Abstract
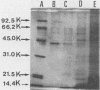
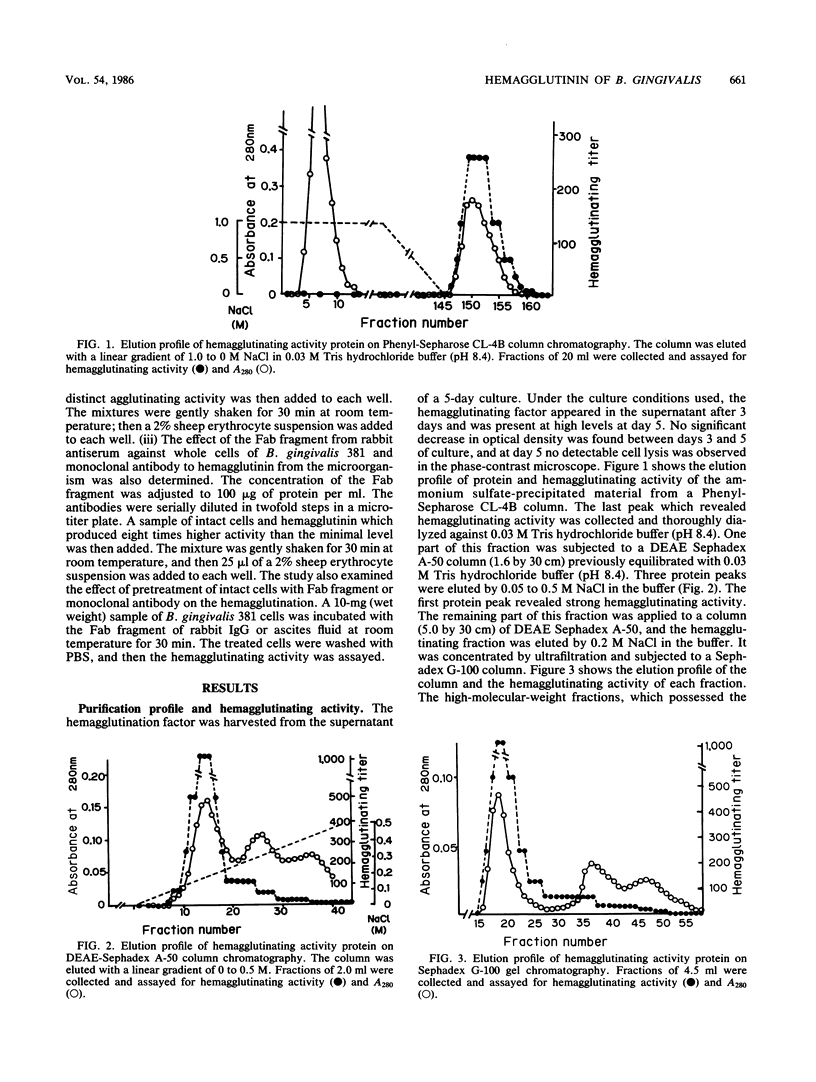
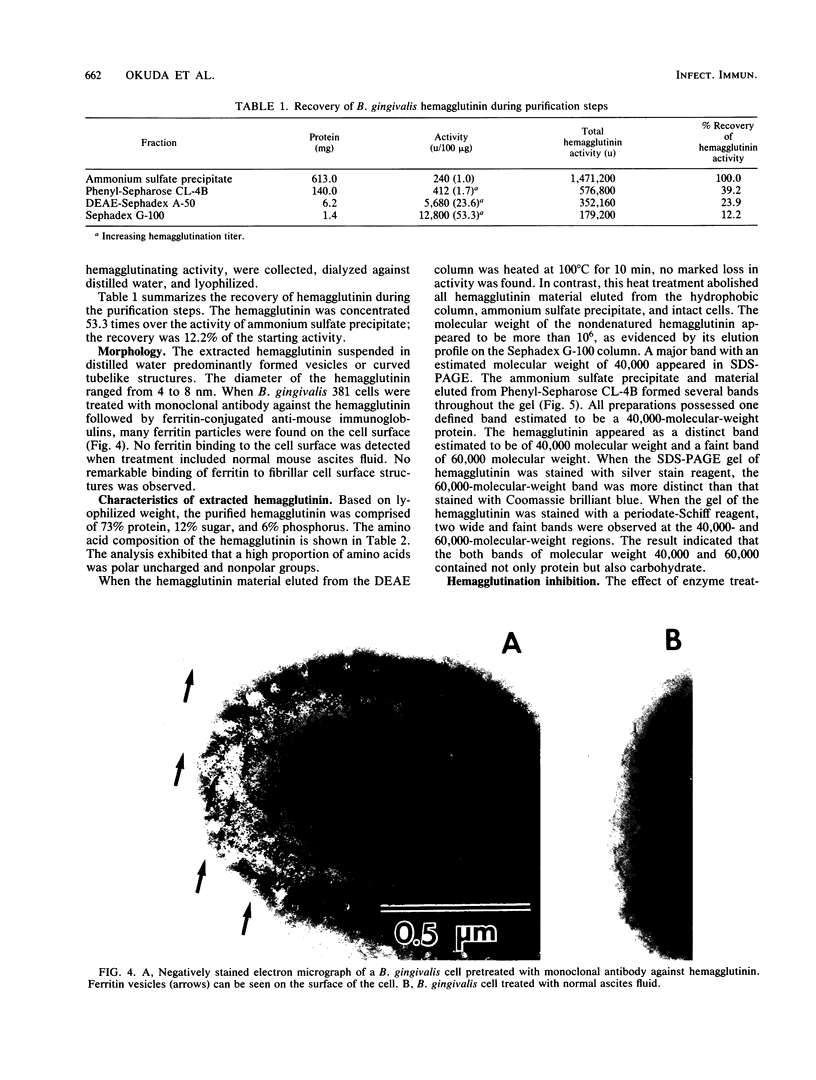
The hemagglutinating factor (hemagglutinin) of Bacteroides gingivalis was prepared from the supernatant of a 5-day diffusate broth culture by ammonium sulfate precipitation and column chromatography with a hydrophobic column of Phenyl-Sepharose CL-4B, DEAE-Sephadex A-50, and Sephadex G-100 gel filtration. The hemagglutinating activity of the preparation was 53.3 times higher than that of ammonium sulfate precipitate. In electron microphotographs, hemagglutinin appears to have a vesicle or tubelike structure. The hemagglutinating activity of intact cells was completely destroyed by heating at 100 degrees C for 10 min, but the activity of extracted hemagglutinin was heat stable. The activity of hemagglutinin was inhibited by L-arginine and L-lysine and partially inhibited by phospholipase D, but it was not affected by proteolytic enzymes, neuraminidase, hyaluronidase, lipase, phospholipase A and C, or sugars. The B. gingivalis hemagglutinin appeared to be comprised mainly of a 40,000-molecular-weight material. The Fab fragment of immunoglobulin G prepared from rabbit antiserum to whole cells of B. gingivalis and monoclonal antibody against the hemagglutinin bound to the cell surface and inhibited the hemagglutinating activity of both the cells and the purified hemagglutinin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J., McBride B. C. Fractionation of hemagglutinating and bacterial binding adhesins of Bacteroides gingivalis. Infect Immun. 1984 Aug;45(2):403–409. doi: 10.1128/iai.45.2.403-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Dehazya P., Coles R. S., Jr Extraction and properties of hemagglutinin from cell wall fragments of Fusobacterium nucleatum. J Bacteriol. 1982 Oct;152(1):298–305. doi: 10.1128/jb.152.1.298-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Heijl L., Wennström J., Lindhe J., Socransky S. S. Periodontal disease in gnotobiotic rats. J Periodontal Res. 1980 Jul;15(4):405–419. doi: 10.1111/j.1600-0765.1980.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Inoshita E., Amano A., Hanioka T., Tamagawa H., Shizukuishi S., Tsunemitsu A. Isolation and some properties of exohemagglutinin from the culture medium of Bacteroides gingivalis 381. Infect Immun. 1986 May;52(2):421–427. doi: 10.1128/iai.52.2.421-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo W., Sato M., Ozawa H. Haemagglutinating activity of Leptotrichia buccalis cells and their adherence to saliva-coated enamel powder. Arch Oral Biol. 1976;21(6):363–369. doi: 10.1016/s0003-9969(76)80004-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mongiello J. R., Falkler W. A., Jr Sugar inhibition of oral Fusobacterium nucleatum haemagglutination and cell binding. Arch Oral Biol. 1979;24(7):539–545. doi: 10.1016/0003-9969(79)90133-x. [DOI] [PubMed] [Google Scholar]
- Mouton C., Hammond P. G., Slots J., Genco R. J. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periondontal disease. Infect Immun. 1981 Jan;31(1):182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahata T., Kiyoshige T., Tomono S., Abe R., Sasaki S., Takazoe I. Oral implantation of Bacteroides asaccharolyticus and Eikenella corrodens in conventional hamsters. Infect Immun. 1982 Apr;36(1):304–309. doi: 10.1128/iai.36.1.304-309.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Okuda K., Kato T., Takazoe I. Monoclonal antibodies against surface antigens of Bacteroides gingivalis. Infect Immun. 1985 Oct;50(1):231–235. doi: 10.1128/iai.50.1.231-235.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Okuda K., Takazoe I. Immunoglobulin G response to subgingival gram-negative bacteria in human subjects. Infect Immun. 1984 Jul;45(1):47–51. doi: 10.1128/iai.45.1.47-51.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Naito Y., Ohta K., Fukumoto Y., Kimura Y., Ishikawa I., Kinoshita S., Takazoe I. Bacteriological study of periodontal lesions in two sisters with juvenile periodontitis and their mother. Infect Immun. 1984 Jul;45(1):118–121. doi: 10.1128/iai.45.1.118-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Takazoe I. Haemagglutinating activity of Bacteroides melaninogenicus. Arch Oral Biol. 1974 May;19(5):415–416. doi: 10.1016/0003-9969(74)90184-8. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peros W. J., Etherden I., Gibbons R. J., Skobe Z. Alteration of fimbriation and cell hydrophobicity by sublethal concentrations of tetracycline. J Periodontal Res. 1985 Jan;20(1):24–30. doi: 10.1111/j.1600-0765.1985.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Hausmann E. Longitudinal study of experimentally induced periodontal disease in Macaca arctoides: relationship between microflora and alveolar bone loss. Infect Immun. 1979 Feb;23(2):260–269. doi: 10.1128/iai.23.2.260-269.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Ebisu S., Okada H. Eikenella corrodens adherence to human buccal epithelial cells. Infect Immun. 1981 Jan;31(1):21–27. doi: 10.1128/iai.31.1.21-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]