Abstract
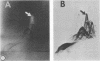
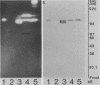
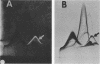
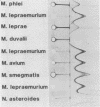
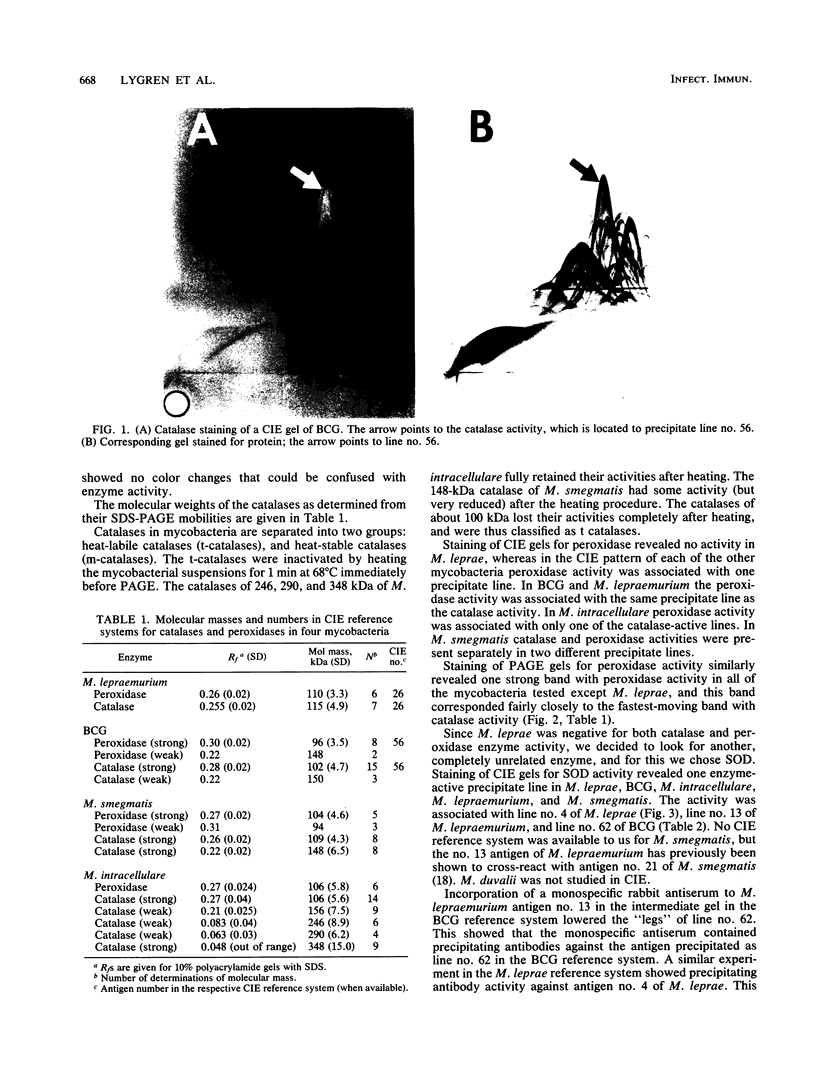
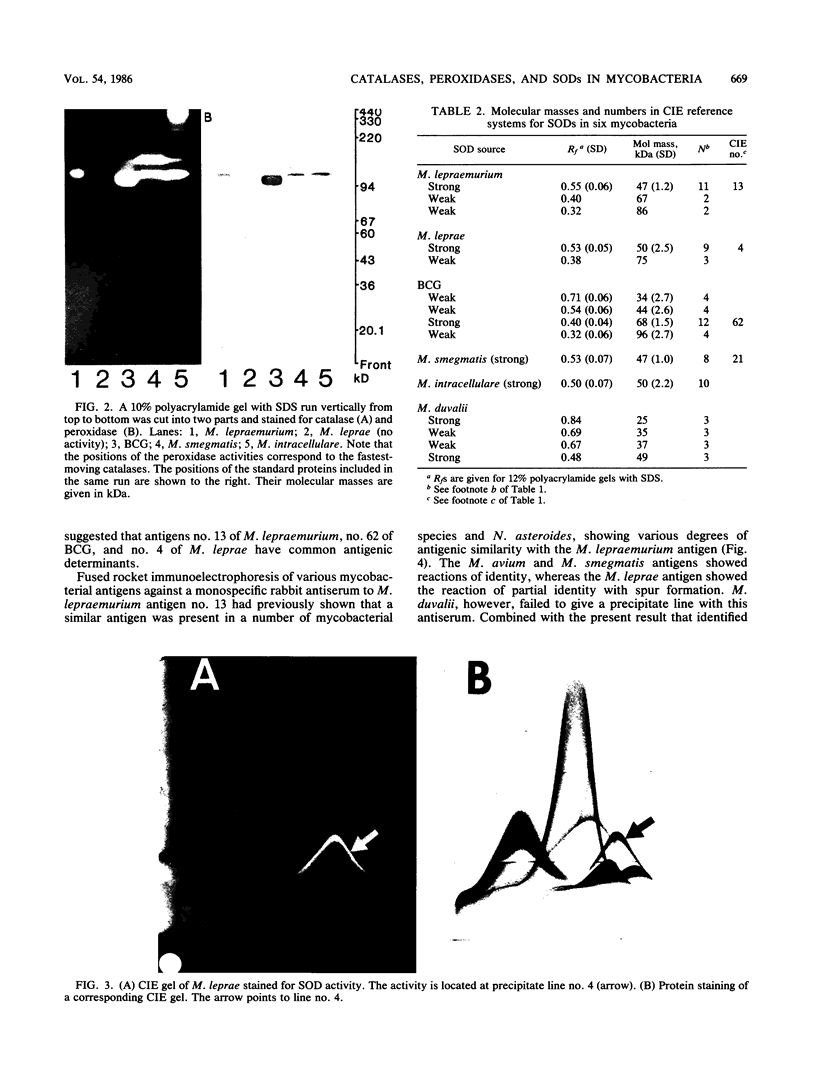
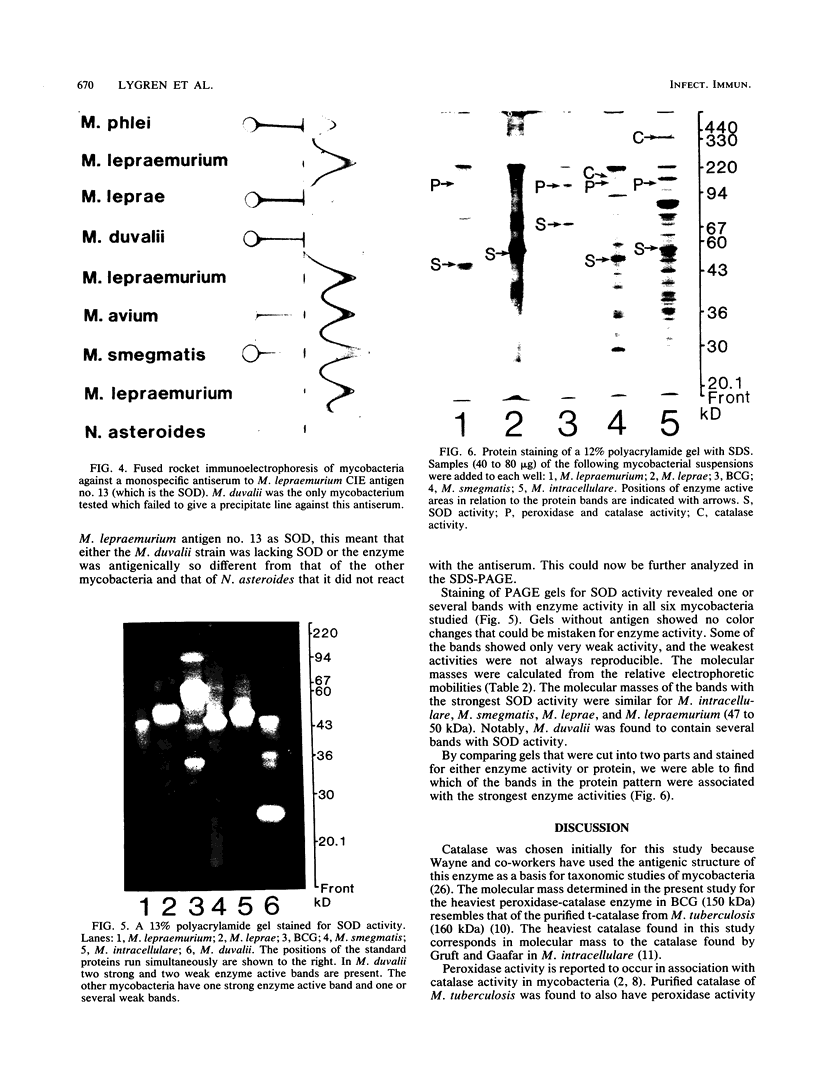
The five mycobacteria Mycobacterium lepraemurium, M. leprae, M. bovis BCG, M. smegmatis, and M. intracellulare were studied. Catalase and peroxidase activities were demonstrated in polyacrylamide and crossed immunoelectrophoresis gels for M. lepraemurium, M. intracellulare, and BCG, but not for M. leprae. Peroxidase and catalase activities were associated with the same precipitate line in crossed immunoelectrophoresis for M. lepraemurium, M. intracellulare, and BCG, showing that in these mycobacteria the two enzyme activities resided in the same molecule. M. smegmatis peroxidase and catalase activities were closely associated on polyacrylamide gel electrophoresis, but on the crossed immunoelectrophoresis catalase and peroxidase activities were associated with two different precipitate lines. Catalases without peroxidase activity were demonstrated in crossed immunoelectrophoresis and polyacrylamide gel electrophoresis in M. intracellulare and M. smegmatis. The catalase without peroxidase activity in M. intracellulare was heat resistant and therefore classified as an m-catalase. In M. smegmatis the catalase without peroxidase activity was only partially heat resistant. All of the catalases with peroxidase activity were heat-sensitive t-catalases. Superoxide dismutase activity in the crossed immunoelectrophoresis was associated with the M. leprae antigen no. 4 and with cross-reacting antigens in the other mycobacteria studied. Several superoxide dismutases were demonstrated in Mycobacterium duvalii. They were antigenically different from the other superoxide dismutases in this study, as shown by lack of reactivity with a monospecific antibody to M. lepraemurium superoxide dismutase. Molecular weights were estimated for all the enzymes in this study by sodium dodecyl sulfate-polyacrylamide gels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen N. H. Intermediate gel in crossed and in fused rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:71–77. doi: 10.1111/j.1365-3083.1973.tb03782.x. [DOI] [PubMed] [Google Scholar]
- Bartholomew W. R. Multiple catalase enzymes in two species of mycobacteria. Am Rev Respir Dis. 1968 Apr;97(4):710–712. doi: 10.1164/arrd.1968.97.4.710. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Closs O., Mshana R. N., Harboe M. Antigenic analysis of Mycobacterium leprae. Scand J Immunol. 1979 Mar;9(3):297–302. doi: 10.1111/j.1365-3083.1979.tb02735.x. [DOI] [PubMed] [Google Scholar]
- Closs O., Reitan L. J. In vitro lymphocyte stimulation using a purified antigen of Mycobacterium leprae and tuberculin PPD. Lepr Rev. 1981 Dec;52 (Suppl 1):251–262. [PubMed] [Google Scholar]
- Davis W. B., Phillips D. M. Differentiation of catalases in Mycobacterium phlei on the basis of susceptibility to isoniazid: association with peroxidase and acquired resistance to isoniazid. Antimicrob Agents Chemother. 1977 Oct;12(4):529–533. doi: 10.1128/aac.12.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi B. G., Shaila M. S., Ramakrishnan T., Gopinathan K. P. The purification and properties of peroxidase in Mycobacterium tuberculosis H37Rv and its possible role in the mechanism of action of isonicotinic acid hydrazide. Biochem J. 1975 Jul;149(1):187–197. doi: 10.1042/bj1490187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz G. A., Wayne L. G. Isolation and characterization of catalase produced by Mycobacterium tuberculosis. Am Rev Respir Dis. 1974 Sep;110(3):312–319. doi: 10.1164/arrd.1974.110.3.312. [DOI] [PubMed] [Google Scholar]
- Gruft H., Gaafar H. A. Multiple catalases of mycobacteria: differences in molecular weight. Am Rev Respir Dis. 1974 Sep;110(3):320–323. doi: 10.1164/arrd.1974.110.3.320. [DOI] [PubMed] [Google Scholar]
- Harboe M., Mshana R. N., Closs O., Kronvall G., Axelsen N. H. Cross-reactions between mycobacteria. II. Crossed immunoelectrophoretic analysis of soluble antigens of BCG and comparison with other mycobacteria. Scand J Immunol. 1979;9(2):115–124. doi: 10.1111/j.1365-3083.1979.tb02713.x. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Kusunose E., Kusunose M., Mori T. Superoxide dismutase from Mycobacterium lepraemurium. J Biochem. 1977 May;81(5):1427–1433. [PubMed] [Google Scholar]
- Katoch V. M., Wayne L. G., Diaz G. A. Serological approaches for the characterization of catalase in tissue-derived mycobacteria. Ann Microbiol (Paris) 1982 Nov-Dec;133(3):407–414. [PubMed] [Google Scholar]
- Kronvall G., Bjune G., Stanford J., Menzel S., Samuel D. Mycobacterial antigens in antibody responses of leprosy patients. Int J Lepr Other Mycobact Dis. 1975 Oct-Dec;43(4):306–306. [PubMed] [Google Scholar]
- Kronvall G., Closs O., Bjune G. Common antigen of Mycobacterium leprae, M. lepraemurium, M. avium, and M. fortuitum in comparative studies using two different types of antisera. Infect Immun. 1977 May;16(2):542–546. doi: 10.1128/iai.16.2.542-546.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Stanford J. L., Walsh G. P. Studies of mycobacterial antigens, with special reference to Mycobacterium leprae. Infect Immun. 1976 Apr;13(4):1132–1138. doi: 10.1128/iai.13.4.1132-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Stanford J. L., Rook G. A., Convit J., Godal T., Kronvall G., Rees R. J., Walsh G. P. Preliminary taxonomic studies on the leprosy bacillus. Br J Exp Pathol. 1975 Dec;56(6):579–585. [PMC free article] [PubMed] [Google Scholar]
- Stavri D., Niculescu D., Stavri H. The Mycobacterium smegmatis peroxidase, cross-reacting antigen with Mycobacterium leprae. Arch Roum Pathol Exp Microbiol. 1981 Apr-Jun;40(2):123–126. [PubMed] [Google Scholar]
- Wheeler P. R., Gregory D. Superoxide dismutase, peroxidatic activity and catalase in Mycobacterium leprae purified from armadillo liver. J Gen Microbiol. 1980 Dec;121(2):457–464. doi: 10.1099/00221287-121-2-457. [DOI] [PubMed] [Google Scholar]
- Widebäck K., Kronvall G., Bjorvatn B., Closs O., Harboe M. Comparative studies of antigen 21 in Mycobacterium and Nocardia species: possible taxonomic relationships with Mycobacterium leprae. Infect Immun. 1980 Nov;30(2):413–420. doi: 10.1128/iai.30.2.413-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury W., Spencer A. K., Stahman M. A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971 Nov;44(1):301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]