Abstract
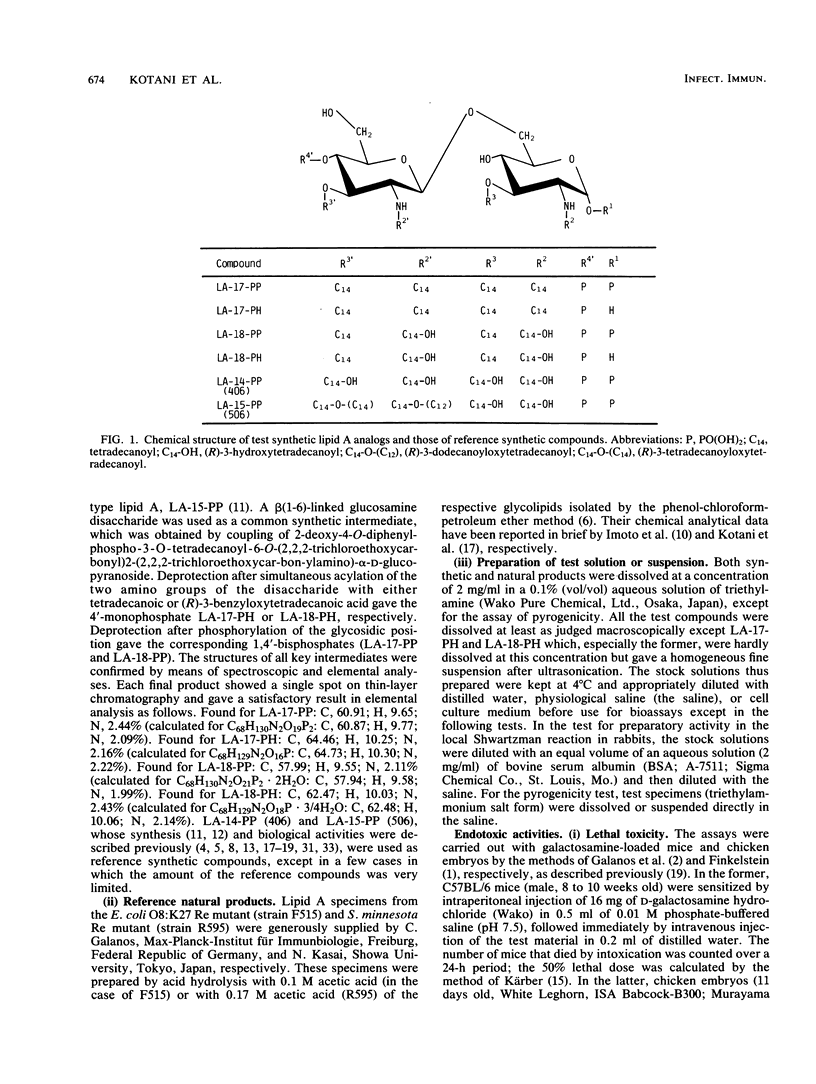
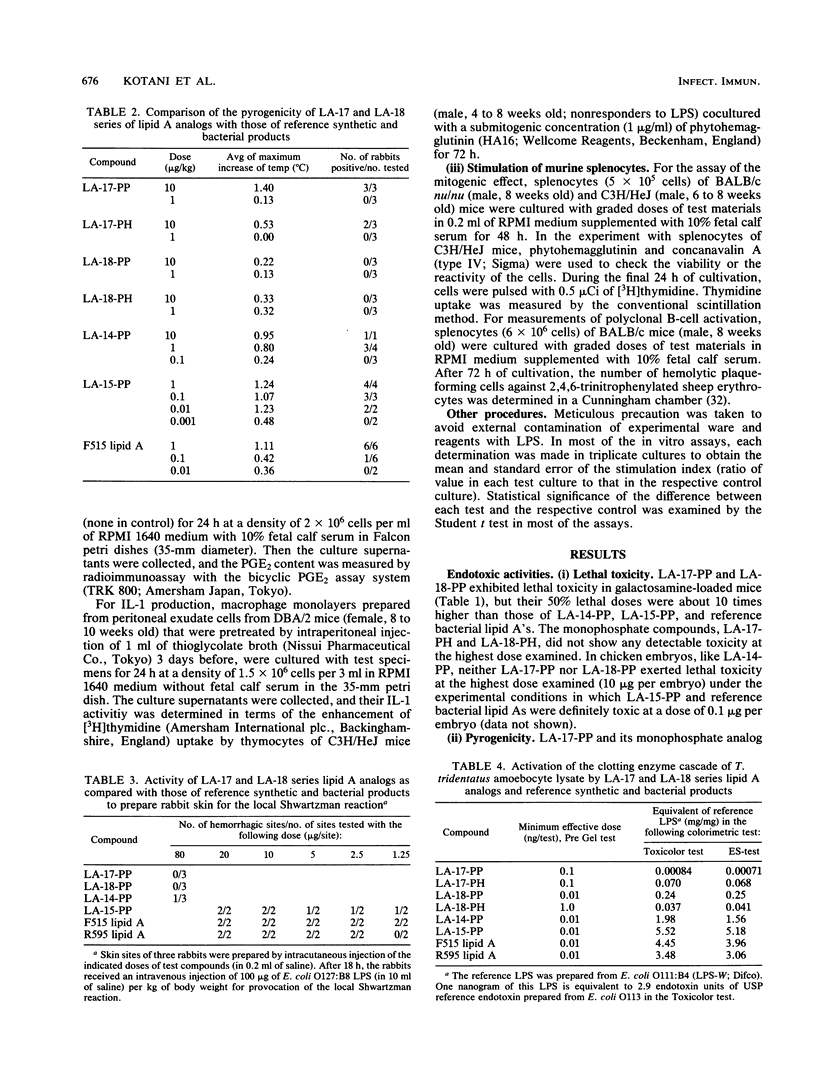
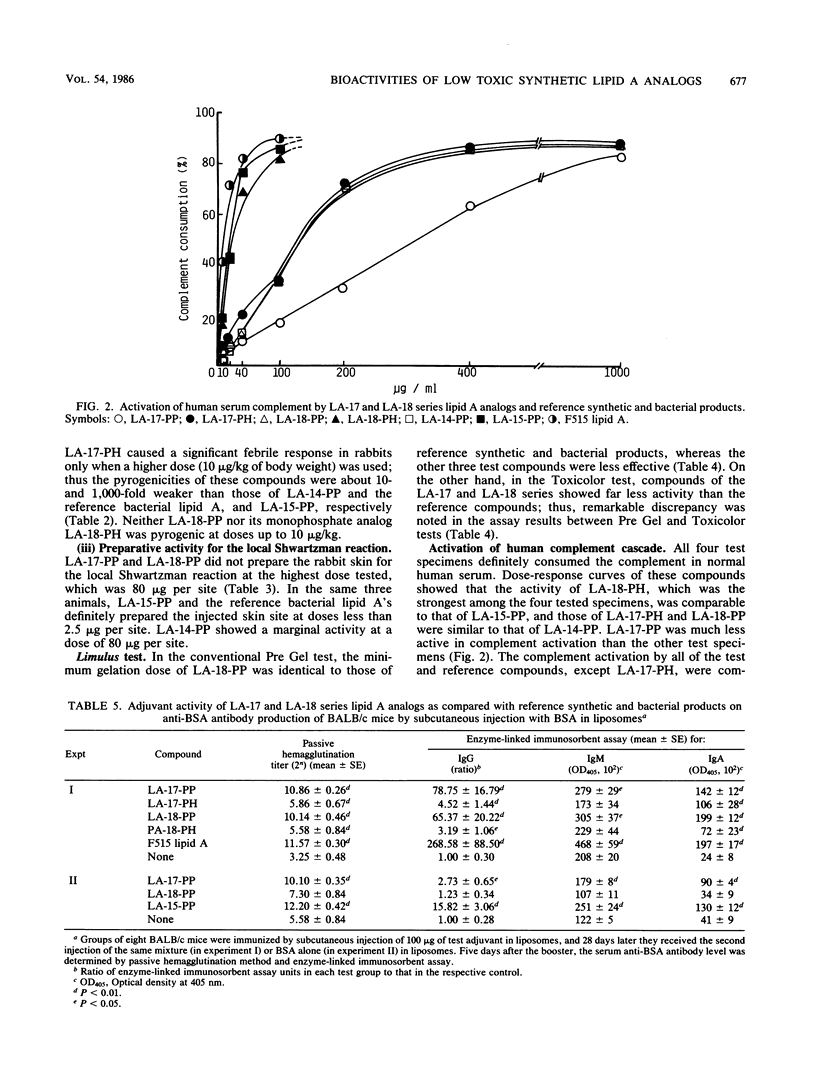
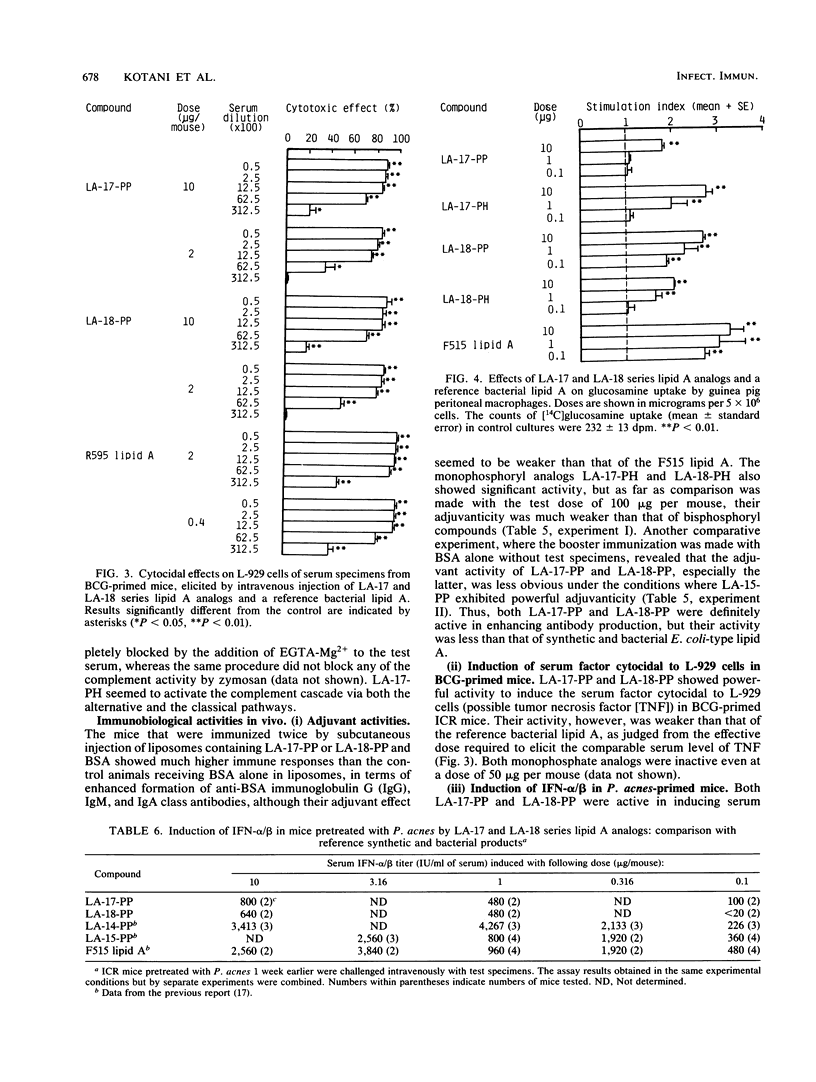
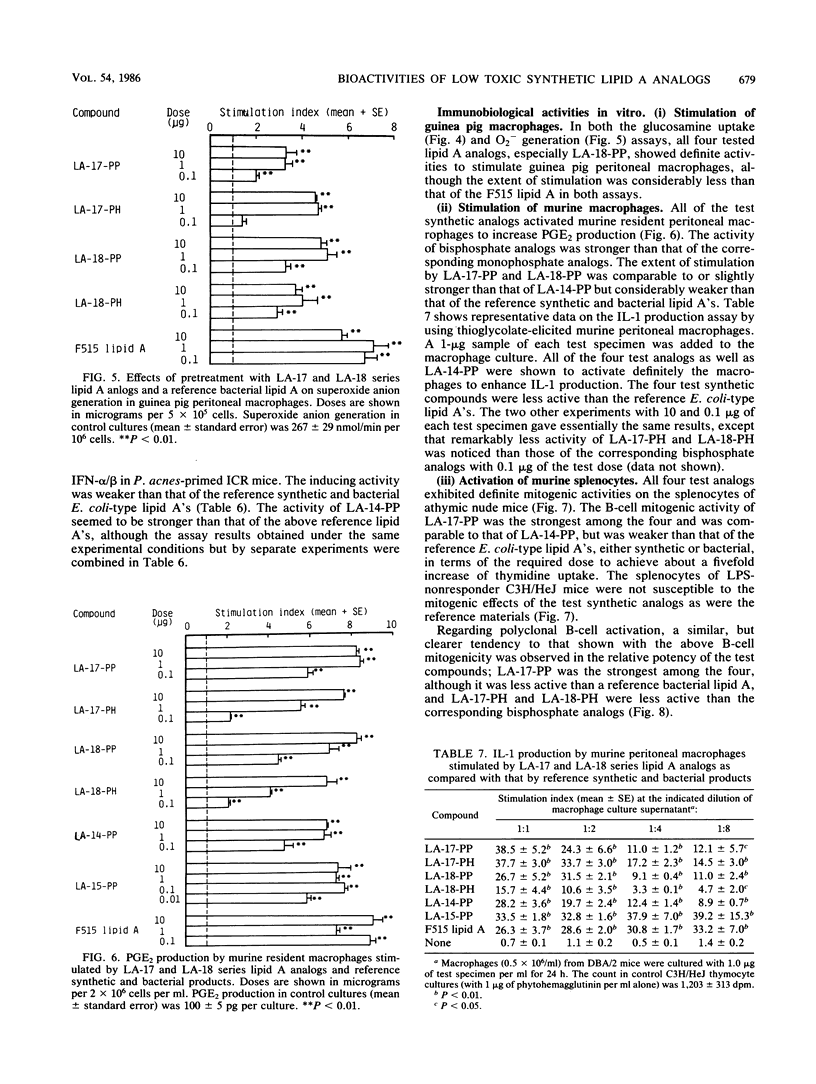
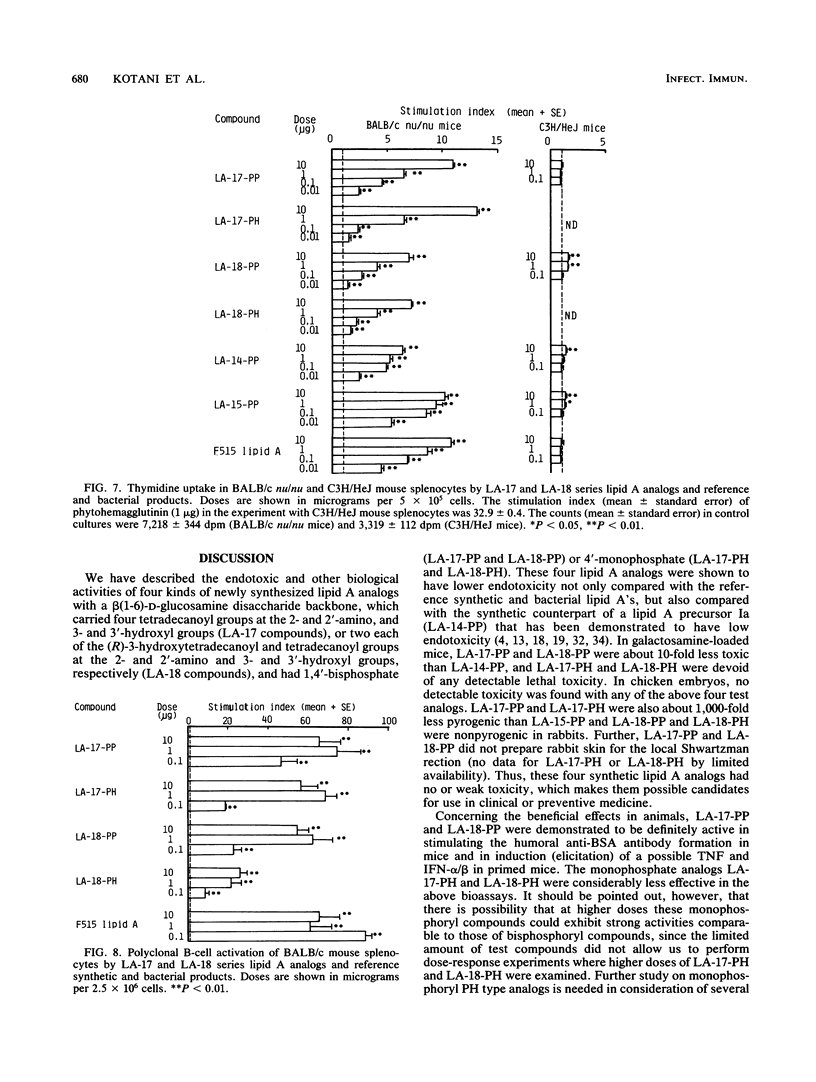
Synthetic lipid A analogs, beta(1-6)glucosamine disaccharide 1,4'-bisphosphates, which possesses four tetradecanoyl groups at the 2- and 2'-amino, and 3- and 3'-hydroxyl groups (LA-17-PP), and each two of the (R)-3-hydroxytetradecanoyl and tetradecanoyl groups at the 2- and 2'-amino and 3- and 3'-hydroxyl groups, respectively (LA-18-PP), were far less endotoxic than synthetic (506, LA-15-PP) and bacterial Escherichia coli type lipid A's; neither compound showed any detectable lethal toxicity in chicken embryos or preparatory activity for the local Shwartzman reaction in rabbits. Also both compounds were only weakly pyrogenic and comparably less lethally toxic in galactosamine-loaded mice than the reference synthetic and bacterial lipid A's and a synthetic counterpart to biosynthetic lipid A precursor Ia (406, LA-14-PP). Nevertheless, LA-17-PP and LA-18-PP exhibited definite in vivo immunoadjuvant activity in mice, and the ability to induce a possible tumor necrosis factor and alpha/beta interferon in Mycobacterium bovis BCG and Propionibacterium acnes-primed mice, respectively, although these activities were weaker than those of the reference lipid A's. 4'-Monophosphate analogs of the above two test compounds exhibited neither endotoxic nor beneficial activities, but they showed remarkable in vitro bioactivities comparable to those of the corresponding bisphosphate compounds; the ability to activate the human complement system and the clotting enzyme cascade of horseshoe crab amoebocyte lysate, stimulatory effects on guinea pig and murine peritoneal macrophages, and murine splenocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FINKELSTEIN R. A. OBSERVATIONS ON MODE OF ACTION OF ENDOTOXIN IN CHICK EMBRYOS. Proc Soc Exp Biol Med. 1964 Mar;115:702–707. doi: 10.3181/00379727-115-29012. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Hansen-Hagge T., Lehmann V., Lüderitz O. Comparison of the capacity of two lipid A precursor molecules to express the local Shwartzman phenomenon. Infect Immun. 1985 May;48(2):355–358. doi: 10.1128/iai.48.2.355-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hansen-Hagge T., Lehmann V., Lüderitz O. Free flow electrophoresis as a tool for enrichment of mutants with temperature-dependent lethal mutations in lipid A synthesis. Eur J Biochem. 1985 Apr 1;148(1):21–27. doi: 10.1111/j.1432-1033.1985.tb08801.x. [DOI] [PubMed] [Google Scholar]
- Hansen-Hagge T., Lehmann V., Seydel U., Lindner B., Zähringer U. Isolation and structural analysis of two lipid A precursors from a KDO deficient mutant of Salmonella typhimurium differing in their hexadecanoic acid content. Arch Microbiol. 1985 May;141(4):353–358. doi: 10.1007/BF00428849. [DOI] [PubMed] [Google Scholar]
- Homma J. Y., Matsuura M., Kanegasaki S., Kawakubo Y., Kojima Y., Shibukawa N., Kumazawa Y., Yamamoto A., Tanamoto K., Yasuda T. Structural requirements of lipid A responsible for the functions: a study with chemically synthesized lipid A and its analogues. J Biochem. 1985 Aug;98(2):395–406. doi: 10.1093/oxfordjournals.jbchem.a135294. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Kojima Y., Matsuura M., Homma J. Y., Yamamoto A., Kumazawa Y., Tanamoto K., Yasuda T., Tsumita T., Imoto M. Biological activities of analogues of lipid A based chemically on the revised structural model. Comparison of mediator-inducing, immunomodulating and endotoxic activities. Eur J Biochem. 1984 Sep 3;143(2):237–242. doi: 10.1111/j.1432-1033.1984.tb08364.x. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Tanamoto K., Yasuda T., Homma J. Y., Matsuura M., Nakatsuka M., Kumazawa Y., Yamamoto A., Shiba T., Kusumoto S. Structure-activity relationship of lipid A: comparison of biological activities of natural and synthetic lipid A's with different fatty acid compositions. J Biochem. 1986 Apr;99(4):1203–1210. doi: 10.1093/oxfordjournals.jbchem.a135583. [DOI] [PubMed] [Google Scholar]
- Kotani S., Takada H., Takahashi I., Tsujimoto M., Ogawa T., Ikeda T., Harada K., Okamura H., Tamura T., Tanaka S. Low endotoxic activities of synthetic Salmonella-type lipid A with an additional acyloxyacyl group on the 2-amino group of beta (1-6) glucosamine disaccharide 1,4'-bisphosphate. Infect Immun. 1986 Jun;52(3):872–884. doi: 10.1128/iai.52.3.872-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Harada K., Mori Y., Kawasaki A., Tanaka A., Nagao S., Tanaka S. Immunobiologically active lipid A analogs synthesized according to a revised structural model of natural lipid A. Infect Immun. 1984 Jul;45(1):293–296. doi: 10.1128/iai.45.1.293-296.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Takahashi I., Ikeda T., Otsuka K., Shimauchi H., Kasai N., Mashimo J. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun. 1985 Jul;49(1):225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T. Addition of perchloric acid to blood samples for colorimetric limulus test using chromogenic substrate: comparison with conventional procedures and clinical applications. J Lab Clin Med. 1984 Sep;104(3):321–330. [PubMed] [Google Scholar]
- Obayashi T., Tamura H., Tanaka S., Ohki M., Takahashi S., Arai M., Masuda M., Kawai T. A new chromogenic endotoxin-specific assay using recombined limulus coagulation enzymes and its clinical applications. Clin Chim Acta. 1985 Jun 30;149(1):55–65. doi: 10.1016/0009-8981(85)90273-6. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Shimauchi H. Enhancement of serum antibody production in mice by oral administration of lipophilic derivatives of muramyl peptides and bacterial lipopolysaccharides with bovine serum albumin. Methods Find Exp Clin Pharmacol. 1986 Feb;8(2):117–125. [PubMed] [Google Scholar]
- Okamura H., Kawaguchi K., Shoji K., Kawade Y. High-level induction of gamma interferon with various mitogens in mice pretreated with Propionibacterium acnes. Infect Immun. 1982 Nov;38(2):440–443. doi: 10.1128/iai.38.2.440-443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Ribi E., Cantrell J. L., Takayama K., Qureshi N., Peterson J., Ribi H. O. Lipid A and immunotherapy. Rev Infect Dis. 1984 Jul-Aug;6(4):567–572. doi: 10.1093/clinids/6.4.567. [DOI] [PubMed] [Google Scholar]
- Schuster B. G., Neidig M., Alving B. M., Alving C. R. Production of antibodies against phosphocholine, phosphatidylcholine, sphingomyelin, and lipid A by injection of liposomes containing lipid A. J Immunol. 1979 Mar;122(3):900–905. [PubMed] [Google Scholar]
- Strain S. M., Armitage I. M., Anderson L., Takayama K., Qureshi N., Raetz C. R. Location of polar substituents and fatty acyl chains on lipid A precursors from a 3-deoxy-D-manno-octulosonic acid-deficient mutant of Salmonella typhimurium. Studies by 1H, 13C, and 31P nuclear magnetic resonance. J Biol Chem. 1985 Dec 25;260(30):16089–16098. [PubMed] [Google Scholar]
- Takada H., Kotani S., Tsujimoto M., Ogawa T., Takahashi I., Harada K., Katsukawa C., Tanaka S., Shiba T., Kusumoto S. Immunopharmacological activities of a synthetic counterpart of a biosynthetic lipid A precursor molecule and of its analogs. Infect Immun. 1985 Apr;48(1):219–227. doi: 10.1128/iai.48.1.219-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Ribi E., Cantrell J. L. Separation and characterization of toxic and nontoxic forms of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):439–443. doi: 10.1093/clinids/6.4.439. [DOI] [PubMed] [Google Scholar]
- Ukei S., Iida J., Shiba T., Kusumoto S., Azuma I. Adjuvant and antitumour activities of synthetic lipid A analogues. Vaccine. 1986 Mar;4(1):21–24. doi: 10.1016/0264-410x(86)90093-9. [DOI] [PubMed] [Google Scholar]



