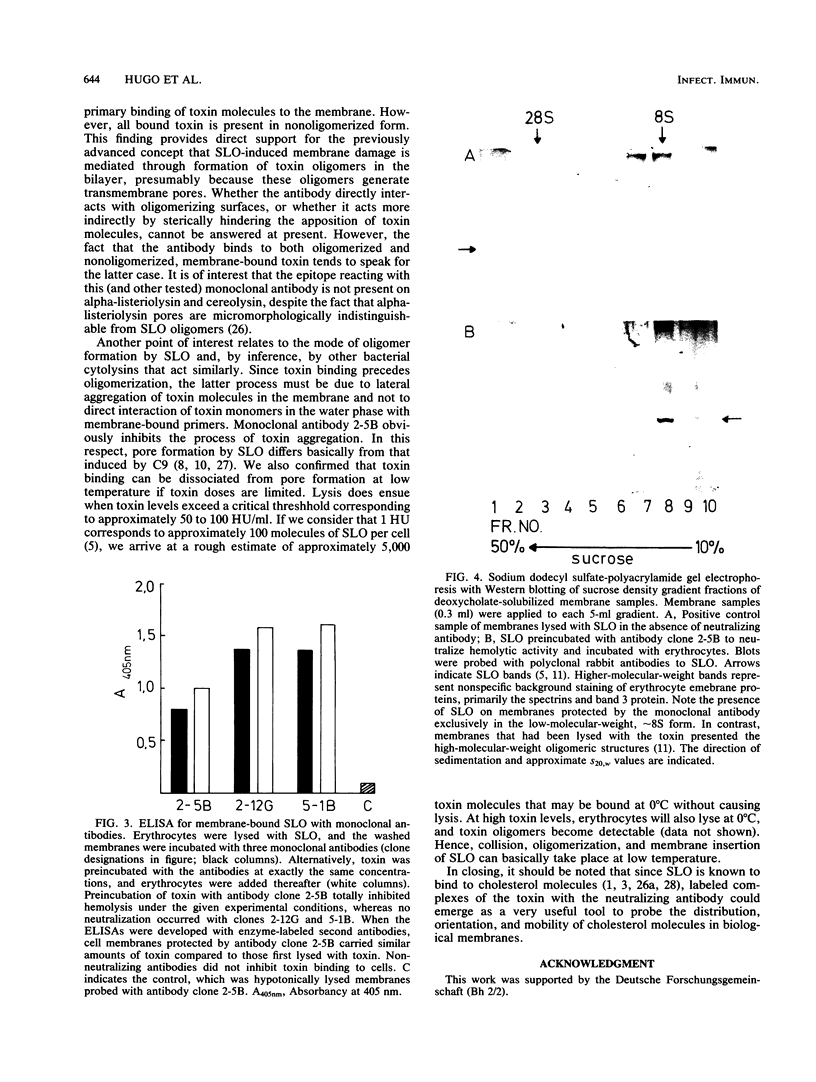
Murine monoclonal antibodies were generated against streptolysin O. One out of 10 tested immunoglobulin clones exhibited strong neutralizing activity; in solution, the presence of approximately two to four antibody molecules per toxin monomer effected 50% neutralization of hemolytic toxin activity. An enzyme-linked immunosorbent assay performed with target cell membranes that were treated with streptolysin O in the presence and absence of neutralizing antibodies showed that the antibodies did not block primary binding of the toxin to the cells. When membranes were solubilized in deoxycholate detergent and centrifuged in sucrose density gradients, those lysed with streptolysin O contained detergent-resistant, high-molecular-weight oligomers identical to the pore lesions, whereas those given toxin and neutralizing antibody contained the toxin exclusively in low-molecular-weight, nonoligomerized form. The process of pore formation by streptolysin O must thus involve two distinct steps, i.e., the primary binding of toxin molecules to the membrane followed by oligomerization of bound toxin monomers by lateral aggregation in the lipid bilayer to form the transmembrane pores.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Patel K., Pasternak C. A. Common action of certain viruses, toxins, and activated complement: pore formation and its prevention by extracellular Ca2+. Biosci Rep. 1984 Sep;4(9):797–805. doi: 10.1007/BF01128822. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Füssle R., Tranum-Jensen J. Staphylococcal alpha-toxin: oligomerization of hydrophilic monomers to form amphiphilic hexamers induced through contact with deoxycholate detergent micelles. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. C5b-9 assembly: average binding of one C9 molecule to C5b-8 without poly-C9 formation generates a stable transmembrane pore. J Immunol. 1986 Apr 15;136(8):2999–3005. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Mechanism of complement cytolysis and the concept of channel-forming proteins. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):311–324. doi: 10.1098/rstb.1984.0092. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by complement. Biochim Biophys Acta. 1983 Aug 11;737(3-4):343–372. doi: 10.1016/0304-4157(83)90006-0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. On the cause and nature of C9-related heterogeneity of terminal complement complexes generated on target erythrocytes through the action of whole serum. J Immunol. 1984 Sep;133(3):1453–1463. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham L., Duncan J. L. Approximate dimensions of membrane lesions produced by streptolysin S and streptolysin O. Biochim Biophys Acta. 1983 Mar 23;729(1):115–122. doi: 10.1016/0005-2736(83)90462-5. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Kim K. S., Bernheimer A. W. Alteration by cereolysin of the structure of cholesterol-containing membranes. Biochim Biophys Acta. 1978 Feb 21;507(2):230–241. doi: 10.1016/0005-2736(78)90419-4. [DOI] [PubMed] [Google Scholar]
- Dourmashkin R. R., Rosse W. F. Morphologic changes in the membranes of red blood cells undergoing hemolysis. Am J Med. 1966 Nov;41(5):699–710. doi: 10.1016/0002-9343(66)90031-3. [DOI] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman S., Sugg N. Effect of calcium ions on staphylococcal alpha-toxin-induced hemolysis of rabbit erythrocytes. Infect Immun. 1985 Jan;47(1):37–40. doi: 10.1128/iai.47.1.37-40.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Hugo F., Jenne D., Bhakdi S. Monoclonal antibodies against neoantigens of the terminal C5b-9 complex of human complement. Biosci Rep. 1985 Aug;5(8):649–658. doi: 10.1007/BF01116996. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Menestrina G. Ionic channels formed by Staphylococcus aureus alpha-toxin: voltage-dependent inhibition by divalent and trivalent cations. J Membr Biol. 1986;90(2):177–190. doi: 10.1007/BF01869935. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Sekiya T., Okamura S., Nozawa Y., Hase J. Ring formation of perfringolysin O as revealed by negative stain electron microscopy. Biochim Biophys Acta. 1979 Dec 12;558(3):307–313. doi: 10.1016/0005-2736(79)90265-7. [DOI] [PubMed] [Google Scholar]
- Parrisius J., Bhakdi S., Roth M., Tranum-Jensen J., Goebel W., Seeliger H. P. Production of listeriolysin by beta-hemolytic strains of Listeria monocytogenes. Infect Immun. 1986 Jan;51(1):314–319. doi: 10.1128/iai.51.1.314-319.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent D., Alouf J. E. Interaction of steptolysin O with sterols. Biochim Biophys Acta. 1976 Aug 16;443(2):288–300. doi: 10.1016/0005-2736(76)90511-3. [DOI] [PubMed] [Google Scholar]
- Silversmith R. E., Nelsestuen G. L. Assembly of the membrane attack complex of complement on small unilamellar phospholipid vesicles. Biochemistry. 1986 Feb 25;25(4):852–860. doi: 10.1021/bi00352a017. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Freer J. H., Arbuthnott J. P. Interaction of Clostridium perfringens theta-haemolysin, a contaminant of commercial phospholipase C, with erythrocyte ghost membranes and lipid dispersions. A morphological study. Biochim Biophys Acta. 1975 Apr 8;382(4):479–493. doi: 10.1016/0005-2736(75)90216-3. [DOI] [PubMed] [Google Scholar]
- Stähli C., Staehelin T., Miggiano V., Schmidt J., Häring P. High frequencies of antigen-specific hybridomas: dependence on immunization parameters and prediction by spleen cell analysis. J Immunol Methods. 1980;32(3):297–304. doi: 10.1016/0022-1759(80)90194-5. [DOI] [PubMed] [Google Scholar]